Abstract
OBJECTIVE
Diabetes is associated with many forms of cancer. Recent evidence has suggested that some treatments for diabetes are associated with an increased cancer risk. Less is known about the association between endogenous insulin in the prediabetes state and cancer risk.
RESEARCH DESIGN AND METHODS
We investigated cumulative cancer incidence and cancer incidence density over 29 years, according to basal insulin, in a cohort of 1,695 nondiabetic men and women of four ethnic origins, aged 51.8 ± 8.0 years at baseline. Total mortality among the 317 subjects (18.7%) who developed cancer at least 2 years after baseline was assessed.
RESULTS
In a Cox proportional hazards model, the all-site hazard ratio of cancer incidence comparing the highest insulin quartile with the other three quartiles was 1.09 (95% CI 0.85–1.40), adjusted for age, sex, and ethnicity. BMI, smoking, and fasting blood glucose were not statistically significant in this model. Basal insulin level was not significantly associated with cancer of specific sites (breast, prostate, colon/rectum, or bladder). Fasting insulin in the upper quartile conferred a 37% increased risk for total mortality among cancer patients, adjusting for age, sex, and ethnic origin (95% CI 0.94–2.00, P = 0.097) compared with that of the lower quartiles. Male sex, older age, and North African origins were associated with a greater risk of mortality during follow-up time.
CONCLUSIONS
This long-term cohort study may suggest a role for basal elevated insulin levels, mainly as a negative predictor in cancer prognosis.
The American Diabetes Association and the American Cancer Society recently issued a consensus report showing cancer incidence to be associated with diabetes (1). Type 2 diabetes has been associated with increased incidence, in the range of 1.2–2.5 of cancers of the pancreas (2), breast (3), colon (4), and bladder (5). In addition, a recent meta-analysis of 23 studies found diabetes to be associated with an increased mortality hazard ratio (HR) of 1.41 (95% CI 1.28–1.55) among individuals with cancer (6). Furthermore, some treatments for diabetes have been implicated in increasing the risk of malignancy (7). The development of some types of insulin has been discontinued secondary to increased mitogenic side effects (8). The affinity of binding to the IGF-1 receptor (IGF-1R) has been implicated.
We and others have shown basal hyperinsulinemia to predict type 2 diabetes (9,10). Further, elevated levels of circulating insulin and C-peptide have been associated with an increased risk of colorectal and pancreatic cancers (11). Though the risk of breast cancer was less certain in the latter study, two recent analyses of the Women's Health Initiative found a positive association between insulin levels and breast cancer (12,13).
Studies in animals (14–17) and in vitro (18,19) suggest a role for insulin in tumor progression. Glucose tolerance status from 2-h glucose tolerance tests has been shown to associate with the risk of cancer mortality (4). However, the effect of elevated basal insulin on cancer prognosis has not been investigated in vivo.
The current study examined the effect, up to 29 years later (mean follow-up time 21.7 ± 6.5 years), of elevated levels of basal insulin on the cumulative incidence of cancer and on cancer survival in a cohort of the Jewish population, representing the four main ethnic origins of immigration to Israel.
RESEARCH DESIGN AND METHODS
The current investigation was based on the Israel Study of Glucose Intolerance, Obesity and Hypertension (The Israel GOH Study), an ongoing nationwide longitudinal study, which was initiated in 1968. The cohort is a random sample (N = 5,711) drawn in 1967 from the Israel Central Population Registry, stratified according to sex, age (subjects born between 1912 and 1941), and ethnicity (European-American, North African, Yemenite, and Other Middle Eastern). European-American origin represents subjects born in Europe or whose ancestors came from Europe either directly to Israel or through America. We note that the Yemenite population was oversampled (20). The detailed sampling procedure and design of this study have been reported elsewhere (20,21).
Between 1977 and 1982 (baseline), 65.3% of the original 5,711 participants were interviewed in their homes. Interviews were not achieved mainly due to shortage of funds (17.7%), reported change of address (7.2%), inability to find a subject at home (3.1%), death during the mean 10 years of follow-up (4.1%), and refusals (2.6%). Consenting individuals were requested to attend a neighborhood medical center; 74% consented. Interviews and laboratory examinations determined weight, height, blood pressure, smoking status, and 12-h fasting plasma glucose. After initiation of this study phase, insulin levels were added to the tests, and were determined in the most recent 1,843 participants of the cohort. The subsample was representative of the parent group in demographic characteristics, BMI, and blood pressure level (20,21).
