Abstract
OBJECTIVE
A common approach to screening for gestational diabetes mellitus (GDM) is the universal testing of all pregnant women with a 1-h, 50-g glucose challenge test (GCT), followed by a diagnostic oral glucose tolerance test (OGTT) in those in whom the GCT is positive (≥7.8 mmol/L). More important, the GCT is performed at any time of day, but there has been limited study of the effect of time of day on test performance. Thus, using their subsequent OGTT (performed in the morning), we sought to characterize the metabolic function of women with positive GCTs in relation to the timing of their test.
RESEARCH DESIGN AND METHODS
A total of 927 women with positive GCTs underwent a 3-h 100-g OGTT. They were stratified into four groups by time of day (hours) of their GCT: <0900 (n = 171), 0900–1059 (n = 288), 1100–1259 (n = 189), and ≥1300 (n = 279).
RESULTS
On the OGTT, the prevalence of GDM progressively decreased across the GCT groups from <0900 h (26.9%) to 0900–1059 h (25.0%) to 1100–1259 h (21.7%) to ≥1300 h (21.5%; P = 0.0022). After adjustment for GDM risk factors, mean adjusted glucose area under the curve (AUCgluc) similarly decreased across the groups, while insulin sensitivity (Matsuda index) and β-cell function (Insulin Secretion-Sensitivity Index-2) progressively increased (all P < 0.0001). In particular, compared with the <0900- and 0900–1059-h groups, women whose positive GCT occurred after 1300 h had superior metabolic function, as evidenced by lower AUCgluc, higher insulin sensitivity, and better β-cell function (all P ≤ 0.0097).
CONCLUSIONS
Among women with a positive GCT, those tested in the afternoon have better metabolic function and a lower risk of GDM on subsequent OGTT.
It is now recognized that many physiologic functions, including energy metabolism, exhibit 24-h circadian rhythms generated by light/dark cycles (1–3). For example, in nonobese humans, glucose tolerance decreases in the afternoon and evening, as detected by oral or intravenous glucose tolerance tests (4,5). Reduced insulin sensitivity and β-cell responsivity to glucose both account for this deterioration in glucose tolerance later in the day (4). The potential importance of this type of circadian cycle is highlighted by the increased risk of obesity and diabetes in shift workers (2). Moreover, sleep restriction or misalignment of circadian rhythm by scheduling a 28-h day diminishes insulin sensitivity in humans (6,7).
A clinical setting in which these circadian changes in glucose metabolism may be particularly relevant is antepartum screening for gestational diabetes mellitus (GDM). Owing to the obstetrical risks associated with maternal hyperglycemia and subsequent fetal overgrowth, screening for GDM has become a standard element of obstetrical practice. Although protocols may vary, a common approach to screening is the universal testing of all pregnant women in the late second trimester with a 1-h, 50-g glucose challenge test (GCT), followed by referral for a diagnostic oral glucose tolerance test (OGTT) in those in whom the GCT is positive (defined as 1-h postchallenge plasma glucose ≥7.8 mmol/L). More important, the GCT is performed throughout the day and without regard to fasting or fed state. Sermer et al. (8) demonstrated that the duration of elapsed time since the last meal could affect the result of the GCT. However, despite emerging recognition of circadian changes in glucose metabolism, there has been limited study of the effect of time of day on the performance of the GCT (9,10). Thus, in this context, we sought to assess the clinical and metabolic phenotype of pregnant women with a positive screening GCT at varying times of the day to better understand the clinical implications of performing this test without regard to time of day.
RESEARCH DESIGN AND METHODS
This analysis was conducted in the setting of an ongoing observational study of early events in the natural history of type 2 diabetes, in which a cohort of women recruited at the time of GDM screening is undergoing longitudinal metabolic characterization (11). In the study, healthy pregnant women are recruited before or just after their 1-h, 50-g GCT, which is typically performed at 24–28 weeks’ gestation. Regardless of the GCT result, all study participants undergo a 3-h, 100-g OGTT for determination of glucose tolerance status in pregnancy. The study protocol was approved by the Mount Sinai Hospital Research Ethics Board and all participants provided written informed consent. The current analysis was restricted to 927 women with singleton pregnancies and a GCT ≥7.8 mmol/L.
