Abstract
DEPTOR [DEP-domain-containing and mTOR (mammalian target of rapamycin)-interacting protein] is a modulator of mTOR signalling that binds to mTORC (mTOR complex) 1 and mTORC2. However, to date, the precise functions of DEPTOR are not fully elucidated, particularly in reproductive tissues where mTOR acts as a placental nutrient sensor. Pregnancy is associated with major physiological and psychosocial changes and adaptation to these changes is crucial for normal fetal development. In the present study, we tested the hypothesis that maternal stress can affect mTOR signalling at term, and, as a result, influence placental growth. We first investigated the expression of DEPTOR, mTOR, rictor (rapamycin-insensitive companion of mTOR) and raptor (regulatory associated protein of mTOR) from human placentas (n=23) using Q-PCR (quantitative PCR), and correlated these data to days of pregnancy and maternal stress, as well as placental and fetal weight. Maternal and fetal cortisol levels were also measured. JEG-3 and BeWo cells, used as placental in vitro models, were treated with cortisol and DEPTOR expression was assessed using Q-PCR. DEPTOR appears to be the predominant transcript in the human placenta compared with mTOR, rictor and raptor in both term (n=13) and preterm (n=10) placentas as assessed by Q-PCR. There was a significantly lower level only of log-DEPTOR gene expression in the high stress group (−1.34) than in the low stress group (0.07; t20=2.41, P=0.026). Interestingly, mothers with high stress had significantly elevated levels of cortisol (8555 pg/ml) compared with those with low stress (4900 pg/ml). We then tested the hypothesis that cortisol can directly affect DEPTOR expression. When BeWo cells were treated with cortisol 10, 100 and 1000 nM, the expression of DEPTOR was significantly down-regulated by 50, 41 and 39% (all P<0.05) respectively when compared with basal levels. Treatment of JEG-3 cells with cortisol, led to a significant decrease of DEPTOR expression at 100 nM (39%, P<0.05) and at 1000 nM (73%, P<0.01). These novel findings are indicative of a higher order of complexity of DEPTOR signalling in the human placenta that is affected by maternal stress, which could affect pregnancy outcome.
Keywords: cortisol, DEP-domain-containing and mammalian target of rapamycin-interacting protein (DEPTOR), mammalian target of rapamycin (mTOR), maternal stress, placenta
Abbreviations: BMI, body mass index; CS, Caesarean section; DAPI, 4′,6-diamidino-2-phenylindole; DEPTOR, DEP-domain-containing and mammalian target of rapamycin-interacting protein; FBS, fetal bovine serum; GβL, G-protein β-like; IUGR, intrauterine growth restriction; MEM, minimal essential medium; mTOR, mammalian target of rapamycin; mTORC, mTOR complex; Q-PCR, quantitative PCR; raptor, regulatory associated protein of mTOR; rictor, rapamycin-insensitive companion of mTOR; RQ, relative quantity S6K1, S6 kinase 1
INTRODUCTION
A vital factor for fetal development is nutrient transport at the placental level. This is because any disturbances in the maternal compartments, for example due to maternal stress or nutritional status, which will affect fetal development, will involve the feto–placental barrier. Interestingly, the mTOR (mammalian target of rapamycin) is a highly conserved serine/threonine protein kinase [1] that functions as an ATP and amino acid sensor to balance nutrient availability and cell growth [2]. mTOR is capable of forming two complexes named mTORC1 and mTORC2, which differ in their composition, are regulated in distinct ways and can exert distinct biological effects. The rapamycin-sensitive mTORC1 contains the following proteins: raptor (regulatory associated protein of mTOR), GβL [G-protein β-like; also known as mLST8 mammalian lethal with SEC13 protein 8)], and PRAS40 (proline-rich Akt substrate of 40 kDa). mTORC1 regulates protein synthesis and cell growth by phosphorylating downstream target proteins such as p70 ribosomal S6K1 (S6 kinase 1) and the eukaryotic initiation factor 4E-BP1 (eukaryotic initiation factor 4E-binding protein 1) [3,4]. The rapamycin-insensitive mTORC2 complex contains: rictor (rapamycin-insensitive companion of mTOR), mSIN1 (stress-activated protein kinase-interacting protein 1), Protor-1 and GβL and functions as an Akt kinase that phosphorylates Ser473 of Akt in the ‘hydrophobic motif’ that is essential for full activation of this kinase [5–7].
Recently, another modulator of the mTOR pathway, termed DEPTOR (DEP-domain-containing mTOR-interacting protein) has been described. DEPTOR binds to both mTORC1 and mTORC2, as evident from co-immunoprecipitation experiments [8]. Its precise function is not fully elucidated, but Peterson et al. [8] have shown in a series of elegant experiments that knocking down DEPTOR leads to activation of signalling through mTORC1 and mTORC2. This is demonstrated both by the observation that there is a change in the phosphorylation status of S6K1 and Akt when DEPTOR levels are decreased (by RNA-based interference) and by the increased in vitro activity against these substrates of mTOR complexes from cells with decreased levels of DEPTOR [8,9]. To date, very little is known about the involvement of DEPTOR in disease pathogenesis. DEPTOR is down-regulated in a number of cancers such as prostate, bladder, cervix and thyroid. Surprisingly, DEPTOR is highly overexpressed in a subset of multiple myelomas harbouring cyclin D1/D3 or c-MAF/MAFB translocations [8]. RSV (resveratrol) is a naturally occurring polyphenol that has been found to exert antioxidant, anti-inflammatory and neuroprotective properties, and inhibits mTOR signalling by promoting the interaction between mTOR and DEPTOR in C2C12 fibroblasts [10]. Results from our laboratory have demonstrated a significant up-regulation of DEPTOR in two paclitaxel-resistant ovarian cancer cell lines when compared with the parental ones [11].