In the current study, we excluded the 77 individuals who used glucose-lowering medications at the time of the baseline basal insulin measurement because such treatment could confound the association between basal insulin and cancer incidence. Despite this exclusion criterion, the fasting plasma glucose values of 19 participants were above 200 mg/dL. These individuals remained in our analysis because their basal insulin values were not confounded by glucose lowering agents; thus the association between endogenous insulin and cancer outcome would not be affected. In total, 65 individuals were diagnosed with cancer prior to or 2 years after the date of the glucose and insulin measurements. To avoid reverse causality, the main analysis excluded these individuals and was based on 1,695 participants. An additional sensitivity analysis, including all cancer incidents, was performed.
Study nurses measured weight and height at the homes of the study participants, using standard calibrated transportable scales and a uniform technique for measuring height. BMI (weight [kg]/height [m2]) was calculated. Blood pressure was measured at the participants’ homes on 2 consecutive days; the mean value was used in the current analysis. Cigarette smoking, assessed from participant report, was categorized as past, current, or never smoked.
Plasma glucose at baseline was determined by the routine automated Technicon AutoAnalyzer II method (Technicon Instruments Corp., Tarrytown, NY), using potassium ferricyanide reduction. Of note, this method overestimates glucose levels by approximately 9% (22). Plasma insulin at baseline was determined in duplicate with the Phadebas Radioimmunoassay kit (Pharmacia Diagnostics Inc., Piscataway, NJ). The within-assay and between-assay coefficients of variation were 4% and 8%, respectively.
Cancer incidence was identified by linkage of the GOH database with the Israel National Cancer Registry (INCR), which was established in 1960. Since 1982, the INCR has received, with high completeness and by law, reports on all malignant tumors diagnosed in Israeli citizens (23). This linkage is possible because of the allocation of a unique personal identification number to every Israel citizen at birth or upon immigration. Vital status and date of death were ascertained through the National Population Registry.
Statistical analysis
Basal insulin, the variable of interest, did not exhibit a normal distribution, despite performance of logarithmic transformation, and was therefore categorized in quartiles. Comparisons of demographic and selected clinical characteristics, according to quartiles of basal insulin, were performed using the χ2 test for categorical variables and one-way ANOVA for continuous variables.
Person-years of follow-up were calculated from the date of basal insulin laboratory test until the date of cancer diagnosis, death, or last follow-up (December 31, 2005), whichever was earliest. In the main analysis, subjects diagnosed with cancer within the first 2 years after baseline were not included. A subsequent sensitivity analysis was performed including all cancer incident patients. All and site-specific cancer incidence density was calculated per 10,000 person-years. The incidence rate for breast cancer was calculated among women only and for prostate cancer among men only. The Cox proportional hazards model, using backward selection, was used to test the association of basal insulin and the risk of cancer development. The covariates included in the final models were those that showed statistical significance at a 10% level in the univariate analysis (age, sex, and origin), are known to be associated with cancer (BMI and smoking), or are strongly related to fasting insulin (fasting blood glucose). We eliminated the least significant variables sequentially until all remaining variables were significant at a 5% level, except for the variable of interest, fasting insulin. The assumption of the Cox proportional hazards model was tested by introducing time-dependent covariates and multiplying each covariate by the time of follow-up. These terms were nonstatistically significant, indicating that the Cox assumption of proportionality over time was met (P = 0.25 for the incidence of cancer, and P = 0.63 for cancer survival). For subjects who were diagnosed with cancer, a Kaplan Meier survival analysis was performed to estimate the risk for total mortality from the date of cancer diagnosis. Differences in cancer survival between quartiles of basal insulin were tested using the log-rank test. To assess basal insulin, as well as other factors that are significantly and independently associated with cancer survival, a methodologically similar backward Cox proportional hazards model was used.
The study was approved by the Institutional Review Board of the Sheba Medical Center. All subjects gave verbal informed consent, as was accustomed at that time.
