Abstract
OBJECTIVE
We evaluated the addition of liraglutide to metformin in type 2 diabetes followed by intensification with basal insulin (detemir) if glycated hemoglobin (A1C) ≥7%.
RESEARCH DESIGN AND METHODS
In 988 participants from North America and Europe uncontrolled on metformin ± sulfonylurea, sulfonylurea was discontinued and liraglutide 1.8 mg/day added for 12 weeks (run-in). Subsequently, those with A1C ≥7% were randomized 1:1 to 26 weeks’ open-label addition of insulin detemir to metformin + liraglutide (n = 162) or continuation without insulin detemir (n = 161). Patients achieving A1C <7% continued unchanged treatment (observational arm). The primary end point was A1C change between randomized groups.
RESULTS
Of 821 participants completing the run-in, 61% (n = 498) achieved A1C <7% (mean change −1.3% from 7.7% at start), whereas 39% (n = 323) did not (−0.6% from 8.3% at start). During run-in, 167 of 988 (17%) withdrew; 46% of these due to gastrointestinal adverse events. At week 26, A1C decreased further, by 0.5% (from 7.6% at randomization) with insulin detemir (n = 162) versus 0.02% increase without insulin detemir (n = 157) to 7.1 and 7.5%, respectively (estimated treatment difference −0.52 [95% CI −0.68 to −0.36]; P < 0.0001). Forty-three percent of participants with insulin detemir versus 17% without reached A1C <7%. Mean weight decreased by 3.5 kg during run-in, then by 0.16 kg with insulin detemir or 0.95 kg without insulin detemir. In the randomized phase, no major hypoglycemia occurred and minor hypoglycemia rates were 0.286 and 0.029 events per participant-year with and without insulin detemir (9.2 vs. 1.3%).
CONCLUSIONS
Supplementation of metformin with liraglutide and then insulin detemir was well tolerated in the majority of patients, with good glycemic control, sustained weight loss, and very low hypoglycemia rates.
Metformin is generally considered to be the most appropriate first-line pharmacotherapy for treating type 2 diabetes (1), but there is no general agreement on how to advance treatment when metformin becomes insufficient. With many drug classes available, translational studies are needed to identify the most effective, safe, and simplest antidiabetes treatment sequences. Practical treatment strategies that achieve and maintain glycated hemoglobin (A1C) levels at <7% while minimizing hypoglycemia and weight gain are especially desirable (2).
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) reduce A1C by 0.8–1.5% and weight by 2–3 kg on average in combination with metformin (depending on study populations and other background therapy), and are associated with a low risk of hypoglycemia (3–6). Although the durability of effectiveness of GLP-1RAs has not yet been established, most other type 2 diabetes drugs fail after several years, such that many patients eventually require insulin treatment to attain and sustain glycemic control. This is typically achieved by adding a basal insulin to previous medications because such insulins are associated with modest hypoglycemia risk and weight gain compared with premixed and prandial insulins (7,8). The side effects of insulin might be mitigated if used with a GLP-1RA; however, to date, there have been only trials adding GLP-1RAs to insulin and no well-controlled trials examining basal insulin added to existing GLP-1RA therapy. In this trial, for the first time, we evaluated a novel treatment intensification sequence: adding a GLP-1RA (liraglutide) to metformin followed by a randomized, open-label investigation of further intensification with systematically titrated basal insulin (insulin detemir) in participants with ≥7% A1C.
RESEARCH DESIGN AND METHODS
Participants
Eligible participants were insulin-naïve adults (18–80 years) with type 2 diabetes treated for ≥3 months with ≥1,500 mg/day metformin and A1C values of 7.0–10.0% or with metformin and sulfonylurea (less than or equal to half of the maximum approved dose) and A1C values of 7.0–8.5% (Supplementary Data online).
Trial design and interventions
The trial was conducted in 202 office- or hospital-based sites in Belgium, Canada, France, Germany, Italy, the Netherlands, Spain, the U.K., and the U.S. between 3 March 2009 and 19 April 2010. Protocol amendments occurring after the study start are summarized in the Supplementary Data online. Protocol, amendments, and informed consent documents were approved by independent local ethics committees and implemented according to good clinical practice (9) and the Declaration of Helsinki (10).
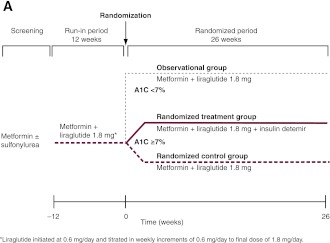
This 38-week, open-label trial comprised a 12-week run-in followed by a 26-week, randomized, two-armed, parallel-group period for participants not achieving <7% A1C. At run-in start, sulfonylurea was discontinued (in approximately one-third of participants), and liraglutide was initiated in 0.6-mg/day weekly increments to a final 1.8-mg/day dose (Fig. 1A). The metformin dose remained unchanged.