The cross-talk between the mother and the fetus is complex as well as bidirectional. Apart from a plethora of hormonal and immunological events that take place during pregnancy, maternal stress is a powerful contributor to birth outcomes. Maternal psychological anxiety can be as a result of pregnancy-related anxiety, depression or distress [12]. Elevated levels of maternal anxiety have been linked with preterm labour and low birth weight babies [13], and there is evidence for a link between higher maternal anxiety and poor offspring development, including problems in cognition and behaviour. Moreover, maternal stress is linked to poor fetal development in utero [14,15]. Since fetal growth is critically dependent on placental nutrient transport, placental mTOR signalling plays an important role in the regulation of fetal growth [16]. Recent findings suggest that mTOR functions as an important placental growth signalling sensor, linking maternal nutrient and growth factor concentrations to amino acid transport [17,18]. Moreover, in placentas from IUGR (intrauterine growth restriction) pregnancies, the protein expression of placental phospho-S6K1 (Thr389), a measure of the activity of mTORC1, was significantly reduced [18]. In an HT (hyperthermia)-induced IUGR model in sheep, a range of changes were apparent regarding the mTOR pathway: there was upregulation of the phosphorylation status of placental mTOR and Akt and decrease of p70 ribosomal S6K [19]. Collectively, these results indicate that fetal stress can affect the placental mTOR signalling pathway [19].
However, the effects of maternal stress on fetal growth are not consistent. In a large cohort study in Denmark, maternal life stress during pregnancy was associated with increased placental weight at birth [20]. Given that mTOR can function as a human placental sensor for nutrients and energy balance, and since maternal stress may influence such factors, we hypothesized that maternal stress can affect mTOR signalling at term, and, as a result, influence placental growth. These issues were investigated both in vivo and in vitro study using clinical samples and placental cell lines respectively.
MATERIALS AND METHODS
Subjects
The study population consisted of pregnant women attending the Department of Obstetrics and Gynaecology, University Hospital, University of Crete. The participants were in the third trimester of their pregnancy. All participants gave informed consent to participate in the study and ethical approval was granted by the local ethics committee of the hospital.
Study design
This was a prospective study in which women's stress levels and attitude towards their pregnancy were measured during the third trimester. All data were collected from September 2007 to March 2008. The women were approached for the first time by the researchers in the prenatal clinic without knowing about the questions. Questions were used to acquire anthropometrical data such as gestational age, weight before conception, current weight and height. After delivery, the weight of the infant was recorded along with the placental weight. A face-to-face interview was conducted with each woman lasting at least 30 min. There were specific questions related to the stress profile, which included: whether the pregnancy was planned or not planned; how stressed each woman was during the pregnancy, with responses ranging from 1 to 4 (1=low, 2=medium, 3=high, 4=very high). This stress questionnaire was based on a study by Wang et al. [21] where women with dysmenorrhoea were asked to describe their stress in preceding cycles as ‘low’, ‘medium’ or ‘high’. The demographic details are given in Table 1.
Table 1. Demographic details of the patients involved in the present study.
Values are means±S.D.
| Parameter | Value |
|---|---|
| Height (cm) | 168±0.04 |
| Pre-pregnancy weight (kg) | 66.0±18.5 |
| BMI (kg/m2) | 23.9±6.80 |
| Age (years) | 29.6±4.30 |
| Pregnancy days | 239.7±42.6 |
| Placental weight (g) | 406.9±94.9 |
| Fetal weight (g) | 2805.2±496.04 |
| Maternal age category | |
| 20–24 years (n=1) | 4.35% |
| 25–35 years (n=22) | 95.65% |
Placental tissue
Placental tissues were obtained from women delivering at term (>37 weeks of gestation) and preterm (<37 weeks of gestation) (total n=23). Placental tissues were obtained from the maternal side of the placentas at a maximum of 30 min after delivery. The tissue samples taken were approximately 0.2–0.5 cm3 in size and were taken from the centre of the cotyledons evenly across the placenta. The tissues were dissected to remove any visible connective tissue and calcium deposits. Following washes in PBS, samples were immediately stored in RNAlater® (Applied Biosystems) at −80°C. Ethical approval was granted from the local ethics authority. Of the 23 placentas collected, 13 were term (average, 268 pregnancy days) and 10 were preterm (average, 211 pregnancy days). None of the term or preterm women were administered any antidepressants or synthetic glucocorticoids during pregnancy.
Of the 13 term placentas, 12 were in labour [mode of delivery: ten CS (Caesarean sections) and two vaginal deliveries] and one non-labour/CS tissue. Of the ten preterm placentas four were non-labour and all CS, and six were from labouring tissues (mode of delivery: three CS and three vaginal deliveries).
Cortisol measurement
Maternal non-fasting blood samples were collected into EDTA tubes between 09.00 and 10.00 hours, and centrifuged immediately at 525 g for 10 min. The resulted plasma was separated and snap frozen in dry ice and stored at −20°C until further use. The maternal blood samples were collected on the same day that the questionnaires were completed. Fetal blood samples were obtained from the umbilical artery at the time of delivery. Identical preparations to maternal samples took place regarding plasma isolation. Plasma cortisol levels were measured using a Cortisol EIA (Enzo Life Sciences), a colorimetric competitive enzyme immunoassay kit according to the manufacturer's instructions. The sensitivity of the assay is 56.72 pg/ml with a range between 156 and 10000 pg/ml. The inter-assay coefficient of variation for cortisol was 7.8% at 969 pg/ml and the intra-assay coefficient of variation for cortisol was 6.6% at 1088 pg/ml.