RESULTS
The follow-up period ranged from 3 to 29 years and comprised 37,276 person-years. At baseline, the mean age of the total sample was 51.8 ± 8.0 years; 50.9% were males, 30.9% active smokers, and 11.9% reported being former smokers. Mean BMI was 25.7 ± 3.4 for males and 26.4 ± 4.6 for females.
Characteristics of the study cohort according to basal insulin quartiles at baseline are presented in the Supplementary Table 1. Fasting glucose, BMI, and blood pressure show significantly higher values with increasing basal insulin quartiles. The mean plasma level of fasting glucose was less than 100 mg/dL in the two lower fasting insulin quartiles and reached 107 mg/dL in the highest quartile. Yemenites contributed most to the first basal insulin quartile and least to the fourth one, whereas the other three origins showed an opposite trend.
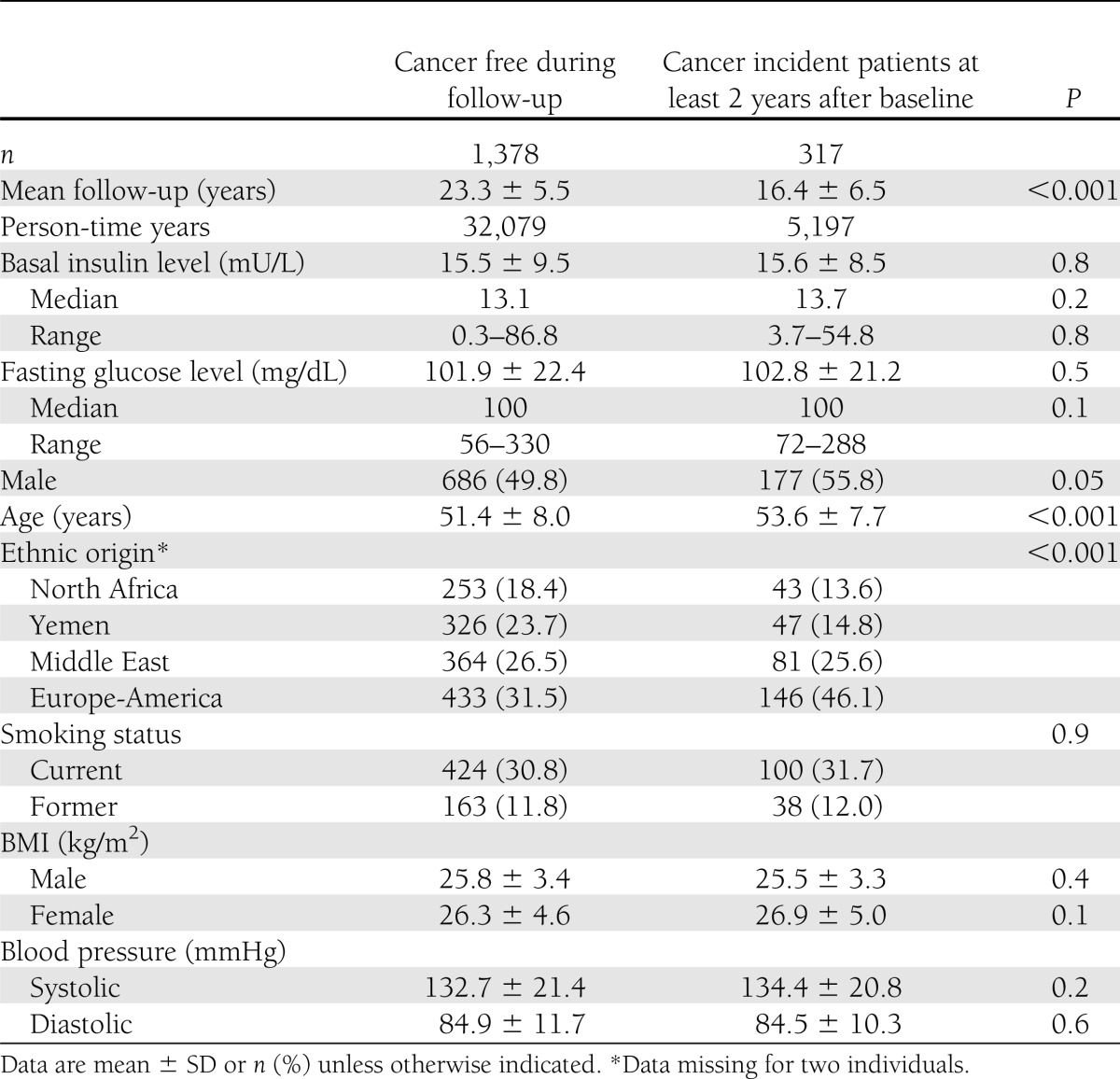
Of the 1,695 individuals in the sample, 317 (18.7%) developed cancer at least 2 years after baseline. Table 1 compares characteristics between subjects who developed cancer during the follow-up period and those who remained cancer free. Neither fasting glucose nor basal insulin levels were associated with cancer incidence. Male sex conferred increased risk for cancer incidence, as did greater age and European-American ethnicity. Individuals of Yemenite and North African ethnicity had lower cancer incidence than did the other ethnic groups. Smoking, BMI, and blood pressure levels did not differ significantly at baseline between those who remained cancer free and those who developed cancer during follow-up.
