Abstract
Chronic kidney disease remains as one of the major complications for individuals with diabetes and contributes to considerable morbidity. Individuals subjected to dialysis therapy, half of whom are diabetic, experience a mortality of ∼20% per year. Understanding factors related to mortality remains a priority. Outside of dialysis units, A1C is unquestioned as the “gold standard” for glycemic control. In the recent past, however, there is evidence in large cohorts of diabetic dialysis patients that A1C at both the higher and lower levels was associated with mortality. Given the unique conditions associated with the metabolic dysregulation in dialysis patients, there is a critical need to identify accurate assays to monitor glycemic control to relate to cardiovascular endpoints. In this two-part point-counterpoint narrative, Drs. Freedman and Kalantar-Zadeh take opposing views on the utility of A1C in relation to cardiovascular disease and survival and as to consideration of use of other short-term markers in glycemia. In the narrative preceeding this counterpoint, Dr. Freedman suggests that glycated albumin may be the preferred glycemic marker in dialysis subjects. In the counterpoint narrative below, Dr. Kalantar-Zadeh defends the use of A1C as the unquestioned gold standard for glycemic management in dialysis subjects.
—William T. Cefalu, MD Editor in Chief, Diabetes Care
Uremia may confound the association of A1C with time-averaged glucose concentration and the ability of A1C to predict clinical outcomes. These nondifferential alterations do not discredit A1C as a reliable long-term marker of glycemic control in dialysis patients as long as appropriate adjustments to interpret A1C values are made. Recent data from large cohorts of diabetic dialysis patients suggest a rather robust association between A1C and glucose concentration (r = 0.51–0.56) and U- or J-shaped A1C-mortality association with a shift to the right. Both very low (<6%) and high (>8%) A1C levels appear incrementally associated with higher all-cause and cardiovascular mortality in peritoneal and hemodialysis patients. These bimodal death risks are robust, especially when longitudinal A1C values are examined. Given the availability of inexpensive and routinely measured A1C assays in virtually all dialysis clinics and given inconsistent data to prove superiority of other glycemic markers such as glycated albumin, there is currently no compelling reason to abandon A1C testing. This article reviews the utility of A1C in diabetic dialysis patients and supports the notion that as long as dialysis patient–specific A1C outcome associations are taken into consideration, A1C meets the clinical criteria of an ideal test for long-term glycemic control in these patients.
DIABETIC DIALYSIS PATIENTS
Type 2 diabetes is the leading cause of chronic kidney disease across the globe (1,2). In the U.S., the cost of diabetic nephropathy is estimated to be over $15 billion annually (2). Despite higher prevalence of comorbid complications in diabetic versus nondiabetic dialysis patients, it is still debatable whether medical management of type 2 diabetes at this advanced disease stage has a significant bearing on outcomes (3). More confusing is the observation that in at least one-third of diabetic dialysis patients recurrent hypoglycemic episodes prompt cessation of antihyperglycemic therapy despite years to decades of needed insulin administration prior to the end-stage kidney disease, a phenomenon also referred to as “burnt-out diabetes” (4,5). Notwithstanding the challenges of the confounding role of nephropathy in the natural history of diabetes, dialysis patient mortality has remained at ∼20% per year, a death rate that is embarrassingly higher than many fatal cancers (2). Given that the consistency of clinical trials targeting such conventional cardiovascular risk factors as serum cholesterol level has minimal, if any, effect on improving survival in diabetic dialysis patients (6), better glycemic control using traditional and/or novel glycemic metrics is of high clinical priority. Whereas A1C remains the most used glycemic marker in dialysis patients, some studies have examined the use of other markers including glycated albumin (7,8) and fructoseamine (9,10) in these patients.
A1C IN DIABETIC DIALYSIS PATIENTS
Over 3 decades of A1C monitoring of diabetic patients by targeting A1C <7% or lower has made this metric the unquestionable foundation of clinical diabetology. However, recent data indicate that even in nonuremic patients A1C exhibits a J-shaped association with outcomes (11). This finding, probably due to the deleterious impact of hypoglycemia, has refuted the original “the lower the better” principle of glycemic control. Some data suggest that A1C has significant limitations and may not be a reliable glycemic metric in several hematological and endocrine disorders, in uremia, and when certain medications are used such as erythropoietin (12). It has even been suggested that A1C has no place in certain populations, including dialysis patients (13,14), and such calls have also served to justify the use of alternative measures of glycemia including fructosamine and glycated albumin (8). The continued discussion about the reliability of A1C in dialysis populations has generated much confusion among both physicians and patients. These suggestions, however, are often based on the flawed assumption that dialysis patients should follow the same A1C target ranges as in nonuremic people. That many studies have not found A1C <7% to be associated with the best dialysis patient outcome does not discredit A1C as a useful glycemic metric.