Cell culture
BeWo and JEG-3 cell lines were purchased from the European Collection of Cell Cultures. The cells were maintained at standard culture conditions of 5% CO2 in air at 37°C. BeWo cells were cultured in Ham's F12 (Sigma) containing 10% heat-inactivated FBS (fetal bovine serum) and 0.5% penicillin/streptomycin, whereas JEG-3 cells were maintained in MEME [MEM (minimal essential medium) Eagle] (Sigma) containing 10% heat-inactivated FBS, 0.5% penicillin/streptomycin, 0.5% L-glutamine, 0.5% sodium pyruvate and 0.5% MEM non-essential amino acids. Prior to treatment, both cell lines were maintained for 3 h in Phenol Red-free medium containing charcoal-stripped FBS.
Cortisol treatments
BeWo and JEG-3 cells were treated overnight with 10, 100 and 1000 nM cortisol in an attempt to mimic stress response in vitro. The concentrations were chosen in accordance with previous studies [22–26] that demonstrate that 10 and 100 nM cortisol doses simulate stress conditions and resemble physiological levels of the circulating steroid, whereas 1000 nM mimics a pharmacological dose. For these treatments, a vehicle-only control was also used.
Immunofluorescent analysis of BeWo and JEG-3 cells
BeWo and JEG-3 cells were fixed in 4% paraformaldehyde for 10 min prior to washes in PBS and incubation with 10% BSA for 1 h. Cells were incubated for 1 h with an anti-DEPTOR antibody (Millipore) at a 1:100 dilution in 1% BSA/PBS. Cells were then washed with PBS prior to a further incubation with a FITC-conjugated anti-(rabbit IgG) antibody (Santa Cruz Biotechnology) for 1 h. Slides were washed with PBS and mounted in Vectashield® Mounting Medium (Vector labs) containing the dye DAPI (4′,6-diamidino-2-phenylindole) to counterstain nuclei. Images were captured using a Plan Apo Neofluor 63× 1.25 NA (numerical apperture) oil objective (Zeiss) on a Zeiss Axiovert 200 M microscope and viewed using AxioVision software.
RNA isolation, cDNA synthesis and PCR
Total ribonucleic acid was isolated using an RNA extraction kit (Sigma), according to the manufacturer's instructions. RNA concentration was determined by spectrophotometric analysis (NanoDrop; Thermo Scientific) and agarose gel electrophoresis. RNA (200 ng from placental tissue and 500 ng from cell lysates) was reverse-transcribed into cDNA using 5 I.U. (international units)/μl RNase H reverse transcriptase (Invitrogen).
Q-PCR (quantitative PCR)
Relative expression of the genes of interest was assessed by Q-PCR on an ABI Prism 7900HT Sequence detection system (Applied Biosystems) using SYBR® Green-PCR reaction mixture (Sigma–Aldrich) and specific primers (Table 2). As a negative control, distilled water was used in place of the cDNA. For the Q-PCR, the following equations were used: ΔCt=Ct (gene of interest)−Ct (housekeeping gene), ΔΔCt=ΔCt (sample)−ΔCt (calibrator), The RQ (relative quantity)=2−ΔΔCt. The RQ value was set up as 1 for the untreated (no supplement) BeWo and JEG-3 cells. We have also calculated the gene expression levels as RQ values, using the untreated control for each cell line as a calibrator. We did so, since RQ values provide a more accurate comparison between the initial amounts of template in each sample, without requiring an exact copy number for analysis. Q-PCR data are reported as means±S.E.M. Statistical analysis of the ΔCt values was performed by one-way ANOVA, followed by Bonferroni multiple comparison as a post-hoc test using GraphPad Prism 5.0.
Table 2. Primers used for Q-PCR studies.
| Gene | Expected size (bp) | Primer sequence |
|---|---|---|
| DEPTOR | 202 | Forward, 5′-CACCATGTGTGTGATGAGCA-3′ |
| Reverse, 5′-TGAAGGTGCGCTCATACTTG-3′ | ||
| Rictor | 117 | Forward, 5′-GGAAGCCTGTTGATGGTGAT-3′ |
| Reverse, 5′-GGCAGCCTGTTTTATGGTGT-3′ | ||
| Raptor | 170 | Forward, 5′-ACTGATGGAGTCCGAAATGC-3′ |
| Reverse, 5′-TCATCCGATCCTTCATCCTC-3′ | ||
| mTOR | 135 | Forward, 5′-TGCCAACTATCTTCGGAACC-3′ |
| Reverse, 5′-GCTCGCTTCACCTCAAATTC-3′ | ||
| β-Actin | 216 | Forward, 5′-AAGAGAGGCATCCTCACCCT-3′ |
| Reverse, 5′-TACATGGCTGGGGTGTTGAA-3′ |
Immunofluorescent analysis of placental sections
Following a series of deparaffinization and dehydration steps, placental tissue sections were incubated with 10% BSA for 1 h. This was followed by incubation for 1 h with antibodies against DEPTOR (Millipore), mTOR, raptor and rictor (Cell Signaling Technology) at a 1:200 dilution in 1% BSA/PBS. Cells were then washed with PBS prior to an incubation with a TRITC (tetramethylrhodamine β-isothiocyanate)-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h. Slides were washed with PBS and mounted in Vectashield® Mounting Medium (Vector labs) containing the dye DAPI to counterstain nuclei. Images were captured using a Plan Apo Neofluor 63× 1.25 NA (numerical apperture) oil objective (Zeiss) on a Zeiss Axiovert 200M microscope and viewed using AxioVision software. The images were then analysed using Image J 1.34s image-analysis software (National Institutes of Health). Results are presented as means±S.D. Statistical comparisons between the BeWo and JEG-3 cells were performed using a Student's t test. Differences between means were considered significant when P<0.05.
