Abstract
OBJECTIVE
Although hyperinsulinemia, a surrogate of insulin resistance, may play a role in the pathogenesis of hypertension (HTN), the longitudinal association between fasting insulin level and HTN development is still controversial. We examined the relation between fasting insulin and incidence of HTN in a large prospective cohort.
RESEARCH DESIGN AND METHODS
A prospective cohort of 3,413 Americans, aged 18–30 years, without HTN in 1985 (baseline) were enrolled. Six follow-ups were conducted in 1987, 1990, 1992, 1995, 2000, and 2005. Fasting insulin and glucose levels were assessed by a radioimmunoassay and hexokinase method, respectively. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% CIs of incident HTN (defined as the initiation of antihypertensive medication, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg).
RESULTS
During the 20-year follow-up, 796 incident cases were identified. After adjustment for potential confounders, participants in the highest quartile of insulin levels had a significantly higher incidence of HTN (HR 1.85 [95% CI 1.42–2.40]; Ptrend < 0.001) compared with those in the lowest quartile. The positive association persisted in each sex/ethnicity/weight status subgroup. A similar dose-response relation was observed when insulin-to-glucose ratio or homeostatic model assessment of insulin resistance was used as exposure.
CONCLUSIONS
Fasting serum insulin levels or hyperinsulinemia in young adulthood was positively associated with incidence of HTN later in life for both men and women, African Americans and Caucasians, and those with normal weight and overweight. Our findings suggested that fasting insulin ascertainment may help clinicians identify those at high risk of HTN.
Hypertension (HTN), a leading cause of cardiovascular morbidity and mortality, has become an important public health burden worldwide (1). It has been well established that HTN tends to coexist with diabetes (2,3), either preceding or being the complication of diabetes. In addition, the risk factors for HTN and diabetes are prone to cluster together, and it has been hypothesized that hyperinsulinemia, a surrogate measure of insulin resistance, might provide the pathophysiological mechanism underlying these observations (4).
Some epidemiological studies, including both cross-sectional and longitudinal studies, have indicated that insulin levels are associated with blood pressure (BP) as well as incidence of HTN (5–7). However, inconsistent findings (8,9), especially in a specific sex or ethnic subgroup (10,11), made this topic a controversy. In addition, among the limited prospective studies on the association of insulin level with incidence of HTN (5,6,9,12–14), most have been conducted in only one sex or one ethnic group (9,12–14). Few studies have examined the association in both men and women, and African Americans (AAs) and Caucasians (5,6). Therefore, we prospectively examined fasting insulin level in relation to incidence of HTN in a large biracial cohort of American men and women over 20 years of follow-up using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study.
RESEARCH DESIGN AND METHODS
Study design
The CARDIA study is an ongoing, prospective, multicenter, observational study of the natural history of the development of cardiovascular disease risk from young adulthood to midlife. In 1985, 5,115 young adults between the ages of 18 and 30 years were randomly selected from four U.S. cities: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. The sampling scheme was designed to achieve a balance at each site by age (18–24 and 25–30 years), sex, ethnicity (AA and Caucasian), and education (high school degree or less and more than high school). To date, six follow-ups have been conducted at examination years 2, 5, 7, 10, 15, and 20. Follow-up rates averaged >90%, and ∼70% of the participants in the original cohort returned at year 20. More details of the study design and recruitment protocol have been previously reported (15). The institutional review committee approval and informed consent was obtained for each examination.
Among those who had serum insulin measured at baseline (n = 3791), we excluded participants who had not fasted for at least 8 h (n = 80) or were pregnant at any examination (n = 200) or were diagnosed as HTN at baseline (n = 98) in a sequential manner. After all these exclusions, 3,413 participants remained in the sample.