Participant assessments, laboratory measurements, and physiologic indices
On the morning of the 3-h 100-g OGTT, data pertaining to personal history of previous GDM and family history of diabetes were collected by interviewer-administered questionnaire. Anthropometric measurements of height and weight were obtained using a medical scale.
All OGTTs were performed in the morning (before 0930 h) after an overnight fast, with venous blood samples drawn for measurement of glucose and insulin at fasting and at 30, 60, 120, and 180 min after ingestion of the glucose load. Specific insulin was measured using the Roche Modular system and the electrochemiluminescence immunoassay kit (Roche Diagnostics catalog number 12017547122). This assay shows 0.05% cross-reactivity to intact human proinsulin and the primary circulating split form (Des 31, 32).
Glycemia, insulin sensitivity, and β-cell function were measured on the OGTT. Glycemia was assessed by glucose tolerance status and by the total glucose area under the curve (AUCgluc) during the test, calculated using the trapezoidal rule. As previously described (11), glucose tolerance status on the OGTT was classified as follows: 1) GDM, defined as two or more glucose values meeting National Diabetes Data Group (NDDG) criteria (12); 2) gestational impaired glucose tolerance (GIGT), defined as only one glucose value meeting NDDG criteria; and 3) normal glucose tolerance (NGT), defined as no glucose values meeting NDDG criteria.
The primary measure of whole-body insulin sensitivity was the insulin sensitivity index (ISOGTT) of Matsuda and DeFronzo (13). ISOGTT is defined as 10,000/√[(FPG × FPI) × (G × I)], where FPG is fasting plasma glucose, FPI is fasting plasma insulin, G is mean glucose during the OGTT, and I is mean insulin. In pregnant women, ISOGTT exhibits better correlation with insulin sensitivity measured by the euglycemic–hyperinsulinemic clamp than the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) or the Quantitative Insulin Sensitivity Check Index (14). Insulin sensitivity (primarily hepatic) was also determined by 1/HOMA-IR, with HOMA-IR calculated as FPG × [FPI/22.5].