Statistical analysis
For the correlation studies, a two-tailed test using SPSS (Version 18) was used. A P≤0.05 was regarded as significant. For the correlation studies between gene expression and clinical data, DEPTOR, mTOR, rictor and raptor expression were log transformed in order to acquire a normal distribution. For analysis of gene expression and categorical data (groups of stress levels), Student's t tests were used. We also tested by the more stringent ANCOVA (analysis of co-variance) the effects of categorical stress (high/low) on levels of DEPTOR, adjusting for confounders either related to DEPTOR or to stress, as covariates.
RESULTS
Expression of DEPTOR, mTOR, rictor and raptor and correlation analyses in human placentas
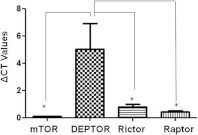
Q-PCR revealed that DEPTOR, mTOR, rictor and raptor are expressed in the human placenta (n=23). DEPTOR is the primary transcript in the total cohort of patients with a ΔCt of 5.021 compared with 0.1040 of mTOR (P=0.0126), 0.7993 of rictor (P=0.0315) and 0.412 of raptor (P=0.0193). From the 23 placentas, 13 were term labour (<37 weeks of gestation) and ten were preterm (>37 weeks of gestation) labour. When ΔCt values were analysed in these two categories, DEPTOR was the predominant transcript. In term labour, ΔCt for DEPTOR was 3.939 compared with 0.09343 for mTOR, 0.7898 for rictor and 0.3345 for raptor (Figure 1). In preterm placentas, the values were 6.428, 0.1178, 0.8117 and 0.5339 for DEPTOR, mTOR, rictor and raptor respectively. There was a significant correlation between log-transformed DEPTOR and raptor (r=0.729, P<0.005), and rictor with raptor (r=0.661, P=0.014) only in the preterm placentas examined. We have also analysed the ΔCt levels in terms of the contractile status (labour, n=18; or non-labour, n=5). In placental tissues obtained during labour the ΔCt for DEPTOR was 5.241 compared with 0.09739 for mTOR, 0.7142 for rictor and 0.4246 for raptor. In non-labouring placentas, the values were 4.228, 0.1280, 1.106 and 0.4027 for DEPTOR, mTOR, rictor and raptor respectively.
Figure 1. Q-PCR analysis of DEPTOR, mTOR, rictor and raptor in human placentas revealed that DEPTOR is the predominant gene among mTOR signalling components.
ΔCt=Ct (gene of interest)−Ct (β-actin); *P<0.05 compared with DEPTOR.
In this cohort (i.e. in 23 patients), 52% reported high and very high levels of stress during pregnancy, and 48% medium and low levels. There was no correlation between maternal stress levels and BMI (body mass index). However, there was a very strong correlation between placental and fetal weight (r=0.986, P<0.0001) and between days of pregnancy and fetal and placental weight (r=0.968 and r=0.942 respectively; P<0.0001). Interestingly, there was a significantly lower level of log-DEPTOR gene expression in the high stress group (−1.34) than in the low stress group (0.07; t20=2.41, P=0.026).
Corresponding fetal plasma cortisol levels were also measured. Mothers with high stress had significantly (P=0.035) elevated levels of cortisol (8555±925 pg/ml) compared with those with low stress (4900±1700 pg/ml.) The average pregnancy days for the high stress group was 223.2±60, whereas the average gestational period for the low stress group was 253.7±23 days. The fetal cortisol from mothers with high stress was measured to be 7440±1349 pg/ml, whereas fetal cortisol from mothers with low stress was measured to be 7525±1173 pg/ml. There was no significance among the two groups (P>0.4). Interestingly, a significant positive correlation between maternal and fetal cortisol was detected in the high but not the low stress group (r=0.678, P=0.022). In the high stress group, there was also a significant inverse correlation between fetal cortisol and placental weight (r=−0.638, P=0.024) as well as fetal weight (r=−0.642, P=0.023). In the low stress group there was a significant correlation between fetal cortisol and placental weight (r=0.804, P=0.029) as well as fetal weight (r=0.784, P=0.037). There was a significant correlation between placental and fetal weight both in the high (r=0.983, P<0.0001) and low maternal stress groups (r=0.870, P<0.011). Finally, umbilical cord (fetal) samples did not vary significantly between the term (8254±949 pg/ml) and preterm groups (6341±1822 pg/ml, P>0.1).
We then assessed whether time of delivery has an effect. In term tissues (n=13) high stress inversely correlated with log-DEPTOR levels (r=−0.772, P=0.002), placental weight (r=−0.591, P=0.03) and fetal weight (r=−0.732, P=0.004). In the same cohort, log-DEPTOR levels positively correlated with placenta weight (r=0.497, P=0.084) and fetal weight (r=0.616, P=0.025). In pre-term placentas (n=10) maternal high stress levels inversely correlated only with placental weight (r=−0.621, P=0.07) and fetal weight (r=−0.695, P=0.038). Interestingly, in this group, log-DEPTOR levels inversely correlated with placental weight (r=−0.622, P=0.055) and fetal weight (r=−0.575, P=0.082). Given the effects of timing of delivery on stress, we retested the main hypothesis. An ANCOVA was used to test whether low/high stress correlates with DEPTOR, controlling for contractile status (labour/non-labour) and timing of labour (term/preterm) entered as covariates. Taking these confounders into consideration, we still found a trend towards a significant relation between high stress and DEPTOR: F1,18=4.12, P=0.055.