Ascertainment of insulin and glucose
Fasting blood samples were collected according to standardized CARDIA procedures and processed at central laboratories (15). Fasting serum insulin was measured originally by a nonspecific insulin assay at baseline and in later examinations by a new radioimmunoassay (Linco Research Inc., St. Charles, MO). To assure comparability of insulin across visits, sera stored from baseline was re-measured by the new assay 8 years later (16). The Pearson correlation of log insulin values for baseline by the original (17) and the new method (18) was 0.81. Fasting glucose was detected by hexokinase method on a Cobas Mira Plus chemistry analyzer at each examination (16). On the basis of re-assays of glucose in 2006 and 2007 in ∼200 samples per examination drawn at years 7, 10, 15, and 20, and of insulin in 100 samples stored since year 15, glucose and insulin were recalibrated to harmonize them with the previous measurements. Recalibrated glucose values were 6.98 + 0.94 × year 7 glucose concentration, 7.15 + 0.96 × year 10 glucose concentration, 6.99 + 1.01 × year 15 glucose concentration, and 4.06 + 0.97 × year 20 glucose concentration. Recalibrated insulin was −0.36 + 0.93 × year 20 insulin concentration (19). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as glucose (mmol/L) × insulin (mU/L)/22.5 (20).
Ascertainment of HTN
BP was measured at the first six examinations using the Hawksley random-zero sphygmomanometer (W.A. Baum Co., Copiague, NY) and at the seventh (examination year 20) using the OmROn HEM907XL by trained and certified technicians (21). Three BP measurements were taken from the right arm of each participant at 1-min intervals after a 5-min seated rest. Systolic BP (SBP) and diastolic BP (DBP) were recorded as phase I and phase V Korotkoff sounds through examination year 15. Based on a study of ∼900 participants, we estimated SBP (random zero) = 3.74 + 0.96 × observed OmROn SBP and estimated DBP = 1.30 + 0.97 × observed OmROn DBP at examination year 20 (22). The second and third of the measurements were averaged for all analyses.
HTN was defined as SBP ≥140 mmHg and/or DBP ≥90 mmHg and/or taking antihypertensive medication at each examination. HTN incidence was defined as the percentage of nonhypertensive participants at baseline who developed HTN at the 20-year follow-up.
Measurement of covariates
Demographic variables, including age, sex, ethnicity, and education level, were collected using a self-administered questionnaire and verified during clinic examinations. Education level was classified as <12, 12, 13–15, and ≥16 years. Anthropometric measures were collected with participants wearing light clothing and no shoes. Body weight was measured to nearest 0.5 lb using a calibrated scale. Height was measured to the nearest 0.5 cm with a vertical ruler. BMI was calculated as weight in kilograms divided by height in meters squared.
Smoking status was determined by an interviewer-administered questionnaire and participants were categorized into three groups: nonsmokers, former smokers, and current smokers. Alcohol consumption was measured by the question, “Do you drink any alcoholic beverages in the past year?” If the answer was yes, it was followed by three questions on how many servings of wine, beer, and liquor. Total ethanol consumption in milliliters per day was estimated from the answers about individual alcoholic beverages using the formula ([beer servings/week × 16.7] + [wine servings/week × 17.02] + [liquor servings/week × 19.09])/7, and was categorized into six groups: 0 (never drink), 0.1–4.9, 5.0–9.9, 10–14.9, 15.0–29.9, and ≥30 mL/day. Physical activity was assessed using the CARDIA Physical Activity History Questionnaire, an interviewer-administered self-report of frequency of participation in 13 categories of recreational sports, exercise, leisure, and occupational activities over the previous 12 months. Physical activity score was calculated in exercise units (EU) based on the frequency and duration of activity over the previous year. A score of 100 EU is roughly equivalent to engaging in vigorous activity 2–3 h/week for 6 months of the year.
Dietary intakes of sodium, potassium, and magnesium were assessed by the CARDIA Diet History Questionnaire, an interviewer-administrated quantitative food frequency questionnaire, which has been evaluated and discussed elsewhere (23). Diet assessment was conducted three times: at baseline and examination years 7 and 20.
Statistical analysis
Participants were divided into quartiles according to insulin levels (μU/mL). Baseline characteristics of participants were expressed as mean (SD), median (interquartile range), or proportion and were compared across quartiles by using ANOVA, Kruskal-Wallis test, or χ2 test as appropriate. We used Cox proportional hazards models to evaluate associations of serum insulin levels, insulin-to-glucose ratio (IGR), and HOMA-IR with incidence of HTN. Follow-up time was calculated as the difference between the baseline examination and the year in which HTN was first identified, year 20, or the year a participant was censored. We used nonparametric splines to examine whether there was a nonlinear relation of exposure of interest with incidence of HTN. We used generalized estimating equations (GEEs) with identity linkage under exchangeable correlation structure assumption to examine exposures of interest in relation to continuous SBP and DBP.