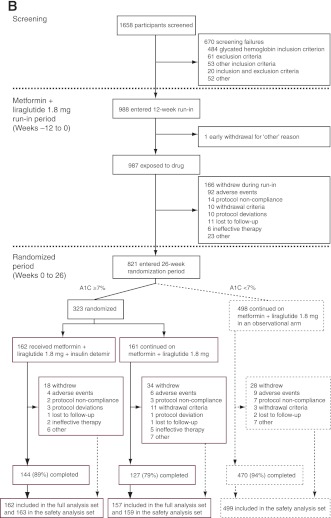
Figure 1.
Trial design (A) and trial flow diagram (B). *Two participants who had been randomized to the metformin and liraglutide control group received the wrong trial treatment. One participant was supplied with insulin detemir but withdrew before administering the treatment. The other participant should not have been randomized as her A1C level at week 0 was <7%. For the full analysis, these participants appear in the randomized control group, and for the safety analysis, they appear in their respective “treatment” groups (i.e., insulin detemir and observational groups, respectively).
Participants with A1C ≥7% at the end of run-in were randomized (1:1) to 26 weeks’ insulin detemir added to metformin + liraglutide 1.8 mg (randomized detemir group) or continued metformin + liraglutide 1.8 mg only (randomized control group) (see Supplementary Data online for randomization details). A double-blind, placebo-controlled approach was not used because titration with detemir placebo was not feasible. To determine the effects of continued treatment on initial responders, participants with A1C <7% after run-in were followed for 26 weeks as a prespecified observational group.
Insulin detemir (100 units/mL) and liraglutide (6.0 mg/mL) (Novo Nordisk A/S, Bagsvaerd, Denmark) were injected subcutaneously once daily with pen devices. Insulin detemir was administered with evening meals or at bedtime; liraglutide was administered at any (consistent) time of day. Insulin detemir treatment was started at 10 units and titrated by investigators on a weekly basis using a specific algorithm targeting 4.1–6.0 mmol/L self-measured fasting plasma glucose (FPG) values (Supplementary Data online).
Assessments and end points
The primary end point was the change in A1C (%) from randomization (week 0) to week 26 to determine whether adding insulin detemir to metformin + liraglutide was superior to continued metformin + liraglutide. Additional efficacy end points included the following: percentage of participants reaching A1C <7 and ≤6.5%, FPG, postprandial plasma glucose from self-measured seven-point glucose profiles, weight, blood pressure, lipids, and percentage of participants reaching the composite end point of A1C <7% with no weight gain or hypoglycemia (during the 26-week period). Safety assessments included adverse events (AEs) and hypoglycemic episodes. Minor hypoglycemic episodes (plasma glucose <3.1 mmol/L) were self-treated. Major episodes required third-party assistance, irrespective of plasma glucose levels.
Statistical analyses
For the primary end point, superiority of the insulin detemir over the control group was concluded if the 95% CI upper limit for the treatment difference was <0. Assuming 20% run-in withdrawals and 40% randomization eligibility, 150 participants per group were needed to detect a 0.5% A1C between-group difference with 90% power.
Unless stated otherwise, efficacy end points were analyzed statistically using the full analysis set (all randomized participants with greater than or equal to one efficacy value) with missing values imputed by carrying forward the last observation. An ANCOVA model with treatment, country, and previous oral antidiabetic therapy as fixed effects and randomization value as covariate was used to analyze change from randomization to week 26. Percentages of participants reaching <7 and ≤6.5% A1C were analyzed using logistic regression with the same fixed effects and covariate. A similar logistic regression model was used for the composite end point (proportion reaching <7% A1C with no weight increase and no hypoglycemia during the 26-week period). The safety analysis set comprised all participants exposed to at least one dose of trial drug. Hypoglycemic episodes were analyzed using a generalized linear model. Descriptive statistics were calculated for the observational group. Run-in results were summarized by the randomized period treatment group (i.e., observational, randomized detemir, or randomized control), although all participants received the same run-in treatment.
RESULTS
Adding liraglutide to metformin: 12-week run-in
During the run-in, 167 of 988 (17%) participants withdrew (Fig. 1B); 76 of 167 (46%) of these withdrew due to gastrointestinal AEs (7.7% of enrolled participants). Of 821 participants completing the run-in, 498 (61%) reached <7% A1C; 323 (39%) did not and were eligible for randomization.