Finally, we have also taken into consideration the smoking status of our patients before and during pregnancy. Of the 23 women, 17 did not smoke (74%) and six did (26%) before pregnancy. During pregnancy, 19 did not smoke (83%), whereas only four kept smoking (17%). Placental DEPTOR levels were not statistically different in the two groups regarding smoking status during pregnancy.
Cellular localization of DEPTOR, mTOR, rictor and raptor in human placenta
Immunofluorescent analysis of the mTOR signalling components in human placental tissue sections revealed the cellular localization and distribution of each of the components individually. The expression of DEPTOR was almost exclusively localized in the syncytium layer exhibiting a strong cytoplasmic localization. Staining was also visible in the cytoplasm of the cytotrophoblast cells beneath the syncytium layer. mTOR staining revealed a cytoplasmic expression not only in the syncytium layer but also in the cytoplasm of the cytotrophoblast cells. rictor appeared to have a similar expression pattern to mTOR, showing expression in the cytoplasm of both the syncytium layer and the cytotrophoblast cells. The expression of raptor was observed mainly in the syncytium layer, with staining observed in the cytoplasm (Figure 2).
Figure 2. Immunofluorescent analysis of DEPTOR, mTOR, rictor and raptor in human placentas.
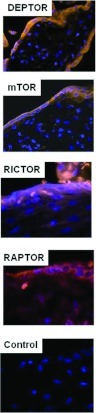
Immunofluorescent analysis showing strong cytoplasmic localization present in the syncytium layer for DEPTOR, whereas mTOR and rictor were expressed in both cytotrophoblasts cells and the syncytium layer with the staining being cytoplasmic. raptor revealed a cytoplasmic staining present in the syncytium layer. Control: immunofluorescent analysis of placental section in which the primary antibody has been omitted indicates specificity of the fluorescent signal detected for the mTOR signalling components. Magnification, ×40.
Expression of DEPTOR in BeWo and JEG-3 cells
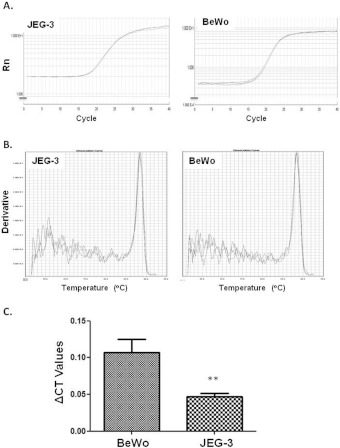
DEPTOR expression in two placental cell lines was then assessed. Prior to Q-PCR analysis, the control gene expression was assessed. An identical amount of RNA (500 ng) was reverse transcribed and analysis of the housekeeping gene β-actin revealed identical Ct for both cell lines (Figure 3A). Moreover, melting curve peak analysis demonstrated an identical peak and area under curve (Figure 3B). These data suggest that, by having an identical control gene, meaningful comparisons between the two cell lines can be made. Q-PCR and immunofluoresce analysis demonstrated for the first time expression of DEPTOR in both cell lines at the mRNA and protein levels. Q-PCR revealed that DEPTOR is overexpressed in BeWo cells when compared with JEG-3 under basal conditions (2.5-fold, P<0.01, Figure 3C).
Figure 3. Differential expression of DEPTOR in BeWo and JEG-3 cells.
(A) Ct values for the housekeeping gene β-actin reveal identical amplification efficiency for both BeWo and JEG-3 cells. (B) Melting curve analysis for β-actin in both cell lines, demonstrates a single melting peak at identical temperature. (C) Q-PCR demonstrates that DEPTOR is significantly expressed in BeWo cells when compared with JEG-3; **P<0.01.
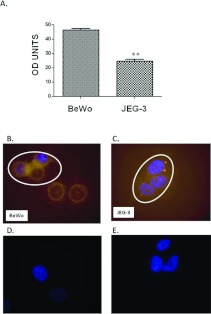
Densitometric analysis of the immunostaining corroborated the Q-PCR data, as it demonstrates higher protein expression of DEPTOR in BeWo (46.4±4.3 units) when compared with JEG-3 cells (24.6±4.8 units; Figure 4A). The cellular distribution of DEPTOR in the two cell lines revealed a cytoplasmic localization. In BeWo cells, DEPTOR is seen mainly around the nucleus, whereas, in JEG-3 cells, a more dispersed cytoplasmic staining is evident (Figures 4B–4E).
Figure 4. Down-regulation of DEPTOR protein in JEG-3 compared with BeWo cells.
(A) Densitometric analysis of DEPTOR revealed a notable up-regulation at the protein level in BeWo cells when compared with JEG-3, mirroring the changes seen at gene level using Q-PCR. (B and C) Immunofluorescent analysis of DEPTOR in BeWo and JEG-3 cells respectively revealed cytoplasmic localization in both cell lines. Circles in (B) and (C) indicate the same amount of cells used for densitometric analysis as in (A). Images were analysed using Image J 1.34s image-analysis software (**P<0.001). Results are presented as means±S.E.M. (D and E) Negative controls of (B and C) where the primary antibody has been omitted, demonstrating staining specificity.
Effects of cortisol on DEPTOR in BeWo and JEG-3 cells
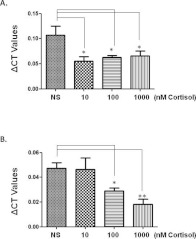
Given the inverse relationship of stress and DEPTOR in our clinical samples, we tested the hypothesis that cortisol might affect DEPTOR expression directly. BeWo and JEG-3 cells were treated overnight with cortisol, in an attempt to resemble a moderate and high stress environment in vitro. When BeWo cells were treated with cortisol 10, 100 or 1000 nM, the expression of DEPTOR was significantly down-regulated by 50, 41 and 39% (all P<0.05) respectively when compared with basal levels (Figure 5A). Treatment of JEG-3 cells with cortisol led to a significant decrease of DEPTOR expression at 100 nM (39%, P<0.05) and at 1000 nM (73%, P<0.01) when compared with basal levels as well (Figure 5B). However, cortisol treatments did not exert any significant changes in the gene expression of mTOR, rictor or raptor in both cell lines (results not shown).