To reduce measurement error caused by within-person variation and to best represent long-term insulin level (IGR or HOMA-IR) prior to HTN, we used cumulative average values of the exposures of interest (24). For example, we related the insulin levels (IGR or HOMA-IR) measured at baseline to new HTN cases identified at examination years 2, 5, and 7, and the average of insulin levels (IGR or HOMA-IR) detected at baseline and years 7, 10, and 15 to new cases identified at year 20. To explore whether the duration of follow-up would affect the associations, we also used the baseline exposure model and the most recent exposure model in the sensitivity analyses (24).
We categorized exposures of interest into quartiles based on their distributions. The initial analysis (model 1) was adjusted for age, sex, ethnicity, and study center. In model 2, we further adjusted for BMI, education, smoking status, alcohol consumption, physical activity, baseline systolic BP, and family history of HTN, and in model 3, we additionally adjusted for dietary intakes of sodium, potassium, and magnesium. Glucose was also adjusted in the final model when insulin was used as exposure. Ordinal variables using the median value in each quartile were created for trend tests. As antihypertensive medication information was used to define incident cases of HTN, we did not include it in Cox models and only included it for GEE analysis.
In addition, we investigated whether sex, ethnicity, and overweight/obese status modified the relations between insulin level and incidence of HTN. These analyses were performed by creating interaction terms of exposure of interest with these potential modifiers. The P values for interaction were calculated from likelihood ratio test by comparing models with and without the interaction terms.
All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC). P ≤0.05 was considered statistically significant.
RESULTS
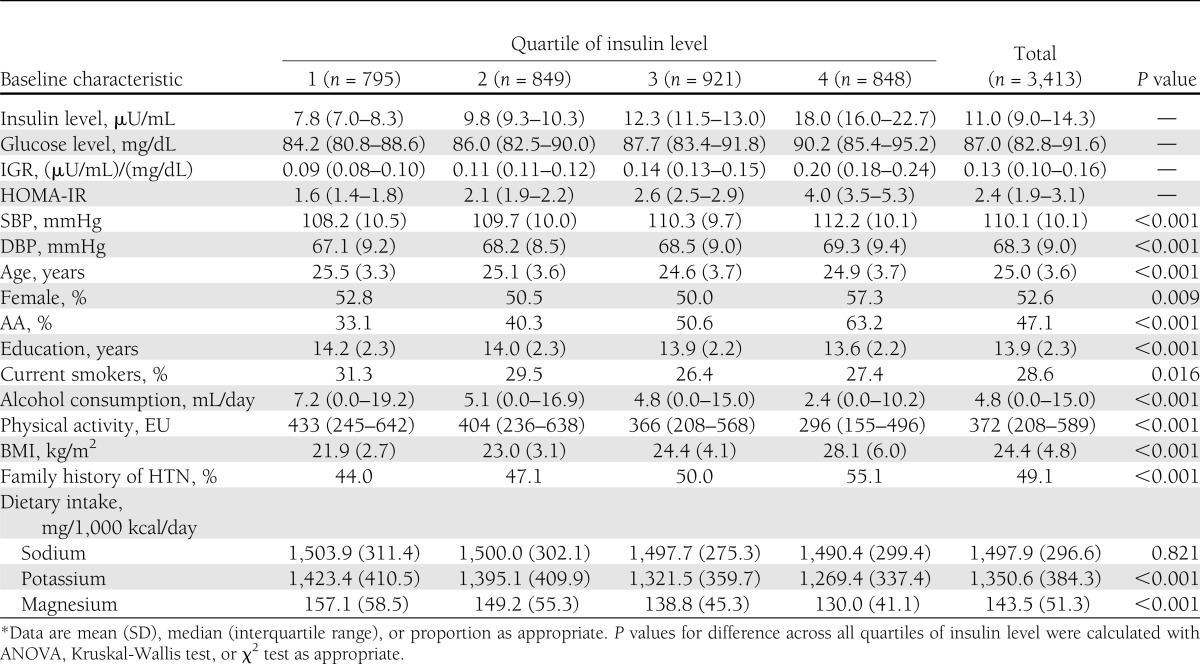
Table 1 shows the baseline characteristics of 3,413 participants according to quartiles of serum insulin level. The cumulative median levels of insulin were 7.8, 9.8, 12.3, and 18.0 μU/mL across quartiles. At baseline, the mean age of study population was 25.0 ± 3.6 years and the mean BMI was 24.4 ± 4.8 kg/m2. Compared with participants in the lowest quartile of insulin level, those in the highest quartile were slightly younger, more likely to be females, AAs, and noncurrent smokers, and had relatively lower education level and lower alcohol consumption. They were less likely to be active and lean.