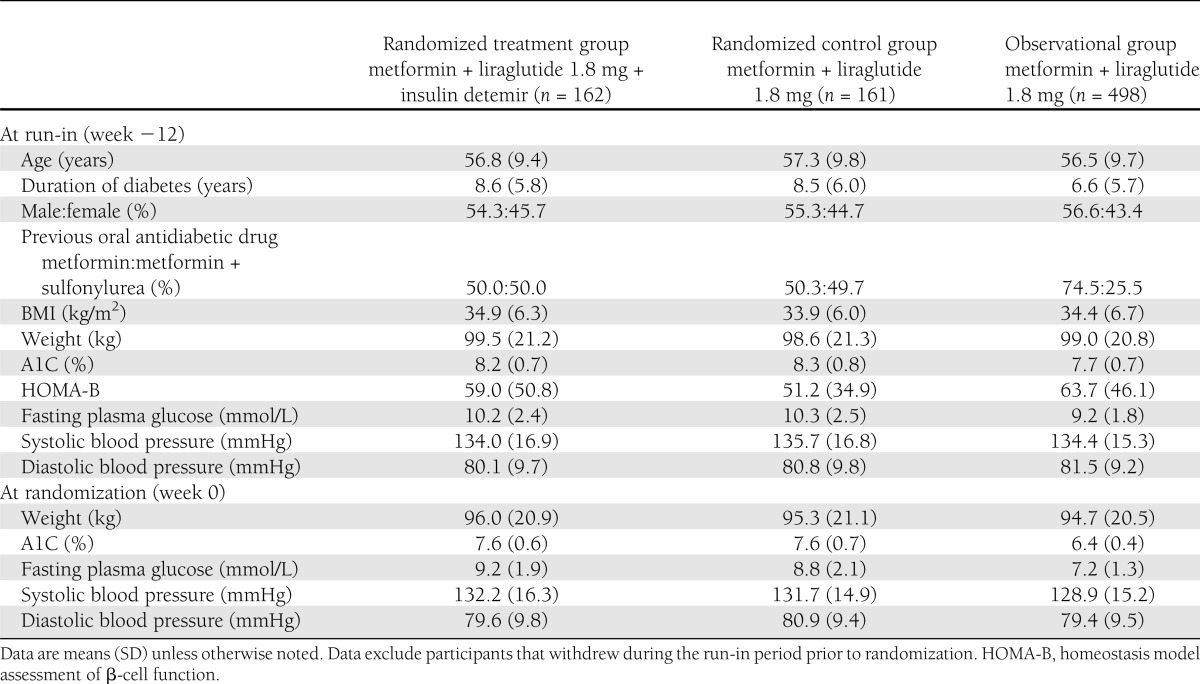
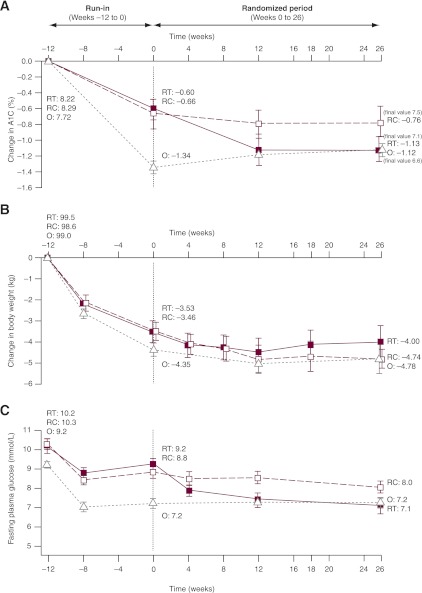
Compared with participants that did not reach the target during run-in, those reaching the target had a shorter type 2 diabetes duration and lower A1C and FPG values and more had been treated with metformin only before enrollment (Table 1). At run-in completion, A1C was reduced by 1.3% in this observational group and by 0.6% in the randomized groups from A1C values at run-in start of 7.7 and 8.3%, respectively (Fig. 2A and Supplementary Table 1); weight decreased by 3.5–4.4 kg (Fig. 2B) and FPG by 1.0–2.0 mmol/L (Fig. 2C). One major hypoglycemic event occurred (blood glucose level, 5.2 mmol/L) and minor hypoglycemia occurred at 0.000–0.372 events per participant-year (Supplementary Table 2).
Table 1.
Demographics and disease characteristics at run-in start (week −12) and randomization (week 0)
Figure 2.