Figure 5. Quantitative analysis of DEPTOR levels in BeWo and JEG-3 cells following 24 h of cortisol treatment.
(A) Cortisol treatment of BeWo cells induced a significant (*P<0.05) down-regulation of DEPTOR (NS=no supplement). (B) In JEG-3 cells, cortisol induced a dose-dependent down-regulation of DEPTOR that reached significance at 100 nM (*P<0.05) and 1000 nM (**P<0.01) when compared with no supplement.
DISCUSSION
In the present study, we provide novel evidence of a bio-behavioural cross-talk of mTOR signalling at placental level. Pregnancy is associated with major physiological and psychosocial changes and adaptation to these changes is crucial for normal fetal development [27,28]. Pregnancy-specific stress may be a more powerful contributor to birth outcomes than general stress [29]. In this study, 23 pregnant women self-reported their stress status ranging from low and medium (low stress response) to high and very high (high stress response). The source of the stress was not reported. Generally, maternal stress could be due to lack of social support, anxiety about the pregnancy outcome or environment-related stressful conditions [28]. Nepomnaschy et al. [30], have suggested that human placentation is an important period for examining the relationship between maternal stress and pregnancy outcome. Indeed a major finding in our study was the significant inverse correlation between mRNA levels of placental DEPTOR and self-reported stress levels. None of the other mTOR components displayed any correlation with stress. It should be noted that the levels of self-reported stress were evaluated only at the third trimester. In a study of 1800 women [12] the different levels of life events stress during pregnancy were analysed. The authors concluded that there were substantial differences of prenatal life events stress in the third trimester of pregnancy, as a function of educational background and maternal age [12]. However, prenatal severe life events in the first and second but not third trimester were associated with an increased risk of preterm birth [12]. Although most studies suggest that prenatal maternal stress predicts adverse infant outcomes [13–15], the large-scale Danish study found maternal stress to predict greater infant birthweight [20]. The exact role of DEPTOR is relatively unknown, but emerging data suggest it plays a role in certain cells' survival [8]. Thus it is possible that, via reduced DEPTOR, stress could lead to alterations in placental growth that can impact on fetal development.
Preterm birth is the most important problem in maternal/child health. Numerous epidemiological studies suggested that maternal stress is associated significantly with the onset of spontaneous preterm birth [31]. Despite extensive research, the mechanisms that drive this are still poorly understood. As a result, the prevalence rate of preterm birth still remains high. In our study, we provide evidence for a dual role of DEPTOR in at-term and preterm placentas. We would like to propose the following model: in at-term placentas (i.e. normal uncomplicated pregnancies) high stress inversely correlates with DEPTOR. Previous evidence suggests that DEPTOR exerts a selective inhibitory effect on mTORC1 signalling relative to mTORC2 [8,9]. Our findings provide novel evidence of this, as in this cohort DEPTOR strongly associates with raptor, a key component of mTORC1 complex, but not with rictor. To the best of our knowledge this is a first time that such an association has been shown in clinical samples. It is possible that the tight association of these molecules compromised the function of mTORC1. The net result still would be a decrease in cell growth that might contribute towards the low placental weight. These findings corroborate a previous study that indeed indicates asymmetry in the actions of DEPTOR [8]. For example, in a vast array of cancers DEPTOR is down-regulated, resulting in an increased rate of tumorigenesis and cell proliferation. However, in multiple myelomas DEPTOR depletion inhibited the proliferation of these cells and led to subsequent apoptotic events. In preterm labour, the dynamics are altered. Stress is no longer inversely associated with DEPTOR and this will result in permanently elevated levels of DEPTOR. Indeed, the Q-PCR data indicate that the expression of DEPTOR is higher in preterm samples. Moreover, there is no association between DEPTOR and raptor. The net effect would be an exacerbated inhibitory action of DEPTOR to mTORC1 accounting for the decrease in placental cell proliferation and thus the overall decrease in placental weight seen in preterm placentas. This dual role provides evidence of a high order of complexity regarding DEPTOR signalling, possibly involving post-translational modifications or co-regulation of mTOR gene complexes as it is evident in these clinical samples.
Glucocorticoids are key mediators of stress responses and can act as mediators of fetal development. They are responsible for the intrauterine maturation of tissues and organs, promoting cellular differentiation [32]. In pregnancies complicated by IUGR, fetal cortisol levels are elevated at term [33], associating reduced fetal growth rates with elevated glucocorticoids. Moreover, alterations in glucocorticoid signalling in early life can induce persistent effects on later phenotype and disease risk [32]. Several studies have also documented an increase of the endogenous levels of cortisol with pregnancy [34,35]. However, due to different methodological approaches, the cortisol values tend to vary. For example, one group has shown that cortisol values varied between 13 and 46 nmol/l when sampled at 37 and 38 weeks of pregnancy. The average cortisol levels were 25.3 nmol/l [36]. However, in another study [37], a greater variation of cortisol levels in late pregnancy was noted, ranging from 3.7 to 55 nmol/l with a median of 11.80 nmol/l. Moreover, no apparent correlation between maternal cortisol levels with preterm labour and preterm birth was noted at any gestational age. In the latter study, cortisol levels peaked towards the end of pregnancy (31–35 weeks), reaching an average of approximately 33 ng/ml for those delivered at term [35].