Table 1.
Baseline characteristics by quartile of fasting insulin level (CARDIA study, 1985–2005*)
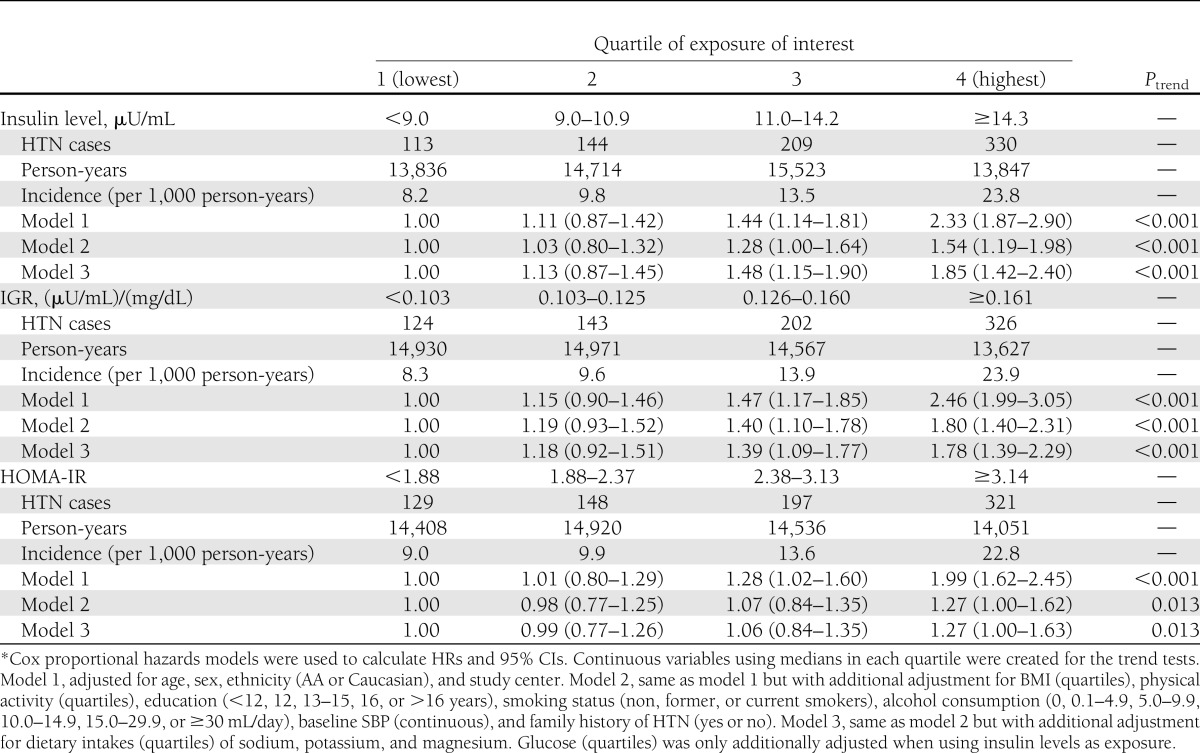
A total of 796 incident cases of HTN were identified (incident rate = 13.7/1,000 person-years) during 57,920 person-years of follow-up. As illustrated in Table 2, insulin levels were positively and significantly associated with incidence of HTN. Participants in the highest quartile of insulin level had a significantly higher incidence of HTN (hazard ratio [HR] 1.85 [95% CI 1.42–2.40]; Ptrend < 0.001) compared with those in the lowest quartile, after adjustment for potential confounders. Nonparametric spline analysis did not suggest any nonlinear association such as a “threshold” relation. Every unit increase of fasting insulin was associated with a 1.03-fold higher risk of incident HTN (95% CI 1.02–1.04). A similar significant dose-response relation was observed for IGR (Q4 vs. Q1: HR 1.78 [95% CI 1.35–2.37]; Ptrend < 0.001) and HOMA-IR (Q4 vs. Q1: 1.27 [1.00–1.63]; Ptrend < 0.001). When we further stratified data by sex or ethnicity, the observed positive associations remained consistent in each subgroup and none of the tests for interactions was statistically significant. Moreover, to test whether the observed positive association was solely driven by overweight/obese status, we did a fully stratified analysis by creating different quartiles within the stratum of normal weight and overweight/obese, and the similar positive associations were documented within each subgroup.