The primary measure of β-cell function was the Insulin Secretion-Sensitivity Index-2 (ISSI-2), an OGTT-derived measure that is analogous to the disposition index and defined as the product of 1) insulin secretion measured by the ratio of the insulin area under the curve (AUCins) to AUCgluc and 2) insulin sensitivity measured by ISOGTT (15). The insulinogenic index divided by HOMA-IR (insulinogenic index/HOMA-IR) was calculated as a secondary measure of β-cell function, with the insulinogenic index defined as the ratio of the incremental change in insulin during the first 30 min of the OGTT to the incremental change in glucose over the same time period (16).
Statistical analyses
All analyses were conducted using SAS 9.2 software (SAS Institute, Cary, NC). Continuous variables were tested for normality of distribution and natural log transformations of skewed variables were used, where necessary, in subsequent analyses. The study population was stratified into the following four groups by the time of ingestion (hours) of the 50-g glucose drink at the GCT: 1) before 0900; 2) between 0900 and 1059 inclusive; 3) between 1100 and 1259 inclusive; and 4) after 1300.
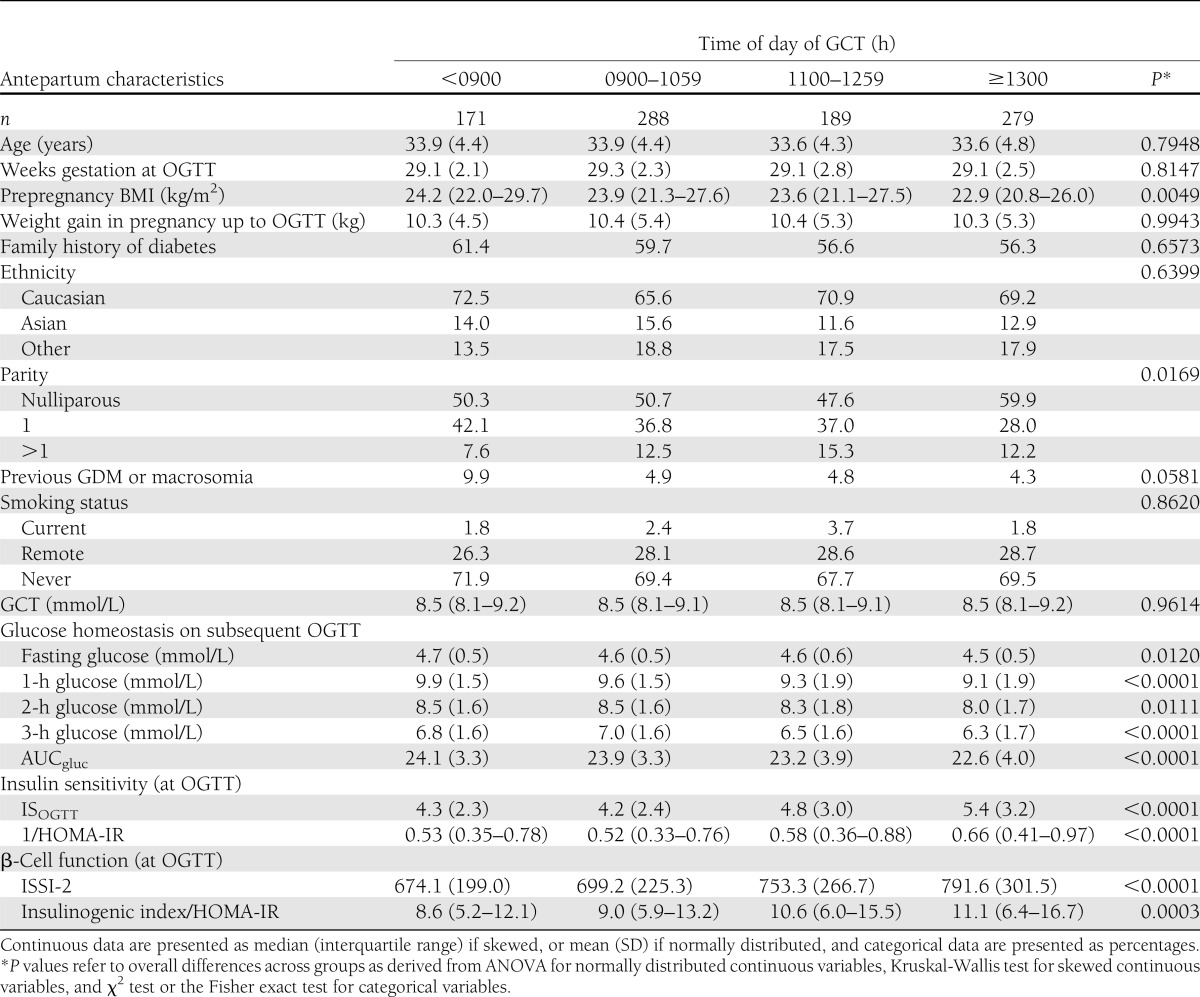
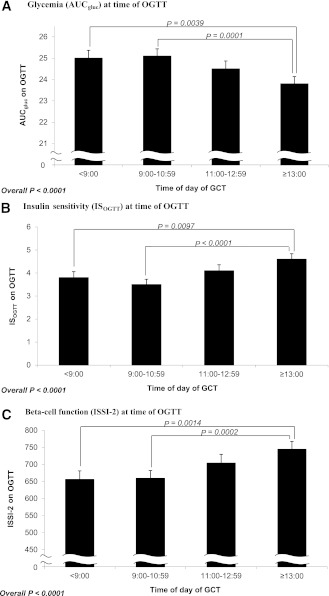
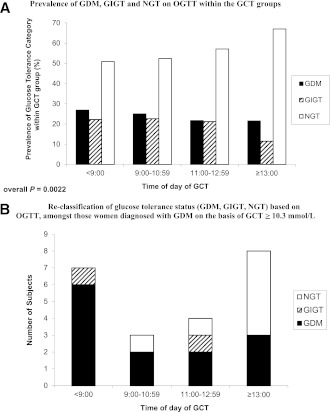
In Table 1, for each study group, continuous variables are presented as median with interquartile range (if skewed) or mean ± SD (if normally distributed), and categorical variables are presented as proportions. Continuous variables were compared across the groups by ANOVA (if normally distributed) or Kruskal-Wallis test (if skewed). Categorical variables were compared by χ2 test or theFisher exact test. In Fig. 1, adjusted mean levels of AUCgluc (Fig. 1A), ISOGTT (Fig. 1B), and ISSI-2 (Fig. 1C) were compared among groups by ANCOVA, after adjustment for age, weeks’ gestation at OGTT, prepregnancy BMI, gestational weight gain up to OGTT, family history of diabetes, ethnicity, parity, and previous GDM/macrosomia. Figure 2A shows the proportion of participants with GDM, GIGT, and NGT within each study group. Figure 2B shows the number of participants with GDM, GIGT, and NGT in each study group among participants with a GCT ≥10.3 mmol/L. Among these women, glucose tolerance status on the OGTT was compared between the <0900- and ≥1300-h groups by the Fisher exact test.