Given the importance of cortisol as a stress marker, we hypothesized that it might affect DEPTOR expression directly. We tested this in vitro, employing two well established choriocarcinoma cell lines (BeWo and JEG-3) that have been used widely to decipher placental signalling. Both cell lines were treated over 24 h with 10, 100 and 1000 nM of cortisol in an attempt to resemble moderate, high and very high stress levels. Treatment of JEG-3 cells with cortisol resulted in a dose-dependent down-regulation of DEPTOR, reaching significance at 100 and 1000 nM. However, when the same treatments were repeated in BeWo cells, dose-dependence was not observed. This could be due to inherent differences that these two cell lines appear to possess. Microarray analyses revealed that up to 2700 genes are differentially expressed between the two cell lines [38]. Interestingly, principal differences observed in various biological processes, including response to stress and signal transduction, where noted [18]. In our study, we demonstrate that DEPTOR is expressed in higher amounts in BeWo cells than in JEG-3 cells. It is possible that this overexpression is responsible for the weaker down-regulation of DEPTOR by cortisol in this cell line.
We would also like to acknowledge that our study has a number of limitations. The clinical sample is relatively small and comes from a relatively homogeneous population of a Greek island. In the study by Ruiz et al. [35], higher maternal cortisol levels were observed in the Hispanic group when compared with Anglo-Americans. To date, there are no data regarding any ethnic variation of maternal cortisol in the Greek population. However, the relative uniformity of our sample could also have certain advantages – we do not need to control for the effects of other factors/variables such as lifestyle, diet and ethnicity, as these may have been rather similar among participants. In addition, the measurement of self-reported stress was rather crude in nature. Given the emerging data of the current study, it will be interesting to dissect the exact nature of the stress response (i.e. life event stress and subtypes, appraisal of stress controllability and pregnancy-related anxiety) as reported previously [31]. Signs of fetal distress should also be taken into account in future studies as our present findings lack an association with the APGAR score. Moreover, our in vitro results were generated by the use of two human choriocarcinoma cell lines (BeWo and JEG-3). These experiments should be repeated in placental explants or primary placental trophoblasts, and perhaps at a wider dose range of cortisol, to gain a better insight into the role of cortisol in such cells. For example, the effect seen in BeWo cells was not dose-dependent as 10 nM of cortisol appeared to suppress DEPTOR expression similarly to 100 and 1000 nM. However, this was not the aim of our study, as we treated both cell lines with concentrations that would simulate stress conditions. Finally, we would also like to acknowledge certain controversy regarding use of housekeeping genes for Q-PCR analysis [39]. We have performed a comparative Q-PCR where we show that in our clinical samples the ΔCt of β-actin and 18 sRNA (small non-coding RNA) were almost identical and therefore the RQ readings and significance (RQ=2−ΔΔCt) were not altered (results not shown). Nevertheless, our findings from two different methodologies (in vivo and in vitro) point to a possible role of prenatal maternal stress in the expression of DEPTOR, and the significance of this finding in fetal development requires further investigation.
Future research should concentrate on dissecting the effects of cortisol at the protein level. For example, it will be of interest to assess effects of cortisol at the post-translational level (i.e. alterations of the phosphorylation status of DEPTOR and other mTOR components). Moreover, the impact of this treatment in the aggregation and subsequent stoichiometry of mTORC1 and mTORC2 complexes should also be elucidated. Collectively, the in vitro findings in combination with the clinical data suggest that stress and the stress hormone cortisol may be potent modulators of DEPTOR at the placental level. As a result, it is attractive to speculate that maternal stress might affect subsequent placental mTOR signalling events, with possible consequences for fetal development.
AUTHOR CONTRIBUTION
Dionisis Mparmpakas performed the RNA extractions, cDNA synthesis, Q-PCR, cortisol measurements and tissue culture. Elena Zachariades contributed to the Q-PCR, cortisol treatments and immunofluorescent experiments. Anastasia Goumenou was involved in tissue acquisition and questionnaire details, as well as provision of the clinical data. Yori Gidron: performed the correlation analyses and contributed intellectually towards the design of the study. Emmanouil Karteris designed the study, and participated in the analyses of data and formatting of the questionnaire.
FUNDING
This work was support, in part, by the Greek State Scholarships Foundation (I.K.Y.).