Table 2.
Multivariable-adjusted HRs (95% CIs) of incidence of HTN by serum levels (quartiles) of insulin, IGR, or HOMA-IR (CARDIA study, 1985–2005*)
Using GEE, we found that insulin level was positively associated with both SBP and DBP. The multivariable-adjusted β coefficients comparing the highest to the lowest quartiles of insulin levels were 3.64 mmHg (95% CI 2.82–4.46 mmHg; Ptrend < 0.001) and 2.34 mmHg (1.65–3.03 mmHg; Ptrend < 0.001) for SBP and DBP, respectively. A similar significant dose-response relation was also observed for the association of IGR or HOMA-IR with SBP and DBP (data not shown). When we examined the associations among normotensive individuals, the positive associations were essentially the same.
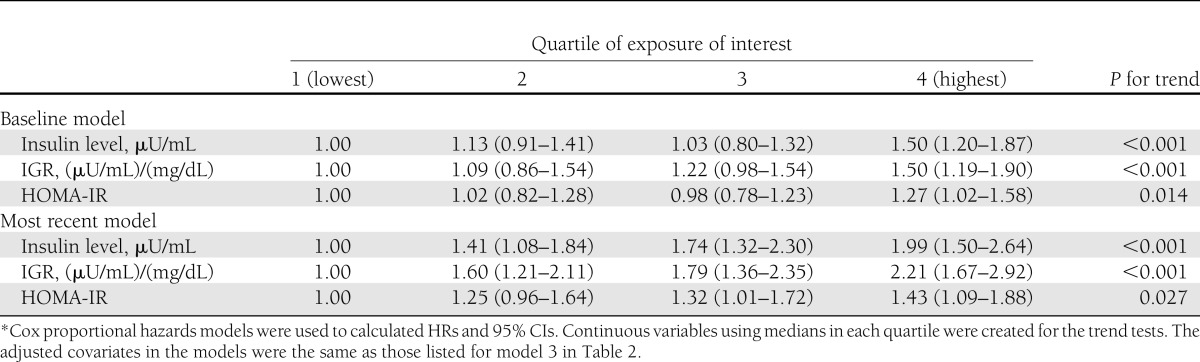
Sensitivity analyses were conducted to test the robustness of our main findings. First, we used the baseline exposure model and the most recent exposure model to explore the possible effect of follow-up time on the observed associations (Table 3). No material differences were found between long-term and short-term exposures. Second, when we further excluded 120 participants with baseline SBP/DBP ≥130/85 mmHg, the multivariable adjusted HR of participants in the highest quartile was 1.83 (95% CI 1.38–2.42; Ptrend < 0.001), as compared with those in the lowest quartile. Third, we additionally excluded 249 participants who were diagnosed as incident HTN only by antihypertensive medication; the results were similar (1.81 [1.30–2.52]; Ptrend < 0.001). In addition, as insulin levels were unavailable at examination years 2 and 5, we re-examined three exposures of interest in relation to incidence of HTN using examination year 7 as baseline. After adjustment for the same covariates listed for model 3 in Table 2, the results remained (data not shown).
Table 3.
A sensitivity analysis of multivariable-adjusted HRs (95% CIs) of incidence of HTN by quartiles of exposure of interest (CARDIA study, 1985–2005*)
CONCLUSIONS
In this unique 20-year follow-up longitudinal study, we found that fasting insulin level and its two derived indices (i.e., IGR and HOMA-IR) were positively associated with incidence of HTN as well as SBP and DBP in a dose-response manner among young AAs and Caucasians. Sex, ethnicity, and BMI status did not significantly modify the observed associations.