Table 1.
Demographic, clinical, and metabolic parameters of study participants stratified by time of day of GCT
Figure 1.
Comparison of adjusted mean levels of AUCgluc (A), ISOGTT (B), and ISSI-2 (C) among study groups defined by time of day of GCT. All analyses adjusted for age, weeks’ gestation at OGTT, prepregnancy BMI, weight gain up to OGTT, family history of diabetes, ethnicity, parity, and previous GDM. The error bars show the SE.
Figure 2.
Prevalence of glucose tolerance categories on OGTT (GDM, GIGT, and NGT) within each GCT group in (A) the entire study population (n = 927) and (B) in those subjects who would be diagnosed with GDM on the basis of GCT >10.3 mmol/L (n = 22).
RESULTS
Clinical and metabolic characteristics of study groups
Table 1 presents the antepartum characteristics of the study participants stratified into four groups by the time of day of the positive GCT. Women with a positive GCT performed later in the day had a lower prepregnancy BMI (P = 0.0049) and were also more likely to be nulliparous (P = 0.0169). Otherwise, there were no significant differences among the groups with respect to age, weeks’ gestation at OGTT, weight gain in pregnancy up to the OGTT, family history of diabetes mellitus, ethnicity, previous GDM/macrosomia, or smoking status.
More important, however, the four study groups (entirely composed of women with an abnormal GCT) showed marked metabolic differences on the subsequent OGTT. Indeed, blood glucose values on the OGTT showed a progressive decrease from the <0900-h GCT group to the 0900–1059-h group to the 1100–1259-h group to the ≥1300-h group (fasting glucose: P = 0.012; 1-h glucose: P < 0.0001; 2-h glucose: P = 0.011; and 3-h glucose: P < 0.0001). Accordingly, AUCgluc during the OGTT progressively declined as the GCT was performed later in the day (P < 0.0001). Furthermore, consistent with these differences, women with a positive GCT later in the day also had greater insulin sensitivity (ISOGTT and 1/HOMA-IR, both P < 0.0001), and better β-cell function (ISSI-2 P < 0.0001; IGI/HOMA-IR P = 0.0003), when assessed on the subsequent OGTT.
Adjusted analyses of metabolic function on OGTT
We next compared mean levels of AUCgluc, ISOGTT, and ISSI-2 among the four GCT groups, after adjustment for GDM risk factors, including age, weeks’ gestation at OGTT, prepregnancy BMI, weight gain up to the OGTT, family history of diabetes, ethnicity, parity, and previous GDM (Fig. 1A–C). There was a significant difference in the adjusted mean AUCgluc across the groups (P < 0.0001; Fig. 1A), with women who had a positive GCT later in the day demonstrating less glycemia on the subsequent OGTT. Of note, this finding was driven by significant pairwise differences between women undergoing the GCT after 1300 h and 1) those tested before 0900 h (P = 0.0039) and 2) those tested between 0900 and 1059 h (P = 0.0001). Similarly, adjusted mean ISOGTT, reflecting insulin sensitivity, was higher among women with a positive GCT later in the day (P < 0.0001, Fig. 1B). This too was driven by significant pairwise differences between women undergoing the GCT after 1300 h and those tested before 0900 h and between 0900 and 1059 h, respectively. Finally, β-cell function, as assessed by ISSI-2, was also higher among women with a positive GCT performed later in the day (P < 0.0001, Fig. 1C), and was again driven by significant pairwise differences among women undergoing the GCT after 1300 h and their peers who completed the GCT before 0900 h and between 0900 and 1059 h, respectively.
It thus emerges that, on the subsequent OGTT, women with a positive result on a screening GCT performed after 1300 h have better metabolic function than their peers who had an abnormal GCT in the morning, after adjustment for relevant GDM risk factors. Furthermore, within the ≥1300-h group, there were no significant differences in mean adjusted AUCgluc, ISOGTT, or ISSI-2 among those women tested from 1300 to 1359 h (n = 112), those tested from 1400 to 1459 h (n = 81), and those tested after 1500 h (n = 86; data not shown).