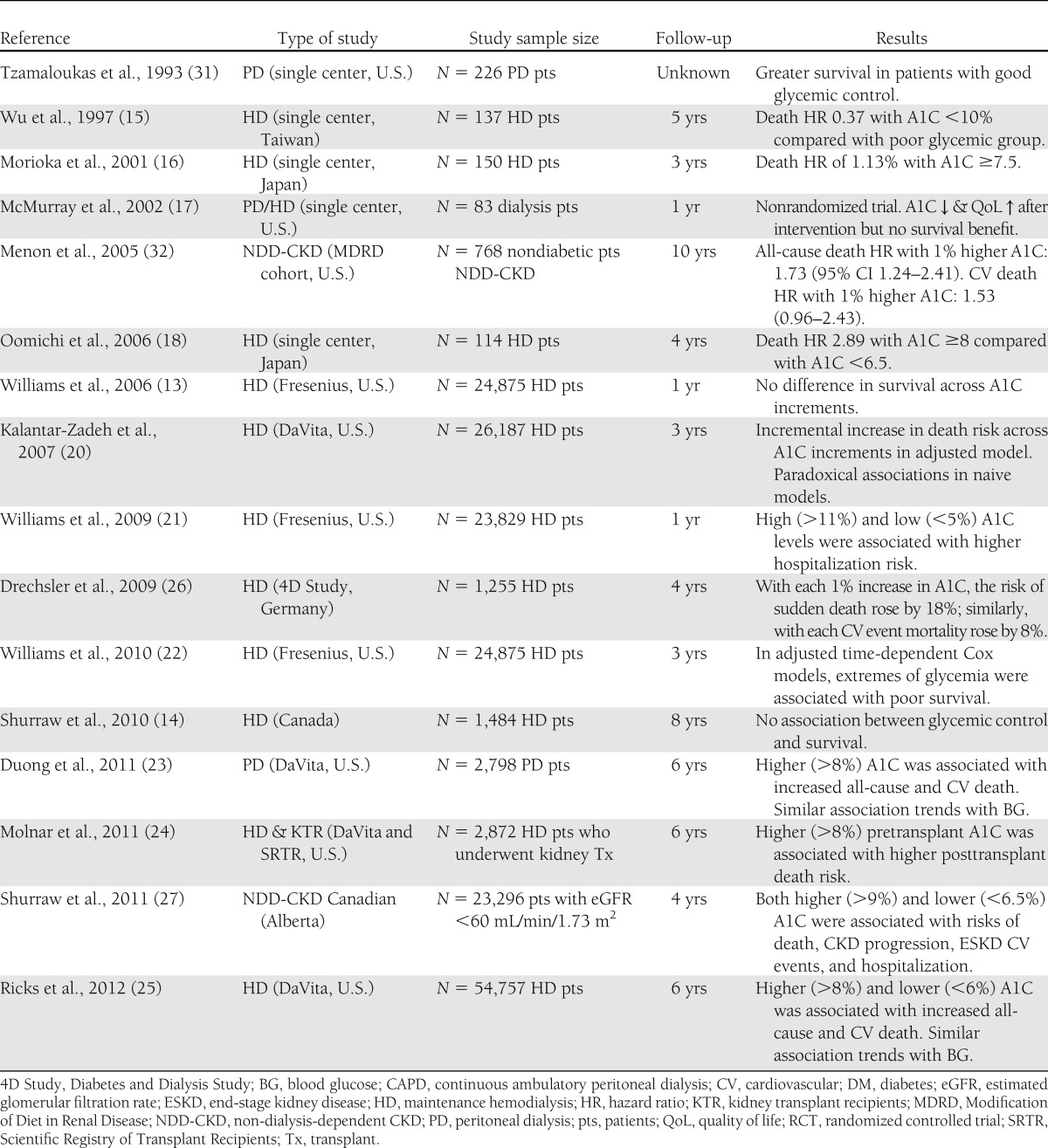
As shown in Table 1, most, but not all, studies support A1C utility for the management of diabetic dialysis patients. From the early 1990s to mid-2000s, several small studies (with <150 subjects) found consistent associations between higher A1C and poorer clinical outcomes in predialysis and dialysis patients with diabetic nephropathy (13,15–18). The first large and nationally representative cohort study of A1C-death association in dialysis patients was published in 2006 by Williams et al. (13) who reported practically no association between one-time measured A1C and survival at 12 months in 24,875 diabetic dialysis patients from the largest dialysis organization in the U.S., i.e., Fresenius Medical Care. The lack of a survival association or trend in this study was likely due to the short-term follow-up and other methodological limitations such as non-time-dependent survival models and lack of stratified analyses to detect interactions (1); however, this study led to some confusion about the role of glycemic control in diabetic dialysis patient care and questioned the validity of A1C as a reliable marker to this end (19). It was then even suggested that the guidelines pertaining to glycemic controls for individuals without advanced nephropathy may not apply to the dialysis population (13,19).