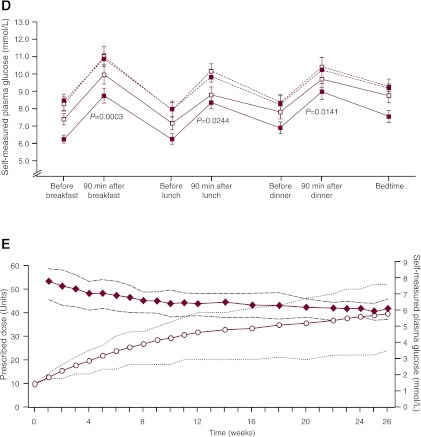
Glycemic efficacy, changes in body weight, and insulin detemir doses. Results from run-in to randomization (vertical dotted line) and from randomization to the end of the trial for change in A1C (A), change in body weight (B), and mean FPG (C). Data are means ± 2 SE from the full analysis set with no imputation. RT, randomized treatment group; RC, randomized control group; O, observational group. D: Self-monitored plasma glucose profiles before and after breakfast, lunch, and dinner and at bedtime for the treatment and control groups at weeks 0 and 26. ■, randomized treatment group; □, randomized control group; △, observational group. Dotted lines, 0 weeks; solid lines, 26 weeks. Vertical bars indicate ± 2 SEM. P values refer to differences between groups in the change from randomization (week 0) to week 26. E: Insulin detemir dose (prescribed dose, ○, left ordinate) and self-measured FPG (♦, right ordinate) during the 26-week randomized period for the insulin detemir treatment group. Dotted lines indicate 25th–75th percentiles.
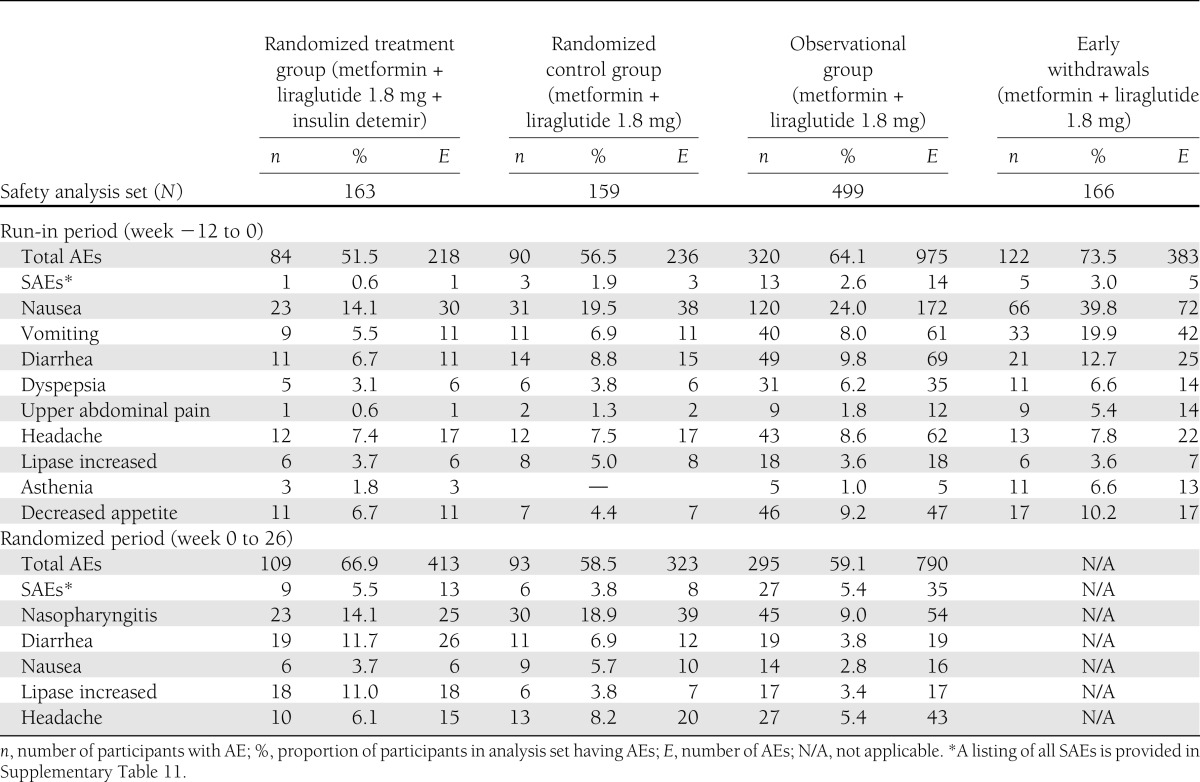
Nausea was the most frequently reported run-in AE (Table 2) but incidences fell to <7% in all groups after 3 weeks (Supplementary Table 3 and Supplementary Fig. 1). One case of acute pancreatitis was reported. In another participant, with elevated calcitonin level (23.5 ng/L) before liraglutide administration, a subsequent thyroidectomy revealed an incidental thyroid neoplasm (1-mm papillary microcarcinoma) (Supplementary Table 4).