Table 1.
Comparison of demographic and clinical characteristics among individuals remaining cancer free and those who developed cancer at least 2 years after baseline
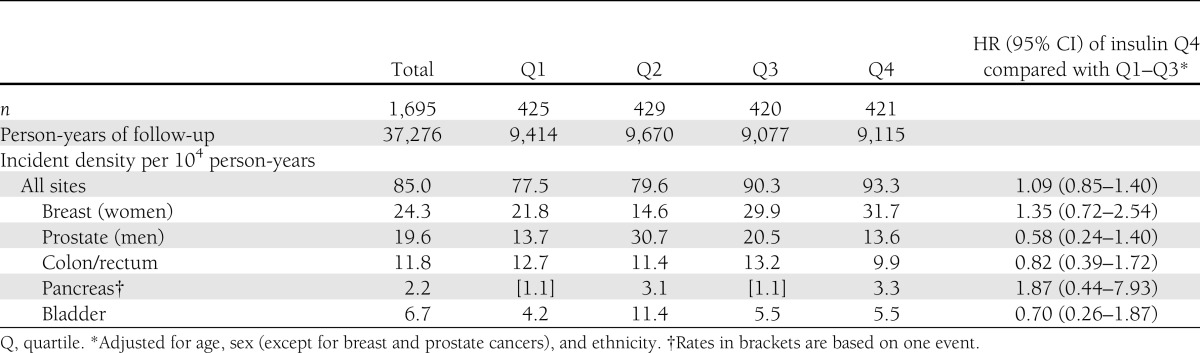
In total, 5.5% of the women were diagnosed with breast cancer, 4.2% of the men with prostate cancer, 2.6% of all the participants with colorectal cancer, and 1.5% with bladder cancer (not shown). Table 2 presents cancer incidence density per 104 person-years of selected sites by fasting insulin quartiles. No differences in site-specific cancer incidence density were noted according to fasting insulin quartiles. The all-site adjusted HR of cancer incidence, comparing the highest insulin quartile to the other three quartiles, was 1.09 (95% CI 0.85–1.40), adjusted for age, sex, and ethnicity. Older age, male sex, and European-American country of origin were found to be significantly associated with cancer incidence. BMI, smoking, and fasting blood glucose did not remain in the final model because they were not statistically significant. The association between basal insulin and cancer incidence did not change (1.11 [0.86–1.44]) when the 17 patients who developed cancer within the first 2 years of follow-up were included. A nonstatistically significant increased HR of 35% (0.72–2.54) was also observed for breast cancer.
Table 2.