Table 1.
Overview of studies examining the utility of A1C in predicting survival in diabetic CKD patients
In 2007, another large and nationally representative cohort study from the second largest U.S. dialysis organization (DaVita) in 23,618 hemodialysis patients who were followed for up to 3 years (2001–2004) (20) showed that in unadjusted survival models lower A1C values appeared paradoxically associated with higher mortality rates; however, after adjusting for potential confounders, A1C >6% was incrementally and linearly associated with increased death risks over 3 years. In 2008, a second cohort study with 23,829 diabetic dialysis patients from Fresenius found that extremely high and low A1C (>11% and <5%) was associated with higher hospitalization risk (21). The third Fresenius dialysis cohort study was published in 2010 (22) to supplement the authors’ prior analyses (which had found no correlation between A1C and mortality at 1 year [13]) by extending the follow-up period to 3 years. In these 24,875 diabetic hemodialysis patients, adjusted time-dependent Cox models indicated that extremes of glycemia were indeed associated with inferior survival (22).
Very recently, however, three additional studies have emerged from DaVita national dialysis cohorts (23–25) indicating that both high and low A1C levels in dialysis patients are associated with poor outcomes. Duong et al. (23) examined mortality predictability of A1C and random serum glucose in a 6-year (2001–2007) cohort of 2,798 diabetic peritoneal dialysis patients with repeated A1C measures. Random serum glucose levels correlated with A1C (r = 0.51). Adjusted all-cause death hazard ratio (HR) for time-varying A1C at 1% increments between <7% and ≥10% exhibited an incremental and linear A1C-death association. Time-averaged blood glucose showed a similar death pattern, corroborating the close correlation between A1C and glycemic exposure (23). In a novel effort to examine the association of pretransplant glycemic control during the dialysis treatment era with posttransplant outcomes in kidney transplant recipients, Molnar et al. (24) recently reported similar pattern of outcome predictability for A1C. Finally, Ricks et al. (25) examined mortality predictability of A1C and random serum glucose over 6 years in 54,757 diabetic hemodialysis patients and found a robust correlation between random serum glucose and A1C (r = 0.56) and a U-shaped mortality association including incrementally higher death risks with A1C >8% and <6%. The identical death predication for time-averaged random glucose confirmed the reliability of A1C as a good marker of glycemic exposure (25). Furthermore, a post hoc analysis of the 1,255 diabetic hemodialysis patients from a large clinical trials in Germany also showed a graded increase in sudden cardiac death with higher A1C levels (26). A U- or J-shaped A1C-death association was recently reported in a large Canadian cohort of 23,296 non-dialysis-dependent diabetic patients with eGFR <60.0 mL/min/1.73 m2 (27); in that A1C levels <6.5% and >9% were associated with poor outcomes (27). It is important to note that in the study by Williams et al. (13), the only large study unable to demonstrate an association between A1C and mortality, only a single baseline measurement of A1C was used for analysis.
IS GLYCATED ALBUMIN A BETTER GLYCEMIC MARKER?