Table 2.
AEs during run-in and randomized period with an incidence ≥5% by system organ class and preferred term and summary of SAEs
Adding insulin detemir to metformin and liraglutide in participants requiring additional glycemic control: the 26-week, randomized period
Participants not reaching glycemic target had a 7.6% mean A1C at completion of the run-in period. They were then randomized to add insulin detemir to metformin + liraglutide (n = 162) or to continue unchanged metformin + liraglutide (randomized control subjects, n = 161). The groups’ characteristics were similar at run-in (Table 1).
Addition of insulin detemir further reduced A1C compared with continued metformin + liraglutide (−0.51% [n = 162] vs. +0.02% [n = 157], respectively; estimated treatment difference [ETD] −0.52 [95% CI −0.68 to −0.36]; P < 0.0001), resulting in A1C values of 7.1 and 7.5%, respectively, at week 26 (Fig. 2A and Supplementary Tables 5 and 6), and meeting the trial’s primary end point. Accordingly, mean FPG decreased more in the detemir (−2.1 mmol/L) than control group (−0.4 mmol/L; ETD −1.7 [−2.2 to −1.3]; P < 0.0001) (Fig. 2C). After a mean 3.5-kg weight loss during run-in, both groups experienced further modest weight reduction over the next 26 weeks: −0.16 kg with detemir and −0.95 kg without (ETD 0.79 [0.08–1.49]; P = 0.03) (Fig. 2B).
Compared with the control group, more than twice as many in the insulin detemir group achieved <7% A1C (17 vs. 43%, respectively; P < 0.0001), and three times as many achieved ≤6.5% (6 vs. 18%, respectively; P = 0.0016; logistic regression estimates) (Supplementary Fig. 2). Similarly, the proportion reaching the composite end point (<7% A1C with no weight gain and no hypoglycemia) after 26 weeks was significantly greater in the insulin detemir (21%) than the control group (9%; P = 0.0016; logistic regression estimates) (Supplementary Fig. 2). Excluding one outlier in the randomized control group with 25 minor hypoglycemic episodes, minor hypoglycemia rates were 0.286 and 0.029 events per participant-year for insulin detemir and control groups, respectively (P = 0.004) (Supplementary Table 2). No major hypoglycemic events occurred during the randomized period.
Self-measured plasma glucose levels decreased in both groups, with significantly greater reductions in postprandial values in the insulin detemir versus control group (Fig. 2D). ETDs ranged from −0.60 mmol/L (95% CI −1.12 to −0.08; P = 0.02) to −1.12 mmol/L (95% CI −1.72 to −0.51; P = 0.0003). Mean prescribed insulin detemir doses increased from 10 units/day to 39.5 units/day (0.41 units/kg), whereas mean self-measured FPG decreased from 7.9 to 6.2 mmol/L (Fig. 2E). Prescribed insulin detemir doses were consistent with algorithm recommendations, indicating good overall adherence to the titration algorithm.
There were no significant differences between groups in changes from randomization for serum lipids, except for free fatty acids (insulin detemir group, −0.11 mmol/L; control group, −0.03 mmol/L; ETD −0.08 [95% CI −0.13 to −0.03]; P = 0.002) (for more information regarding changes in lipid levels, see Supplementary Table 7).
A total of 67% (109 of 163) and 59% (93 of 159) of participants had one or more AEs, and 5.5% (9 participants; 13 events) and 3.8% (6 participants; 8 events) had serious AEs (SAEs), in the insulin detemir and control groups, respectively (Table 2). No pattern or clustering of SAEs was observed, with most being considered unlikely to be related to treatment (Supplementary Table 8). Although more AEs of increased lipase were reported with insulin detemir, a minor increase in the median serum lipase levels (below the upper limit of normal) was observed across all groups, without apparent treatment differences (Supplementary Table 9). One case of chronic pancreatitis occurred (control group) (Supplementary Table 4).
Efficacy and safety over 38 weeks: the observational group
The 498 participants achieving the <7% target with metformin + liraglutide at the end of run-in were followed for another 26 weeks. This group experienced a mean 1.3% A1C reduction by the end of run-in, and 1.1% overall from start of run-in to week 26 (from 7.7% at run-in start to 6.6% at study end). The group also experienced overall reductions from start of run-in to week 26 in FPG of 2.1 mmol/L (from 9.2 mmol/L at run-in start to 7.2 mmol/L) and weight of 4.8 kg (from 99.0 kg at run-in start to 94.6 kg) (Fig. 2), and improved seven-point blood glucose profiles (completers) (Fig. 2D).