The findings of our study are generally in accordance with the results of previous studies. Serum insulin was found to be positively associated with incident HTN in 3,513 middle- or elder-aged individuals with a 5-year follow-up in Multi-Ethnic Study of Atherosclerosis (MESA) (6). Likewise, the positive association was found in 377 middle-aged participants with a 7-year follow-up from the Baltimore, MD, Clinical Center of Trials of HTN Prevention, Phase 1 (5). There were still a number of other supportive studies (9,12–14,25), although they were either relatively short-term (9,13), using a retrospective design (12,14,25), or only focusing on one sex or ethnic group (9,12,14).
However, unlike in our study, the positive association was not consistently observed in some ethnicity/sex subgroups in other studies. For example, positive association of fasting insulin level with SBP and DBP was documented in non-Hispanic whites but not in Mexican Americans in 8-year follow-up San Antonio Heart Study (26). The racial difference was also found in another study, in which positive association was only found in Caucasians but not in AAs and Pima Indians (11). In National Health and Nutrition Examination Survey (NHNES) 1999–2002, higher fasting insulin >12.2 mU/mL or HOMA-IR ≥2.6 was found to be positively associated with pre-HTN in men but not in women. In addition, sex difference in insulin-HTN relation was reported in 527 AAs aged 18–55 (10). Moreover, some studies reported findings contrary to our observations. Acute physiological increases in plasma insulin did not elevate arterial pressure of 13 borderline hypertensive young adults (27). In a double-blind crossover study, administration of insulin exerted a small BP-lowering effect on 23 nondiabetic, untreated patients with essential HTN (28). Of note, both of these small-sized studies investigated the short-term effect of insulin on pre-HTN or HTN. By contrast, our present study is elucidating a long-term association of insulin with incident HTN among normotensive participants.
The findings from this study are biologically plausible. Insulin can increase BP through all primary components that determine BP, including cardiac output, blood volume, and vascular flexibility. Insulin, a well-established inotropic agent (29), can increase cardiac output. Insulin can increase blood volume through stimulating secretion of vasopressin (an antidiuretic) (30) and renal sodium retention (31). Insulin can increase vascular tone through several mechanisms. First, it can increase basal level of calcium in cytosol of vascular smooth muscle cells by promoting the voltage-dependent Ca2+ influx and inhibiting Na+/Ca2+ exchanger–dependent Ca2+ extrusion, leading to increased vascular tone (32). Second, it can stimulate the rennin-angiotensin system (33). Third, it can stimulate the secretion of endohelin-1 (34), a vascular constrictor. Therefore, insulin can increase BP in many different ways. However, this primary role of insulin in increasing BP has not been fully appreciated due to the popular perception that insulin is not functional in the presence of insulin resistance, which is tightly linked to increased BP. Nevertheless, studies have shown that insulin is functional at an increased basal level in the presence of insulin resistance and hyperinsulinemia (35).
Some researchers argued that the relation between insulin resistance and HTN is probably not unidirectional (36). However, the likelihood of high BP causing elevated insulin levels is presumably very low, because changes in insulin level usually precede the presence of obvious HTN in metabolic diseases (37). In addition, a large body of evidence lends credence to the increase of BP by insulin (38,39), but no existing evidence, especially in mechanism, supports that HTN might cause hyperinsulinemia.
There are several strengths that need to be emphasized in this study. First, although randomized, placebo-controlled trials are the best approach to establish causal inference, it may not be feasible to conduct one on this topic, which makes the results from a well-designed prospective cohort study with relatively large sample size and long-term follow-up highly valuable. Second, the BP of our participants was measured by trained personnel using standardized procedures rather than self-reported, which may substantially reduce possible measurement error in BP. Importantly, the participants were young (at their ages of 18–30 years) at baseline, which enables us to investigate the evolution of cardiovascular disease risk by following the course of BP and the appearance of incident HTN in young adulthood.
Our study also has several limitations. First, insulin levels were not obtained at examination years 2 and 5. However, our results were unlikely to be biased substantially because we used accumulative models to approach the long-term exposure. Of note, when we used examination year 7 as baseline, the results were consistent. Another concern is that the assays of baseline insulin were measured again 7 years later. However, this would not materially alter our results. In one study, no significant change was detected in repeated assays of 34 samples stored at −20°C over an 8-year period (40). Also, the correlation between log insulin values from these two methods was high (r = 0.81) and would have little effect on the association between insulin levels and incident HTN. In fact, the re-assay of baseline insulin levels with the same method used in later examinations may reduce the measurement error and strengthen our study. Third, the CARDIA cohort represents young AAs and Caucasians recruited from four metropolitan areas in the U.S. and was not nationally representative, which may limit the generalizability of our findings.