Glucose tolerance status on OGTT
Having demonstrated better glycemia, insulin sensitivity, and β-cell function on subsequent OGTT among women with an abnormal GCT performed in the afternoon, we next compared the study groups with respect to glucose tolerance status on the OGTT (Fig. 2A). Indeed, consistent with the metabolic differences noted above, the true prevalence of GDM on the OGTT progressively decreased from the ≤0900-h group (26.9%) to the 0900–1059-h group (25.0%) to the 1100–1259-h group (21.7%) to the ≥1300-h group (21.5%; overall P = 0.0022). A similar pattern was observed regarding the prevalence of GIGT. Conversely, there was a higher prevalence of NGT in those whose abnormal GCT was performed later in the day, with a prevalence of 50.9% in the ≤0900-h group that increased to 67.0% in the ≥1300-h group.
Lastly, we evaluated glucose tolerance on the OGTT among women with a GCT ≥10.3 mmol/L, a threshold at which Canadian clinical practice guidelines recommend that GDM can be diagnosed without proceeding to an OGTT (17). These women were of interest because the preceding findings suggest that, in the absence of the OGTT, those assessed in the afternoon potentially could be at risk for being misclassified as having GDM. There were 22 participants (2.4%) with a GCT ≥10.3 mmol/L. In seven of these women the GCT was performed before 0900 h, of six of whom GDM was confirmed on the OGTT (while the other woman had GIGT). In contrast, of the eight women whose GCT exceeded 10.3 mmol/L while tested after 1300 h, only three had GDM on the OGTT, whereas five had completely normal glucose tolerance on the OGTT (Fig. 2B). Indeed, comparison of the <0900- and ≥1300-h groups revealed significant differences in their subsequent glucose tolerance findings on the OGTT (P = 0.026), consistent with an increased likelihood of women being misclassified as having GDM if diagnosed solely on the basis of an afternoon GCT ≥10.3 mmol/L.
CONCLUSIONS
In this report, we demonstrate a graded relationship between time of day of a positive GCT and metabolic function on the subsequent OGTT. In particular, women with a positive GCT performed in the afternoon have a superior metabolic phenotype characterized by lesser glycemia, higher insulin sensitivity, and better β-cell function compared with women with a positive GCT documented earlier in the day. Thus, women with a positive GCT in the afternoon were less likely to have GDM than those with a positive GCT in the morning. These data suggest that for optimal performance, testing conditions and interpretation of the GCT should be modified to account for the effect of the time of day when the test is performed.
The circadian changes in glucose tolerance observed in our study likely reflect the action of molecular clocks present in most cells (1,2,18). The core clock components, the transcription factors, CLOCK and BMAL1, participate in negative feedback loops with other transcription factors, leading to oscillations in the expression of proteins involved in metabolism (1,2,18). The master regulators of this system are the clock proteins in the suprachiasmatic nuclei of the anterior hypothalamus that respond to light sensed by the retina and relay signals to the other parts of the hypothalamus and brain that synchronize metabolism (1). The suprachiasmatic nuclei are required for impaired afternoon/evening insulin sensitivity in rats (19), but the subsequent pathways and target organs, such as liver, muscle, or pancreas, have not yet been clearly delineated. Consistent with this physiologic mechanism, glucose tolerance decreases in the afternoon in nonobese individuals due to reductions in insulin sensitivity and insulin secretion (4,20). In light of these data, we reasoned that the screening GCT in pregnancy is a specific clinical setting in which these circadian changes may be relevant in practice because this test is performed at any time of day.
Although diurnal variation in glucose tolerance in pregnancy has been reported (21), there has been limited previous study of its impact on the GCT (9,10). McElduff and Hitchman (10) studied 646 Australian women undergoing the GCT in a morning (0930–1200 h) or afternoon (1205–1710 h) clinic and demonstrated that the percentage of women with a positive GCT screening test was higher during the afternoon compared with the morning (31.1 vs. 17.0%). In addition, a positive screening test in the afternoon had a lower positive-predictive value for GDM (10). Similarly, a second study also showed that the positive-predictive value of the GCT was consistently lower when performed in the afternoon (9).