AEs for the 38-week period are summarized in Supplementary Table 10. In the observational group, 81% (402 of 499) of participants had AEs and 7.8% (39 of 499) experienced 49 SAEs, with 45 considered unlikely to be related to study drug and without obvious pattern (Supplementary Table 8). No major hypoglycemic episodes occurred, whereas 9.0% (45 of 499) of participants experienced minor hypoglycemia (0.211 events per participant-year). See Supplementary Table 9 for additional safety assessments.
Systolic blood pressure and heart rate: run-in to 26 weeks
At 26 weeks, systolic blood pressure was reduced from run-in start by 3.13 mmHg in the control group, 1.65 mmHg in the insulin detemir group, and 3.33 mmHg in the observational group (Supplementary Table 11). Heart rate was increased from run-in start to 26 weeks in all treatment groups: by 3.62 beats per minute (bpm) in the control group, 3.87 bpm in the insulin detemir group, and 3.64 bpm in the observational group (Supplementary Table 12).
CONCLUSIONS
This large, prospective clinical study provides support for a new sequential treatment paradigm in type 2 diabetes, that is, adding a GLP-1RA (liraglutide) to metformin followed (if necessary) by a basal insulin (insulin detemir) to achieve and maintain glycemic targets. With 12 weeks of liraglutide + metformin treatment, 61% of run-in completers reached an A1C <7%. For the 39% of completers not initially achieving target, adding insulin detemir provided clinically relevant further reductions of mean A1C from 7.6 to 7.1%. A total of 43% of insulin detemir recipients then achieved the <7% A1C target, compared with 17% continuing treatment with metformin + liraglutide. In the observational group, all of whom achieved A1C <7% after the 12-week run-in, 74% remained at target after a further 26 weeks’ treatment. Translated into clinical practice, and extrapolating from all patients who completed run-in, sequential intensification enabled approximately three-quarters of patients to achieve an A1C <7%. Importantly, overall, the hypoglycemia risk was very low, and insulin detemir recipients maintained the significant weight reductions achieved during liraglutide run-in rather than gaining weight as typically occurs with insulin.
Traditional clinical practice initiates insulin when multiple oral therapies are no longer effective. However, after metformin, commonly used oral agents such as sulfonylureas and thiazolidinediones carry side effects, particularly hypoglycemia and/or weight gain; the exceptions are dipeptidyl peptidase-4 inhibitors and α-glucosidase inhibitors, which are weight-neutral but appear to show less glucose-lowering activity than other agents (1,11). Because GLP-1RAs provide greater glycemic efficacy and weight loss (6), they may be more beneficial than these oral agents after metformin, but less acceptable in some patients because of gastrointestinal side effects and the injection barrier. Insulin initiation also usually causes weight gain and an increased hypoglycemia risk (1,12). In this study, however, the insulin detemir group maintained the initial weight loss achieved by adding liraglutide to metformin. Subsequent intensification with insulin detemir allowed 43% of the group to reach the glycemic target. It is conceivable that more aggressive insulin titration, giving higher insulin doses, would allow even more patients to reach A1C target, but perhaps at the expense of more hypoglycemia. The incidence of confirmed minor hypoglycemia with insulin detemir in our study, 0.286 events per participant-year, was considerably lower than the 1.3–3.67 events per participant-year in previous trials with insulin detemir added to oral agents (13,14). Although withdrawing sulfonylureas (as in the run-in) is associated with decreased hypoglycemia risks (3.2 vs. 1.6 events per participant-year, despite an increased insulin requirement) (15), initiating liraglutide before detemir may further reduce the hypoglycemia risk, perhaps by modulating endogenous glucose-dependent insulin secretion and glucagon secretion as well as body weight, thereby lowering exogenous insulin requirements.
It remains unknown whether an association exists between incretin-based therapies and pancreatitis (16), but acute pancreatitis is known to be about three times more common in patients with type 2 diabetes than in the general population (17). In the present trial, two cases of pancreatitis were reported (one acute and one chronic) and neither case of pancreatitis was preceded by elevated lipase levels. Reports of pancreatitis were similarly uncommon in other liraglutide studies, although imbalances in pancreatitis are noted for liraglutide and other incretin-based therapies (16,18). An initial small increase in median serum lipase below the upper limit of normal was observed across groups in the current study, and substantial fluctuations occurred over time. Interpreting these changes is difficult in the absence of an active comparator or a placebo group (i.e., a group not receiving liraglutide). Elevated lipase (or amylase) levels by themselves are insufficient to make a diagnosis of pancreatitis without the presence of abdominal pain and ideally confirmation with imaging studies. Most commonly, no gastrointestinal symptoms were associated with fluctuations in lipase observed during this trial.