Cancer incidence density (per 104 person-years) of selected sites and HR (95% CI) for cancer events among 1,695 men and women according to quartiles of plasma values of basal insulin* (1, lowest quartile; 4, highest quartile)
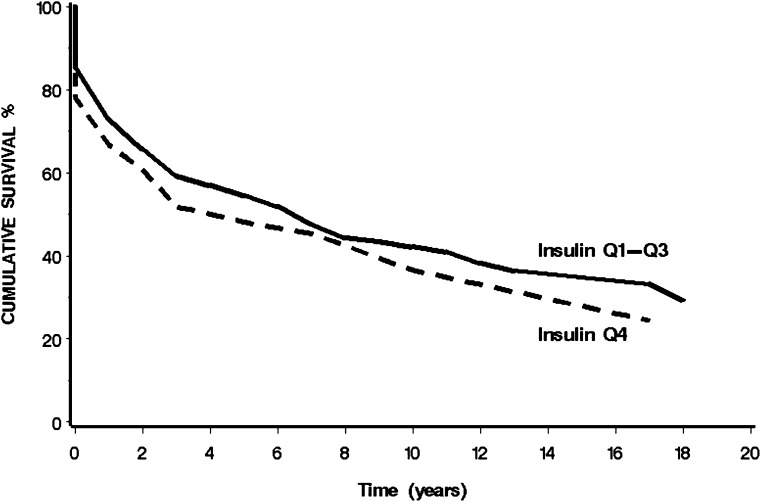
Figure 1 presents the age-adjusted cumulative survival of the 317 individuals who were diagnosed with cancer during the 3–29 year follow-up according to basal insulin quartiles. The median survival time of the fourth quartile was 3 years (95% CI 2–10) compared with 6 years (3–8) in the first three quartiles (P = 0.2).
Figure 1.
Age-adjusted cumulative survival among cancer incident patients by basal insulin. The curves represent the cumulative survival of cancer incident patients over 18 years of follow-up according to baseline basal insulin in the lower three quartiles (Q1-Q3) and the upper quartile (Q4).
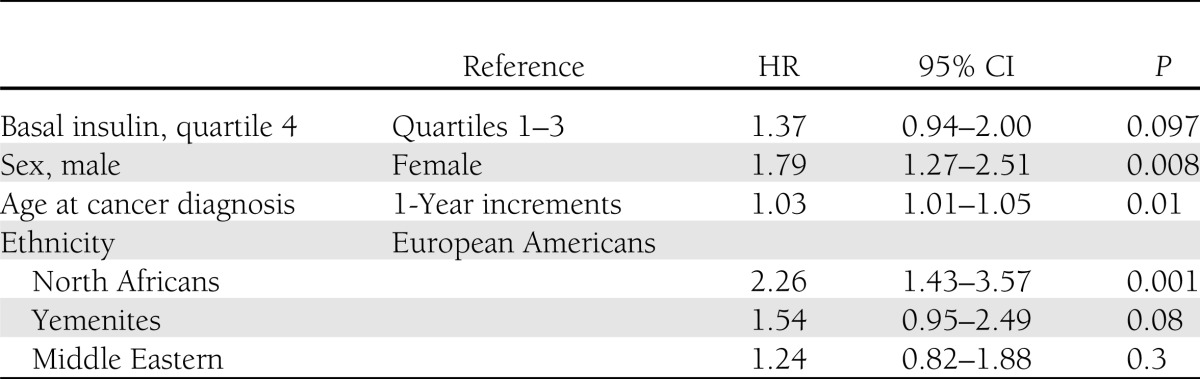
Table 3 presents a Cox proportional hazards model of mortality for cancer patients according to insulin quartiles. No differences were observed in HRs between the first three quartiles of basal insulin. The upper quartile of fasting insulin (≥18.3 mU/L) confers a 37% increased hazard for total mortality compared with the other three quartiles (P = 0.097), adjusting for age, sex, and ethnic origin. Male sex (HR 1.79 [95% CI 1.2–2.51]), older age at cancer diagnosis (an additional 3% increased risk for every year of age), and North African origin were significantly associated with increased risk for all-cause mortality. BMI and smoking were not found to be significantly associated with overall survival, controlling for the other covariates in the final model.
Table 3.
Cox proportional hazards model of factors affecting overall survival of cancer patients
In a subsequent subanalysis, which included cancer events diagnosed within the first 2 years of follow-up, the HR of the fourth quartile, compared with the other three combined, was 1.41 (95% CI 0.98–2.03, P = 0.066).
CONCLUSIONS
In this follow-up of up to 29 years of a multiethnic cohort with 37,276 person-years accumulated, basal insulin in the upper quartile of nondiabetic individuals did not confer a risk for total cancer incidence. Higher breast cancer incidence rates were observed in the two upper quartiles of basal insulin. Among those who developed cancer, basal insulin in the upper quartile—assessed at least 2 years prior to diagnosis—was associated with increased total mortality; statistical significance was borderline. In the absence of cause-specific mortality, we used total mortality as a surrogate for cancer mortality. Our data thus suggest that hyperinsulinemia may be a prognostic factor in cancer patients. The lack of statistical significance for the 37% increased risk of mortality conferred by basal insulin in the upper quartile may be due to the relatively limited number of cancer patients.