In conclusion, this prospective study suggests that fasting serum insulin levels are positively and longitudinally associated with BP and incidence of HTN in apparently healthy American young adults, including both men and women, and AAs and Caucasians. The findings from the current study may help clinicians identify those who are at high risk of HTN, and support that lifestyle modification to decrease insulin concentrations may be of great importance for HTN prevention.
Acknowledgments
P.X. and K.H. were partially supported by grant R01-HL-081572 from the National Institutes of Health. CARDIA was supported by grants N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095 from the National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
P.X. researched data, contributed to discussion, wrote the draft, and reviewed and edited the manuscript. K.L., S.S., and O.D.W. contributed to discussion and reviewed and edited the manuscript. W.C. contributed to discussion, wrote the draft (mechanism part), and reviewed and edited the manuscript. K.H. created the concept and design, contributed to discussion, wrote the draft, and reviewed and edited the manuscript. All authors read and approved the final version of the manuscript. K.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Drs. Mercedes R. Carnethon (Northwestern University) and Young-il Kim (University of Alabama at Birmingham, Birmingham, AL) for their valuable comments. The authors also thank the other investigators and the staff of the CARDIA study for their valuable contributions.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–223 [DOI] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Cantey P, Smiley D, et al. Primary aldosteronism in diabetic subjects with resistant hypertension. Diabetes Care 2007;30:1699–1703 [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173–194 [DOI] [PubMed] [Google Scholar]
- 4.Ferrannini E. Metabolic syndrome: a solution in search of a problem. J Clin Endocrinol Metab 2007;92:396–398 [DOI] [PubMed] [Google Scholar]
- 5.He J, Klag MJ, Caballero B, Appel LJ, Charleston J, Whelton PK. Plasma insulin levels and incidence of hypertension in African Americans and whites. Arch Intern Med 1999;159:498–503 [DOI] [PubMed] [Google Scholar]
- 6.Levin G, Kestenbaum B, Ida Chen YD, et al. Glucose, insulin, and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2010;172:1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Lee ET, Fabsitz RR, et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension 2006;47:403–409 [DOI] [PubMed] [Google Scholar]
- 8.Mbanya JC, Thomas TH, Wilkinson R, Alberti KG, Taylor R. Hypertension and hyperinsulinaemia: a relation in diabetes but not essential hypertension. Lancet 1988;1:733–734 [DOI] [PubMed] [Google Scholar]
- 9.Skarfors ET, Lithell HO, Selinus I. Risk factors for the development of hypertension: a 10-year longitudinal study in middle-aged men. J Hypertens 1991;9:217–223 [DOI] [PubMed] [Google Scholar]
- 10.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Hypertension, insulin resistance, and aldosterone: sex-specific relationships. J Clin Hypertens (Greenwich) 2009;11:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad MF, Lillioja S, Nyomba BL, et al. Racial differences in the relation between blood pressure and insulin resistance. N Engl J Med 1991;324:733–739 [DOI] [PubMed] [Google Scholar]
- 12.Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med 2009;169:155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh K, Imai K, Masuda T, et al. Association between blood pressure and insulin resistance in obese females during weight loss and weight rebound phenomenon. Hypertens Res 2001;24:481–487 [DOI] [PubMed] [Google Scholar]
- 14.Salomaa VV, Strandberg TE, Vanhanen H, Naukkarinen V, Sarna S, Miettinen TA. Glucose tolerance and blood pressure: long term follow up in middle aged men. BMJ 1991;302:493–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116 [DOI] [PubMed] [Google Scholar]
- 16.Folsom AR, Jacobs DR, Jr, Wagenknecht LE, et al. Increase in fasting insulin and glucose over seven years with increasing weight and inactivity of young adults. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol 1996;144:235–246 [DOI] [PubMed] [Google Scholar]