Recent data by Freedman and colleagues (7,8) suggest that glycated albumin may be superior to A1C for monitoring glycemic control in diabetic dialysis patients because glycated albumin is a measure of shorter-term glycemic control (2 to 3 weeks) than A1C (1 to 2 months). Freedman et al. (8) reported that each 5% increase in quarterly measured glycated albumin was associated with 14% higher risk for all-cause mortality in 444 prevalent diabetic dialysis patients during 2.3 years, whereas there was no association between A1C or blood glucose and death risk in this cohort. The reported lack of glucose-death association in this study, however, cast doubt on the validity of the glycated albumin as a true glycemic marker and may indicate that the positive finding was essentially a reflection of the inherent mortality predictability of serum albumin in any dialysis patient population (28). It is also argued that the ratio of A1C to glycated albumin is 30–40% higher in dialysis patients than in nonuremic patients (7) making the point that A1C underestimates the degree of hyperglycemia. However, albumin homeostasis is often abnormal in dialysis patients, many of whom have hypoalbuminemia (28); hence, potential inaccuracies with glycated albumin are probably even worse than the known limitations of A1C and may be a potential explanation for the higher A1C:glycated albumin ratio in dialysis patients (29). Among the potential reasons for the discrepancy between the study by Freedman et al. (8) and the positive A1C studies in Table 1 is the fact that the risk with glycemic control is cumulative and best captured in a time-dependent or time-averaged analysis; the greater the number of measurements of the risk factor of interest, the better captured is the cumulative risk (29). In the study by Freedman et al. (8), the median number of A1C measurements was less than glycated albumin (3 vs. 8), and the number of events was rather small, limiting the statistical power. Adequate monitoring of glycemic control would require a monthly measurement of glycated albumin compared with a quarterly measurement of A1C, which would add to the overall expense (29). Given the limitations of the existing data, it seems premature to abandon A1C in favor of glycated albumin.
RECOMMENDATIONS AND CONCLUSIONS
Given the preponderance of supportive data reviewed above and summarized in Table 1, A1C remains the ideal glycemic metric and outcome predictor in diabetic dialysis patients as long as the target range is carefully tailored for this unique patient population. We suggest considering the following points in the utility and interpretation of A1C:
Both high (>8% or more unequivocally >9%) and very low (<6%) A1C levels appear associated with poor outcomes in diabetic dialysis patients. Hence, the conventional A1C target ranges recommended for the nonuremic diabetic population (e.g., <7%) should not be extrapolated to dialysis patients.
The A1C-death association appears more robust in patients with higher hemoglobin levels (>10 g/dL) and better nutritional status (albumin >3.8 g/dL). In more severely anemic patients or those with protein-energy wasting, low A1C may be a surrogate of poor nutritional status and, hence, a mortality predictor. Indeed, a provocative cohort study in chronic heart failure patients found a paradoxically inverse association between higher A1C and greater survival (30). Given the interaction of nutrition, inflammation, and anemia with indices of glycemic control, an unusually low A1C (<6%) may warrant additional work-up rather than being considered as a “favorable” range.
A1C monitoring in dialysis patients should be based on repeated measures and examining the moving averages and trends rather than a single measurement. The DaVita cohort studies are consistent in showing a more robust, linear, and incremental outcome predictability of time-averaged A1C (23–25). Indeed, even in the Fresenius cohort studies, which initially showed no A1C-death association over a short follow-up period with a single A1C measurement (13), a statistically significant mortality trend with high A1C values was observed when time-varying models using multiple A1C values were used (22).
Given the well-studied and well-established associations of A1C and clinical outcomes including all-cause and cardiovascular mortality, hospitalization, and risk of nephropathy progression in the majority of studies (Table 1), dismissing the inexpensive and conveniently available A1C in favor of such other glycemic metrics as glycated albumin is immature at this time. Comparative outcome studies between A1C and these glycemic markers in diabetic dialysis patients are needed before they can be recommended for clinical use.
Acknowledgments
The work is supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants K24-DK091419, R0-DK078106, and R21-DK077341 and by a philanthropic grant from Mr. Harold Simmons.