We found no other published, prospective, controlled trials studying insulin added to a GLP-1RA in type 2 diabetes. A few previous studies have investigated the alternative intensification sequence, adding a GLP-1RA (specifically, twice-daily exenatide) to insulin (19,20–22). In the only randomized, controlled trial to date, patients with more advanced disease at baseline (compared with the current study) already receiving 0.5 units/kg of insulin glargine were randomized to either add-on exenatide or continuation of basal insulin only (19). The insulin doses were optimized in both groups by further rigorous titration postrandomization. In this setting, add-on exenatide with insulin optimization was associated with a 1.7% reduction in A1C levels (from a baseline of 8.3–6.7% vs. 1.0% A1C reduction with basal insulin optimization only), modest weight loss, and no significant increase in risk of minor hypoglycemia. In other nonrandomized studies, A1C reductions ranged from 0.0 to 0.9% (20–22). In the current study, however, adding a GLP-1RA before basal insulin was also effective, offering significantly improved glycemic control, substantial weight loss, and a very low hypoglycemia rate. This intensification sequence may therefore be preferable provided that potential gastrointestinal AEs can be accepted initially. It may also be preferable to initiate with a longer-acting once-daily GLP-1RA, such as liraglutide, rather than a twice-daily shorter-acting GLP-1RA to facilitate better compliance.
We acknowledge that our trial has some limitations. More participants may have reached glycemic targets with a lower FPG target for insulin titration (23). As some participants randomized to continued liraglutide + metformin subsequently reached target without dose adjustments, it is also conceivable that fewer patients would have needed insulin with a longer run-in period. Two factors may have influenced the outcome of the run-in period. First, the study used the higher (1.8 mg) of the two liraglutide doses. The higher dose may have favored efficacy outcomes and weight loss but adversely affected tolerability (11,24). Potentially, fewer patients may have withdrawn early due to gastrointestinal AEs if they had been permitted to return to the 1.2-mg dose or if upward titration was conducted more slowly. Second, one-third of participants were taking a sulfonylurea before the study; this was discontinued at the start of run-in and may have negatively influenced improvements in glycemic control but favored weight loss. However, neither factor should have affected the 26-week randomized-group comparisons. Moreover, discontinuation of sulfonylureas increases the trial’s translational validity because many physicians add an injectable therapy only after failure of more than one oral agent, and then tend to withdraw sulfonylureas. We recognize that the randomized control group did not have a masked placebo or active comparator; this was mainly due to logistic limitations with insulin comparators. However, we also chose not to include an active comparator to retain the focus on the efficacy and safety of adding a basal insulin to a GLP-1RA in combination with metformin. It is possible that some participants were disappointed with their treatment allocation and withdrew, an effect that might not have occurred with an active comparator. It would be informative, however, if future trials adhered to the comparative effectiveness concept (25). Future studies might also be of a longer duration and consider the overall costs of diabetes care (particularly the balance between the higher prices of new medications versus any potential for reduced costs associated with diabetes complications and less self-monitoring of blood glucose). A 26-week extension to the current study has been undertaken, primarily to assess longer-term treatment safety.
In conclusion, in type 2 diabetes with inadequate glycemic control on metformin ± sulfonylurea, the addition of liraglutide was effective and safe, enabling the majority of participants to reach A1C levels <7%, with sustained weight loss and low hypoglycemia risk. For those not reaching glycemic targets, intensification with insulin detemir provided clinically relevant additional glycemic control, sustaining previous weight loss and the very low hypoglycemia risk.
Acknowledgments
This study was funded by Novo Nordisk. J.H.D. is an advisory board member for Eli Lilly & Co.; has received grant support from and has attended speakers’ bureau for Medtronic; is an advisory board member and has attended speakers’ bureau for Novo Nordisk; has received grant support from Novartis; has attended speakers’ bureau for Roche; and is an advisory board member, has attended speakers’ bureau, and has received grant support from sanofi-aventis. S.C.B. has received lecture fees and research, educational, and clinical grants from Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, sanofi-aventis, Schering-Plough, Servier, and Takeda. H.W.R. is a consultant for Abbott Laboratories, GlaxoSmithKline, MannKind Corporation, and Takeda Pharmaceuticals America; has attended speakers’ bureau for Amylin Pharmaceuticals, Bristol-Myers Squibb, and Eli Lilly & Co.; is a consultant for and has attended speakers’ bureau for AstraZeneca and Merck Sharp & Dohme; is a consultant for and has received grant support from Biodel, Merck, and TolerX; has received grant support from MacroGenics; and is a consultant for, has received grant support from, and has attended speakers’ bureau for Novo Nordisk and sanofi-aventis. J.S. has attended speakers’ bureau for AstraZeneca, Bayer, Berlin Chemie, Bristol-Myers Squibb, Eli Lilly & Co., GlaxoSmithKline, LifeScan, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, sanofi-aventis, and Takeda. D.D. is a consultant for and has received grant support from Amylin; is a consultant for Ethicon, Merck Sharp & Dohme, Novo Nordisk, and Takeda; and has received grant support from Johnson & Johnson, MannKind Corporation, and sanofi-aventis. A.B.T. and M.Z. are full-time employees of Novo Nordisk A/S. J.R. is an advisory board member and consulant for and has received grant support from Amylin, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Merck Sharp & Dohme, Eli Lilly & Co., GlaxoSmithKline, Johnson & Johnson, Novartis, Novo Nordisk, Pfizer, sanofi-aventis, Roche, Intarcia, and Takeda and has received grant support from AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
The funder participated in trial design and collection, review, and analysis of data. Liraglutide and insulin detemir are proprietary compounds manufactured and marketed by Novo Nordisk A/S.