Many theories have been hypothesized to explain the significant risk of malignancy associated with diabetes. Erickson et al. (24) recently demonstrated an association between chronic hyperglycemia and overall survival of early stage breast cancer. According to the Warburg effect, malignant cells rely on aerobic glycolysis in contrast to normal cells, which use oxidative phosphorylation to generate energy (25). An environment rich in glucose and insulin could therefore promote malignant potential in cells.
Possible means by which hyperinsulinemia could affect cancer progression include enhancing tumor aggressiveness (6,26), decreasing resistance to a tumor (26), and decreasing the effectiveness of medications. It has been suggested that insulin cannot be considered a carcinogen because it does not induce somatic cell mutations (27). However, insulin may be one of a number of interacting factors that determine whether premalignant lesions, which may be present in a high proportion of healthy individuals, progress to invasive metastatic fatal cancers.
IGF acts synergistically with other hormones, such as insulin and estradiol, and with other risk factors for cancer; circulating IGF-1 levels are inversely correlated with age (28,29). Endogenous estradiol enhances the stimulatory action of IGF-1 in breast cells (30). Insulin increases the bioavailability of IGF-1 by enhancing its synthesis and by inhibiting the production of its plasma carriers IGF binding protein (IGFBP) -1 and IGFBP-2 in the liver and in other tissues (31,32). Furthermore, insulin and IGF-1 both stimulate the synthesis of sex steroids and inhibit the synthesis of their plasma carriers, thus enhancing the bioavailability of sex hormones (31). Mechanisms for an association between hyperinsulinemia and cancer progression have been demonstrated in animal models. Exogenous insulin has long been known to increase tumor growth in rats (14). Recently, insulin-sensitizing treatment was shown sufficient to abrogate mammary tumor progression in mice (17).
Cannata et al. (32) summarized the mitogenic effects of insulin through different pathways associated with the insulin receptor (IR). The IR has two isoforms: IR-A and IR-B. Although both bind insulin, IR-A also binds to IGF-1, which is a powerful stimulator of cell proliferation (33) and also a mediator of antiapoptotic and mitogenic effects. Enhanced IR expression is common in cancer, particularly IR-A (33). Biological plausibility and correlative studies in humans imply that insulin may, via its receptors, influence for example breast cancer risk and progression. IR level was assessed as more than six times higher in malignant breast tissue as in healthy breast tissue, with positive correlation to tumor size and histological grading (34). Cancer cells appear to lose their ability to downregulate IRs in response to hyperinsulinemia (19).
IR and IGF-1R share a high degree of homology and thus can form hybrid receptors. With a higher affinity for IGF-1 than for insulin, hybrid receptors act more like IGF-1R than IR. IGF-1R mediates body growth through the effects of IGF-1 on cell proliferation, differentiation, and apoptosis. Insulin decreases IGFBP-1, and perhaps also IGFBP-2 (35). The result is increased free IGF-1, the biologically active form of the growth factor.
Importantly, the effects of hyperinsulinemia may occur through less direct means. Both insulin activation of the inflammatory system and enhancement of adiposity can induce insulin resistance, which may then promote cancer development and progression (27). BMI in the current study was associated with basal insulin quartiles but did not differ between those who developed cancer and those who remained cancer free.
As in ours, other studies have found increased cancer incidence in men, as well as increased mortality. A large study found a stronger association between the metabolic syndrome and colorectal cancer in men than in women (36). However, as in our study, menopausal status of women or the use of hormone replacement therapy was not considered. Perhaps a protective effect of estrogen or progesterone masks the true effects of the metabolic syndrome on colorectal cancer development. In our study, incidence of colorectal cancer in men was 3.0% (26 patients) and in women, 2.2% (18 patients); yet the HR for colorectal cancer among men who belonged to the upper insulin quartile during baseline was lower than in women, with age- and ethnicity-adjusted HRs of 0.73 (95% CI 0.27–1.95) and 0.86 (0.28–2.66), respectively. High circulating levels of insulin reduce hepatic synthesis and release of sex hormone–binding globulin, thus increasing levels of bioavailable estrogen in both sexes and of bioavailable testosterone in women but not in men. Insulin promotes androgen synthesis in the ovaries (and possibly the adrenal glands) in women before menopause, and elevated androgynous sex steroid levels are associated with increased risk of breast and endometrial cancer in postmenopausal women. Likewise, a recent study provided evidence for involvement of estrogen and androgen signaling in the etiology of colorectal cancer (37). Our study population demonstrated a nonstatistically significant lower risk of prostate cancer, consistent with the theory that increased circulating insulin levels in men decrease circulating testosterone. Despite previous studies demonstrating an increased risk of bladder cancer in association with diabetes (5), our study showed a nonstatistically significant lower risk of bladder cancer.