References
- 1.Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 2.Dennis P. B., Jaeschke A., Saitoh M., Fowler B., Kozma S. C., Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 3.Wullschleger S., Loewith R., Hall M. N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Guertin D. A., Sabatini D. M. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Laplante M., Sabatini D. M. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong L. Q., Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am. J. Physiol. Endocrinol. Metab. 2005;289:E187–E196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 7.Oh W. J., Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson T. R., Laplante M., Thoreen C. C., Sancak Y., Kang S. A., Kuehl W. M., Gray N. S., Sabatini D. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proud C. G. Dynamic balancing: DEPTOR tips the scales. J. Mol. Cell Biol. 2009;1:61–63. doi: 10.1093/jmcb/mjp012. [DOI] [PubMed] [Google Scholar]
- 10.Liu M., Wilk S. A., Wang A., Zhou L., Wang R. H., Ogawa W., Deng C., Dong L. Q., Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster H., Coley H., Goumenou A., Pantos G., Harvey A., Karteris E. Expression of mTOR signalling components during drug resistance in ovarian cancer. Anticancer Res. 2010;30:3529–3534. [PubMed] [Google Scholar]
- 12.Zhu P., Tao F., Hao J., Sun Y., Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. Am. J. Obstet. Gynecol. 2010;203:34.e1–34.e8. doi: 10.1016/j.ajog.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Rini C. K., Dunkel-Schetter C., Wadhwa P. D., Sandman C. A. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18:333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 14.Edwards C. H., Cole O. J., Oyemade U. J., Knight E. M., Johnson A. A., Westney O. E., Laryea H., West W., Jones S., Westney L. S. Maternal stress and pregnancy outcomes in a prenatal clinic population. J. Nutr. 1994;124:1006–1021. doi: 10.1093/jn/124.suppl_6.1006S. [DOI] [PubMed] [Google Scholar]
- 15.Dole N., Savitz D. A., Hertz-Picciotto I., Siega-Riz A. M., McMahon M. J., Buekens P. Maternal stress and preterm birth. Am. J. Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 16.Roos S., Powell T. L., Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem. Soc. Trans. 2009;37:295–298. doi: 10.1042/BST0370295. [DOI] [PubMed] [Google Scholar]
- 17.Wen H. Y., Abbasi S., Kellems R. E., Xia Y. mTOR: a placental growth signalling sensor. Placenta. 2005;26:S63–S69. doi: 10.1016/j.placenta.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Roos S., Jansson N., Palmberg I., Säljö K., Powell T. L., Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007;582:449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arroyo J. A., Brown L. D., Galan H. L. Placental mammalian target of rapamycin and related signaling pathways in an ovine model of intrauterine growth restriction. Am. J. Obstet. Gynecol. 2009;201:616.e1–616.e7. doi: 10.1016/j.ajog.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tegethoff M., Greene N., Olsen J., Meyer A. H., Meinlschmidt G. Maternal psychosocial stress during pregnancy and placenta weight: evidence from a national cohort study. PLoS ONE. 2010;5:e14478. doi: 10.1371/journal.pone.0014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Wang X., Wang W., Chen C., Ronnennberg A. G., Guang W., Huang A., Fang Z., Zang T., Wang L., Xu X. Stress and dysmenorrhoea: a population based prospective study. Occup. Environ. Med. 2004;61:1021–1026. doi: 10.1136/oem.2003.012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flint M. S., Kim G., Hood B. L., Bateman N. W., Stewart N. A., Conrads T. P. Stress hormones mediate drug resistance to paclitaxel in human breast cancer cells through a CDK-1-dependent pathway. Psychoneuroendocrinology. 2009;34:1533–1541. doi: 10.1016/j.psyneuen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Rupprecht M., Salzer B., Raum B., Hornstein O. P., Koch H. U., Riederer P., Sofic E., Rupprecht R. Physical stress-induced secretion of adrenal and pituitary hormones in patients with atopic eczema compared with normal controls. Exp. Clin. Endocrinol. Diabetes. 1997;105:39–45. doi: 10.1055/s-0029-1211725. [DOI] [PubMed] [Google Scholar]
- 24.Rupprecht R., Koch M., Montkowski A., Lancel M., Faulhaber J., Harting J., Spanagel R. Assessment of neuroleptic-like properties of progesterone. Psychopharmacology. 1999;143:29–38. doi: 10.1007/s002130050916. [DOI] [PubMed] [Google Scholar]
- 25.Bernabé D. G., Tamae A. C., Biasoli E. R., Oliveira S. H. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav. Immun. 2011;25:574–83. doi: 10.1016/j.bbi.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Miller D. B., O'Callaghan J. P. Neuroendocrine aspects of the response to stress. Metab. Clin. Exp. 2002;51:5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- 27.Sandman C. A., Davis E. P., Buss C., Glynn L. M. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2011 doi: 10.1159/000327017. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas A. J. Mother-offspring dialogue in early pregnancy: impact of adverse environment on pregnancy maintenance and neurobiology. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;35:1167–1177. doi: 10.1016/j.pnpbp.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Lobel M., Cannella D. L., Graham J. E., DeVincent C., Schneider J., Meyer B. A. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 30.Nepomnaschy P. A., Welch K. B., McConnell D. S., Low B. S., Strassmann B. I., England B. G. Cortisol levels and very early pregnancy loss in humans. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3938–3942. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadhwa P. D., Culhane J. F., Rauh V., Barve S. S., Hogan V., Sandman C. A., Hobel C. J., Chicz-DeMet A., Dunkel-Schetter C., Garite T. J., Glynn L. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr. Perinat. Epidemiol. 2001;15:17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 32.Cottrell E. C., Seckl J. R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goland R. S., Jozak S., Warren W. B., Conwell I. M., Stark R. I., Tropper P. J. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J. Clin. Endocrinol. Metab. 1993;77:1174–1179. doi: 10.1210/jcem.77.5.8077309. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberger S. E., Resch F., Doszpod N., Moehler E. Prenatal stress and infant affective reactivity at five months of age. Early Hum. Dev. 2011;87:129–136. doi: 10.1016/j.earlhumdev.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz R. J., Fullerton J., Brown C. E., Schoolfield J. Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biol. Res. Nurs. 2001;3:39–48. doi: 10.1177/109980040100300106. [DOI] [PubMed] [Google Scholar]
- 36.de Weerth C., van Hees Y., Buitelaar J. K. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum. Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 37.Obel C., Hedegaard M., Henriksen T. B., Secher N. J., Olsen J., Levine S. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology. 2005;30:647–656. doi: 10.1016/j.psyneuen.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Burleigh D. W., Kendziorski C. M., Choi Y. J., Grindle K. M., Grendell R. L., Magness R. R., Golos T. G. Microarray analysis of BeWo and JEG3 trophoblast cell lines: identification of differentially expressed transcripts. Placenta. 2007;28:383–389. doi: 10.1016/j.placenta.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Cleal J. K., Day P., Hanson M. A., Lewis R. M. Measurement of housekeeping genes in human placenta. Placenta. 2009;30:1002–1003. doi: 10.1016/j.placenta.2009.09.002. [DOI] [PubMed] [Google Scholar]