- 17.Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 1965;25:1375–1384 [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM, Bowsher RR, Mykkänen L, et al. Proinsulin and specific insulin concentration in high- and low-risk populations for NIDDM. Diabetes 1994;43:1490–1493 [DOI] [PubMed] [Google Scholar]
- 19.Park K, Lee DH, Erickson DJ, Himes JH, Shikany JM, Jacobs DR., Jr Association of long-term change in waist circumference with insulin resistance. Obesity (Silver Spring) 2010;18:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 21.Gunderson EP, Chiang V, Lewis CE, et al. Long-term blood pressure changes measured from before to after pregnancy relative to nonparous women. Obstet Gynecol 2008;112:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xun P, Hou N, Daviglus M, et al. Fish oil, selenium and mercury in relation to incidence of hypertension: a 20-year follow-up study. J Intern Med 2011;270:175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu K, Slattery M, Jacobs D, Jr, et al. A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis 1994;4:15–27 [PubMed] [Google Scholar]
- 24.He K, Rimm EB, Merchant A, et al. Fish consumption and risk of stroke in men. JAMA 2002;288:3130–3136 [DOI] [PubMed] [Google Scholar]
- 25.Selby JV, Friedman GD, Quesenberry CP., Jr Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. Am J Epidemiol 1990;131:1017–1027 [DOI] [PubMed] [Google Scholar]
- 26.Mitchell BD, Haffner SM, Hazuda HP, Valdez R, Stern MP. The relation between serum insulin levels and 8-year changes in lipid, lipoprotein, and blood pressure levels. Am J Epidemiol 1992;136:12–22 [DOI] [PubMed] [Google Scholar]
- 27.Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 1992;19:621–627 [DOI] [PubMed] [Google Scholar]
- 28.Heise T, Magnusson K, Heinemann L, Sawicki PT. Insulin resistance and the effect of insulin on blood pressure in essential hypertension. Hypertension 1998;32:243–248 [DOI] [PubMed] [Google Scholar]
- 29.Rieker RP, Lee JC, Downing SE. Positive inotropic action of insulin on piglet heart. Yale J Biol Med 1975;48:353–360 [PMC free article] [PubMed] [Google Scholar]
- 30.Paulmyer-Lacroix O, Anglade G, Grino M. Insulin-induced hypoglycaemia increases colocalization of corticotrophin-releasing factor and arginine vasopressin mRNAs in the rat hypothalamic paraventricular nucleus. J Mol Endocrinol 1994;13:313–320 [DOI] [PubMed] [Google Scholar]
- 31.Kageyama S, Yamamoto J, Isogai Y, Fujita T. Effect of insulin on sodium reabsorption in hypertensive patients. Am J Hypertens 1994;7:409–415 [DOI] [PubMed] [Google Scholar]
- 32.Villa-Abrille MC, Sidor A, O’Rourke B. Insulin effects on cardiac Na+/Ca2+ exchanger activity: role of the cytoplasmic regulatory loop. J Biol Chem 2008;283:16505–16513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 1975;55:845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferri C, Pittoni V, Piccoli A, et al. Insulin stimulates endothelin-1 secretion from human endothelial cells and modulates its circulating levels in vivo. J Clin Endocrinol Metab 1995;80:829–835 [DOI] [PubMed] [Google Scholar]
- 35.Liu HY, Hong T, Wen GB, et al. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am J Physiol Endocrinol Metab 2009;297:E898–E906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu FB, Stampfer MJ. Insulin resistance and hypertension: the chicken-egg question revisited. Circulation 2005;112:1678–1680 [DOI] [PubMed] [Google Scholar]
- 37.Barnard RJ, Roberts CK, Varon SM, Berger JJ. Diet-induced insulin resistance precedes other aspects of the metabolic syndrome. J Appl Physiol 1998;84:1311–1315 [DOI] [PubMed] [Google Scholar]
- 38.Brands MW, Hildebrandt DA, Mizelle HL, Hall JE. Sustained hyperinsulinemia increases arterial pressure in conscious rats. Am J Physiol 1991;260:R764–R768 [DOI] [PubMed] [Google Scholar]
- 39.Juan CC, Fang VS, Kwok CF, Perng JC, Chou YC, Ho LT. Exogenous hyperinsulinemia causes insulin resistance, hyperendothelinemia, and subsequent hypertension in rats. Metabolism 1999;48:465–471 [DOI] [PubMed] [Google Scholar]
- 40.Perry IJ, Wannamethee SG, Whincup PH, Shaper AG, Walker MK, Alberti KG. Serum insulin and incident coronary heart disease in middle-aged British men. Am J Epidemiol 1996;144:224–234 [DOI] [PubMed] [Google Scholar]