References
- 1.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis 2008;52:766–777 [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Kalantar-Zadeh K. Enter the dragon: a Chinese epidemic of chronic kidney disease? Lancet 2012;379:783–785 [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl 2003;87:S24–S31 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr 2009;19:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial 2010;23:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wanner C, Krane V, März W, et al. ; German Diabetes and Dialysis Study Investigators Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238–248 [DOI] [PubMed] [Google Scholar]
- 7.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 2008;73:1062–1068 [DOI] [PubMed] [Google Scholar]
- 8.Freedman BI, Andries L, Shihabi ZK, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol 2011;6:1635–1643 [DOI] [PubMed] [Google Scholar]
- 9.Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int Suppl 2010;117:S41–S45 [DOI] [PubMed] [Google Scholar]
- 10.Lamb E, Venton TR, Cattell WR, Dawnay A. Serum glycated albumin and fructosamine in renal dialysis patients. Nephron 1993;64:82–88 [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinow KB, Hirsch IB. Reexamining metrics for glucose control. JAMA 2011;305:1132–1133 [DOI] [PubMed] [Google Scholar]
- 13.Williams ME, Lacson E, Jr, Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: Characteristics, glycemic control, and survival. Kidney Int 2006;70:1503–1509 [DOI] [PubMed] [Google Scholar]
- 14.Shurraw S, Majumdar SR, Thadhani R, Wiebe N, Tonelli M; Alberta Kidney Disease Network Glycemic control and the risk of death in 1,484 patients receiving maintenance hemodialysis. Am J Kidney Dis 2010;55:875–884 [DOI] [PubMed] [Google Scholar]
- 15.Wu MS, Yu CC, Yang CW, et al. Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant 1997;12:2105–2110 [DOI] [PubMed] [Google Scholar]
- 16.Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care 2001;24:909–913 [DOI] [PubMed] [Google Scholar]
- 17.McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002;40:566–575 [DOI] [PubMed] [Google Scholar]
- 18.Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care 2006;29:1496–1500 [DOI] [PubMed] [Google Scholar]
- 19.Feldt-Rasmussen B. Is there a need to optimize glycemic control in hemodialyzed diabetic patients? Kidney Int 2006;70:1392–1394 [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007;30:1049–1055 [DOI] [PubMed] [Google Scholar]
- 21.Williams ME, Lacson E, Jr, Teng M, Hakim RM, Lazarus JM. Extremes of glycemic control (HbA1C) increase hospitalization risk in diabetic hemodialysis patients in the USA. Am J Nephrol 2009;29:54–61 [DOI] [PubMed] [Google Scholar]
- 22.Williams ME, Lacson E, Jr, Wang W, Lazarus JM, Hakim R. Glycemic control and extended hemodialysis survival in patients with diabetes mellitus: comparative results of traditional and time-dependent Cox model analyses. Clin J Am Soc Nephrol 2010;5:1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duong U, Mehrotra R, Molnar MZ, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 2011;6:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molnar MZ, Huang E, Hoshino J, et al. Association of pretransplant glycemic control with posttransplant outcomes in diabetic kidney transplant recipients. Diabetes Care 2011;34:2536–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricks J, Molnar MZ, Kovesdy CP, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes 2012;61:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drechsler C, Krane V, Ritz E, März W, Wanner C. Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation 2009;120:2421–2428 [DOI] [PubMed] [Google Scholar]
- 27.Shurraw S, Hemmelgarn B, Lin M, et al. ; Alberta Kidney Disease Network Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med 2011;171:1920–1927 [DOI] [PubMed] [Google Scholar]
- 28.Mehrotra R, Duong U, Jiwakanon S, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis 2011;58:418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clin J Am Soc Nephrol 2011;6:1520–1522 [DOI] [PubMed] [Google Scholar]
- 30.Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1C levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J 2006;151:91. [DOI] [PubMed] [Google Scholar]
- 31.Tzamaloukas AH, Murata GH, Zager PG, Eisenberg B, Avasthi PS. The relationship between glycemic control and morbidity and mortality for diabetics on dialysis. ASAIO J 1993;39:880–885 [PubMed] [Google Scholar]
- 32.Menon V, Greene T, Pereira AA, et al. Glycosylated hemoglobin and mortality in patients with nondiabetic chronic kidney disease. J Am Soc Nephrol 2005;16:3411–3417 [DOI] [PubMed] [Google Scholar]