In this context, the current analysis extends these data by stratifying a large sample of women (n = 927) into four GCT timing groups and demonstrating a graded relationship between time of day and metabolic function. Furthermore, the detailed clinical and metabolic characterization of study participants made it possible to adjust for GDM risk factors and demonstrate independent relationships between time of day and glycemia, insulin sensitivity, and β-cell function (Fig. 1), thereby providing insight into the pathophysiologic basis behind the effect of time of day on test performance. We speculate that in some pregnant women, the normal circadian decline in insulin sensitivity and/or β-cell responsivity to glucose later in the day may contribute to a positive GCT in the afternoon that might not have been positive if performed earlier in the day. As such, when all participants subsequently underwent the OGTT in the morning, those with positive GCTs later in the day had better insulin sensitivity and β-cell function than those with a positive GCT in the morning.
Our findings hold clinical implications for antepartum GDM testing. First, these data suggest that with the current application of a single screening GCT threshold across the day, there will be a greater number of women tested in the afternoon who go on to have a diagnostic OGTT unnecessarily. Second, our results suggest that the time of day of testing may lead to the misclassification of patients with markedly elevated GCT results when a threshold is applied at which GDM can be diagnosed without proceeding to the OGTT. A previous study suggested that a GCT ≥11.0 mmol/L had a positive-predictive value for GDM of 90.7% when performed before mid-day, which decreased to 80.8% when performed after mid-day (9). We show that this effect extends to the current Canadian Diabetes Association Clinical Practice Guidelines threshold of 10.3 mmol/L and may even be more pronounced at this level, as evidenced by our finding that only three of eight patients with an afternoon GCT ≥10.3 mmol/L actually had GDM on the OGTT (compared with six of seven such patients among those tested before 0900 h). Furthermore, the other five women with an afternoon GCT ≥10.3 mmol/L had completely normal glucose tolerance on the OGTT. These data suggest that failure to consider the effect of time of day in clinical practice is likely leading to a higher rate of misdiagnosis of GDM in women diagnosed solely on the basis of an afternoon GCT.
The current study comes in the context of ongoing controversy regarding GDM screening practices (22). The International Association of Diabetes in Pregnancy Study Groups (IADPSG) recently recommended that all pregnant women not known to have pre-existing diabetes proceed directly to an OGTT, eliminating a need for the GCT (23). Two-step testing, starting with the GCT, has some advantages over the one-step OGTT proposed by the IADPSG: it is easier to perform, may induce less vomiting (24), and may reduce costs by eliminating the need for a large number of OGTT tests in normal patients. However, the use of the GCT may delay treatment and is associated with a false-negative rate that holds important clinical consequences (25,26). In this context, implementation of the IADPSG guidelines with an OGTT performed in all women under standardized conditions in the morning would obviate our concern regarding the effect of time of day on GCT performance. However, if the GCT continues to be used as a screening test for GDM, then our results suggest that the test could be refined by standardizing the timing of the GCT or adjusting cutoff points by the time of day of testing.
A limitation to the current analysis is the lack of data on the time since the participants’ last meal. Sermer et al. (8) showed that time since the last meal affects the result of the GCT, leading to their suggestion of cut points of 8.2, 7.9, and 8.3 mmol/L for elapsed postprandial times of <2, 2–3, and >3 h, respectively. It is possible that participants undergoing GCT testing in the afternoon in our study were >3 h removed from their lunch and, therefore, had correspondingly elevated GCT results that led to misclassification. However, the graded relationship across the day between time of the GCT and metabolic profile, the robust effect of time of day on GCT performance, the lack of differences between the hourly strata within the afternoon group (1300–1359, 1400–1459, and ≥1500 h), and the well-established diurnal variation in glucose metabolism all argue against this possibility as the basis for our findings.
Another limitation is that this analysis was conducted in participants with a positive GCT ≥7.8 mmol/L, as opposed to a population-based study, and thus cannot be used to generate new cutoff points depending on the time of day of testing. Nevertheless, this study has demonstrated that time of day of GCT testing is an important factor that affects test performance and highlighted the potentially important clinical implications of this phenomenon.
In summary, the metabolic phenotype of pregnant women with a positive GCT varies in relation to the time of day when the test is performed. In particular, when assessed on subsequent OGTT, patients with a positive GCT in the afternoon really have a superior metabolic profile compared with those with positive tests earlier in the day. The clinical implications of this phenomenon are that some patients will undergo unnecessary further testing by OGTT while others may be overtly misclassified as having GDM as a result of GCT testing in the afternoon. Overall, these data suggest that GDM screening protocols that include the GCT should be refined to account for the effect of time of day on test performance.