In the current study, baseline glucose was found to associate with insulin quartiles, but not with cancer incidence. In a previous publication, which studied the same cohort 10 years earlier (38), we reported a modest association between glucose intolerance or type 2 diabetes and 20-year cancer incidence (HR 1.32 [95% CI 0.96–1.81] and HR 1.24 [95% CI 0.96–1.62] for glucose intolerance and diabetes, respectively).
We cannot exclude the possibility of hyperinsulinemia being a risk factor for total mortality due to noncancer-related effects, such as coronary heart disease (39). Though the lack of data for cancer-specific mortality is a limitation of this study, cause-specific mortality statistics have limitations, such as underestimation of cancer as the cause of mortality (40).
Although we excluded from the study individuals who used glucose-lowering medications, some nonpharmacologically treated individuals with diabetes may have remained in the cohort.
The lack of data on hormone replacement therapy and C-peptide are other limitations of this study. Information regarding hormone replacement therapy was not available in the study database. It is noteworthy that baseline period was 1980, a time when this treatment was not yet common in Israel. C-peptide, which is secreted simultaneously and in equimolar quantities with insulin but has a longer plasma half-life, may provide a more effective index of insulinemia—one that is less subject to individual variation (27). In a recent meta-analysis, C-peptide was the most common marker of insulin levels in epidemiological studies. Assessment of fasting insulin, measured at baseline in the current study, was rarely investigated in recent years (11).
The long-term follow-up is a strength of this study, as is the availability of data on cancer incidence and not just mortality. Of importance, the INCR is a highly validated data source that undergoes periodic quality assurance testing (23). In contrast, other studies often use cancer mortality to determine cancer incidence. Further, basal insulin was measured directly, as compared with studies that have assessed a cluster of markers associated with hyperinsulinemia and insulin resistance, without directly measuring insulin.
Although the impact of hyperinsulinemia on the progression of dysglycemia has recently become a popular subject of research, the impact of hyperinsulinemia on cancer incidence and prognosis has been less investigated. This long-term study suggests that elevated levels of basal insulin, presenting many years prior to cancer diagnosis, may impact prognosis in cancer patients.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
R.D. and A.C. conceived and designed the study, analyzed and interpreted data, drafted the manuscript, and revised the manuscript critically for important intellectual content. L.K.-B. contributed to interpretation of the data, provided important intellectual content to the study, and drafted the manuscript. M.H.S. and C.C. contributed to discussion and reviewed and edited the manuscript. R.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge Dr. Pesach Segal, The Sheba Medical Center, Ramat Gan, Israel, for his contribution in the baseline phase of this study.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/DC11-1513/-/DC1.