J.H.D., S.C.B., H.W.R., J.S., D.D., and J.R. planned the trial, determined the trial design, and analyzed and interpreted data. A.B.T. implemented the study, oversaw the conduct of the trial, and cooperated with the medical writers and the trial statistician regarding statistical analysis, data management, and writing the study report. M.Z. planned the trial and determined the trial design, implemented the study, and analyzed and interpreted data. All authors participated in drafting the manuscript and all have approved the final version. J.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011, and in poster form at the Diabetes UK Annual Professional Conference, London, U.K., 30 March–1 April 2011.
The authors gratefully acknowledge the contribution of the investigators and their staff and of the participants of this trial. The authors thank Dr. Tulay T. Cushman, Dr. Jen Faleska, Dr. John Smith, and Dr. Helle Frobøse Sørensen for medical writing support; Rie Elvang Søndergaard for statistical support; and Mia Skettrup Fitsios for statistical programming support in the preparation of this manuscript (all from Novo Nordisk). The authors acknowledge Watermeadow Medical (Witney, U.K.), supported by Novo Nordisk, for providing writing and editing support.
Footnotes
Clinical trial reg. no. NCT00856986, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1928/-/DC1.
*A complete list of the members of the Liraglutide-Detemir Study Group can be found in Supplementary Table 13.
References
- 1.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Frid A, Hermansen K, et al. LEAD-2 Study Group Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell-Jones D, Vaag A, Schmitz O, et al. ; Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomized controlled trial. Diabetologia 2009;52:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 8.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 9.International Conference on Harmonisation. ICH Harmonised Tripartite Guideline. Good Clinical Practice [Internet], 1996. Available from http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf Accessed 9 August 2011
- 10.World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects [Internet], 2008. Available from http://www.wma.net/en/30publications/10policies/b3/index.html Accessed 9 August 2011
- 11.Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456 [DOI] [PubMed] [Google Scholar]
- 12.Holman RR, Thorne KI, Farmer AJ, et al. 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 13.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 14.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006;28:1569–1581 [DOI] [PubMed] [Google Scholar]
- 15.Swinnen SG, Dain MP, Mauricio D, DeVries JH, Hoekstra JB, Holleman F. Continuation versus discontinuation of insulin secretagogues when initiating insulin in type 2 diabetes. Diabetes Obes Metab 2010;12:923–925 [DOI] [PubMed] [Google Scholar]
- 16.Raschi E, Piccinni C, Poluzzi E, Marchesini G, De Ponti F. The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol. 19 October 2011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009;32:834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010;362:774–777 [DOI] [PubMed] [Google Scholar]
- 19.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 20.Samarasinghe YP, Shlomowitz A, Munro N, Feher MD. The short term effects of exenatide therapy in insulin treated patients with type 2 diabetes mellitus–a new option to achieve weight loss (Abstract). Diabetologia 2008;51(Suppl. 1):S353 [Google Scholar]
- 21.Sheffield CA, Kane MP, Busch RS, Bakst G, Abelseth JM, Hamilton RA. Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract 2008;14:285–292 [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P, Dandona P. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract 2007;13:444–450 [DOI] [PubMed] [Google Scholar]
- 23.Blonde L, Merilainen M, Karwe V, Raskin P, TITRATE Study Group Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab 2009;11:623–631 [DOI] [PubMed] [Google Scholar]
- 24.Zinman B, Gerich J, Buse JB, et al. LEAD-4 Study Investigators Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan DM. Time for clinically relevant comparative effectiveness studies in type 2 diabetes. Ann Intern Med 2011;154:131–132 [DOI] [PubMed] [Google Scholar]