Acknowledgments
This study was supported by operating grants from the Canadian Institutes of Health Research (CIHR) (MOP-84206) and the Canadian Diabetes Association (CDA) (OG-3-08-2543-RR).
R.R. holds a CIHR Clinical Research Initiative New Investigator Award and CDA Clinician-Scientist incentive funding. A.J.G.H. holds a Tier-II Canada Research Chair in Diabetes Epidemiology. B.Z. holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and University of Toronto.
No potential conflicts of interest relevant to this article were reported.
R.J.G. researched the data, contributed to the analysis, wrote the manuscript, and contributed to critical revision. C.Y. performed the statistical analyses and contributed to critical revision. M.S., P.W.C., A.J.G.H., and B.Z. participated in the design and implementation of the overall study and contributed to critical revision. R.R. participated in the design and implementation of the overall study, designed the analysis plan, supervised the analysis and manuscript, and contributed to critical revision. R.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this work were presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, PA, 8–12 June 2012.
The authors thank Mount Sinai Hospital Department of Pathology and Laboratory Medicine and Patient Care Services.
References
- 1.Kalsbeek A, Yi CX, La Fleur SE, Fliers E. The hypothalamic clock and its control of glucose homeostasis. Trends Endocrinol Metab 2010;21:402–410 [DOI] [PubMed] [Google Scholar]
- 2.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 2010;106:447–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasai MJ, Pernicova I, Grant PJ, Scott EM. An endocrinologist’s guide to the clock. J Clin Endocrinol Metab 2011;96:913–922 [DOI] [PubMed] [Google Scholar]
- 4.Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes 1992;41:750–759 [DOI] [PubMed] [Google Scholar]
- 5.Carroll KF, Nestel PJ. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes 1973;22:333–348 [DOI] [PubMed] [Google Scholar]
- 6.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009;106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009;5:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sermer M, Naylor CD, Gare DJ, et al. Impact of time since last meal on the gestational glucose challenge test. The Toronto Tri-Hospital Gestational Diabetes Project. Am J Obstet Gynecol 1994;171:607–616 [DOI] [PubMed] [Google Scholar]
- 9.Wong VW, Garden F, Jalaludin B. Hyperglycaemia following glucose challenge test during pregnancy: when can a screening test become diagnostic? Diabetes Res Clin Pract 2009;83:394–396 [DOI] [PubMed] [Google Scholar]
- 10.McElduff A, Hitchman R. Screening for gestational diabetes: the time of day is important. Med J Aust 2002;176:136. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Pre-gravid physical activity and reduced risk of glucose intolerance in pregnancy: the role of insulin sensitivity. Clin Endocrinol (Oxf) 2009;70:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 14.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001;24:1602–1607 [DOI] [PubMed] [Google Scholar]
- 15.Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 2009;26:1198–1203 [DOI] [PubMed] [Google Scholar]
- 16.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 17.Canadian Diabetes Association. 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2008;32(Suppl. 1):1–215 [DOI] [PubMed] [Google Scholar]
- 18.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab 2011;13:125–137 [DOI] [PubMed] [Google Scholar]
- 19.la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 2001;50:1237–1243 [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996;45:1044–1050 [DOI] [PubMed] [Google Scholar]
- 21.Aparicio NJ, Joao MA, Cortelezzi M, et al. Pregnant women with impaired tolerance to an oral glucose load in the afternoon: evidence suggesting that they behave metabolically as patients with gestational diabetes. Am J Obstet Gynecol 1998;178:1059–1066 [DOI] [PubMed] [Google Scholar]
- 22.Karakash SD, Einstein FH. Diabetes in pregnancy: glycemia control guidelines and rationale. Curr Opin Endocrinol Diabetes Obes 2011;18:99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: problems associated with the oral glucose tolerance test. Diabetes Res Clin Pract 2004;63:73–74 [DOI] [PubMed] [Google Scholar]
- 25.Moses RG, Cheung NW. Point: universal screening for gestational diabetes mellitus. Diabetes Care 2009;32:1349–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moses RG. Gestational diabetes: what is the relevance of the glucose challenge test? Med J Aust 2002;176:354. [DOI] [PubMed] [Google Scholar]