References
- 1.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856–862 [DOI] [PubMed] [Google Scholar]
- 4.Zhou XH, Qiao Q, Zethelius B, et al. DECODE Study Group Diabetes, prediabetes and cancer mortality. Diabetologia 2010;53:1867–1876 [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006;49:2819–2823 [DOI] [PubMed] [Google Scholar]
- 6.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008;300:2754–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009;52:1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drejer K. The bioactivity of insulin analogues from in vitro receptor binding to in vivo glucose uptake. Diabetes Metab Rev 1992;8:259–286 [DOI] [PubMed] [Google Scholar]
- 9.Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care 2009;32:1464–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 2000;49:2094–2101 [DOI] [PubMed] [Google Scholar]
- 11.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem 2008;114:63–70 [DOI] [PubMed] [Google Scholar]
- 12.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2009;101:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabat GC, Kim M, Caan BJ, et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer 2009;125:2704–2710 [DOI] [PubMed]
- 14.Heuson JC, Legros N, Heimann R. Influence of insulin administration on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in intact, oophorectomized, and hypophysectomized rats. Cancer Res 1972;32:233–238 [PubMed] [Google Scholar]
- 15.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev 1996;5:1013–1015 [PubMed] [Google Scholar]
- 16.Novosyadlyy R, Lann DE, Vijayakumar A, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res 2010;70:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D. Insulin-sensitizing therapy attenuates type 2 diabetes-mediated mammary tumor progression. Diabetes 2010;59:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong M, Holdaway IM. Insulin binding by normal and neoplastic colon tissue. Int J Cancer 1985;35:335–341 [DOI] [PubMed] [Google Scholar]
- 19.Mountjoy KG, Finlay GJ, Holdaway IM. Abnormal insulin-receptor down regulation and dissociation of down regulation from insulin biological action in cultured human tumor cells. Cancer Res 1987;47:6500–6504 [PubMed] [Google Scholar]
- 20.Modan M, Halkin H, Almog S, et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest 1985;75:809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modan M, Halkin H, Fuchs Z, et al. Hyperinsulinemia—a link between glucose intolerance, obesity, hypertension, dyslipoproteinemia, elevated serum uric acid and internal cation imbalance. Diabete Metab 1987;13:375–380 [PubMed] [Google Scholar]
- 22.Passey RB, Gillum RL, Fuller JB, Urry FM, Giles ML. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974). Clin Chem 1977;23:131–139 [PubMed] [Google Scholar]
- 23.Fishler Y, Chetrit A, Barchana M, Modan B. Assessment of the completeness of the Israel Cancer Registry Database: methods and findings [Internet], c2003 [in Hebrew]. Ramat Gan, Israel, Israel Center for Disease Control. Available from www.health.gov.il Accessed May 2011
- 24.Erickson K, Patterson RE, Flatt SW, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 2011;29:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warburg O. On the origin of cancer cells. Science 1956;123:309–314 [DOI] [PubMed] [Google Scholar]
- 26.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103–1123 [DOI] [PubMed] [Google Scholar]
- 27.Godsland IF. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin Sci (Lond) 2009;118:315–332 [DOI] [PMC free article] [PubMed]
- 28.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med 1997;336:633–640 [DOI] [PubMed] [Google Scholar]
- 29.Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol 1997;145:970–976 [DOI] [PubMed] [Google Scholar]
- 30.Sachdev D, Yee D. The IGF system and breast cancer. Endocr Relat Cancer 2001;8:197–209 [DOI] [PubMed] [Google Scholar]
- 31.Kaaks R. Nutrition, hormones and breast cancer: is insulin the missing link? Cancer Causes Control 1996;7:605–625 [DOI] [PubMed] [Google Scholar]
- 32.Cannata D, Fierz Y, Vijayakumar A, LeRoith D. Type 2 diabetes and cancer: what is the connection? Mt Sinai J Med 2010;77:197–213 [Review] [DOI] [PubMed] [Google Scholar]
- 33.Frasca F, Pandini G, Scalia P, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 1999;19:3278–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papa V, Pezzino V, Costantino A, et al. Elevated insulin receptor content in human breast cancer. J Clin Invest 1990;86:1503–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 2001;60:91–106 [Review] [DOI] [PubMed] [Google Scholar]
- 36.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev 2002;11:385–391 [PubMed] [Google Scholar]
- 37.Slattery ML, Sweeney C, Murtaugh M, et al. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol Biomarkers Prev 2005;14:2936–2942 [DOI] [PubMed] [Google Scholar]
- 38.Dankner R, Chetrit A, Segal P. Glucose tolerance status and 20 year cancer incidence. Isr Med Assoc J 2007;9:592–596 [PubMed]
- 39.Casassus P, Fontbonne A, Thibult N, et al. Upper-body fat distribution: a hyperinsulinemia-independent predictor of coronary heart disease mortality. The Paris Prospective Study. Arterioscler Thromb 1992;12:1387–1392 [DOI] [PubMed] [Google Scholar]
- 40.Welch HG, Black WC. Are deaths within 1 month of cancer-directed surgery attributed to cancer? J Natl Cancer Inst 2002;94:1066–1070 [DOI] [PubMed] [Google Scholar]