Abstract
OBJECTIVE
Older adults with type 2 diabetes are at high risk of fractures and falls, but the effect of glycemic control on these outcomes is unknown. To determine the effect of intensive versus standard glycemic control, we assessed fractures and falls as outcomes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) randomized trial.
RESEARCH DESIGN AND METHODS
ACCORD participants were randomized to intensive or standard glycemia strategies, with an achieved median A1C of 6.4 and 7.5%, respectively. In the ACCORD BONE ancillary study, fractures were assessed at 54 of the 77 ACCORD clinical sites that included 7,287 of the 10,251 ACCORD participants. At annual visits, 6,782 participants were asked about falls in the previous year.
RESULTS
During an average follow-up of 3.8 (SD 1.3) years, 198 of 3,655 participants in the intensive glycemia and 189 of 3,632 participants in the standard glycemia group experienced at least one nonspine fracture. The average rate of first nonspine fracture was 13.9 and 13.3 per 1,000 person-years in the intensive and standard groups, respectively (hazard ratio 1.04 [95% CI 0.86–1.27]). During an average follow-up of 2.0 years, 1,122 of 3,364 intensive- and 1,133 of 3,418 standard-therapy participants reported at least one fall. The average rate of falls was 60.8 and 55.3 per 100 person-years in the intensive and standard glycemia groups, respectively (1.10 [0.84–1.43]).
CONCLUSIONS
Compared with standard glycemia, intensive glycemia did not increase or decrease fracture or fall risk in ACCORD.
Older adults with type 2 diabetes are at higher risk of fractures but have higher bone density compared with those without diabetes (1). Falls are more frequent in those with diabetes, which may contribute to their higher fracture risk (2,3). Few studies have examined the effect of glycemic control on fractures or falls in adults with type 2 diabetes. While better glycemic control reduces microvascular complications (4–9) and could potentially reduce fracture and fall risk (1,4,7–10), associated hypoglycemia may increase the risk of injurious falls (11).
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) BONE ancillary study was designed to determine the effect of a strategy of intensive glycemia therapy on occurrence of fractures and falls. ACCORD is a randomized trial of cardiovascular outcomes, designed to compare an intensive therapeutic strategy targeting normal A1C levels (i.e., <6.0%), with a standard strategy targeting A1C levels from 7.0 to 7.9% in a population with long-standing type 2 diabetes and a history of cardiovascular disease (CVD) or significant cardiovascular risk factors. The design and main results have previously been published (12). We report here the effect of the intensive, compared with standard, glycemia therapy on nonspine fractures, height change as a surrogate for vertebral fracture, falls, and bone mineral density (BMD).
RESEARCH DESIGN AND METHODS
ACCORD trial design and participants
The ACCORD trial has previously been described (13). In brief, inclusion criteria for participants included type 2 diabetes, an A1C of 7.5–11%, and an age of 40–79 years with a history of CVD or an age of 55–79 years with subclinical evidence of CVD or significant risk factors for CVD. Exclusion criteria included frequent serious hypoglycemia, BMI of ≥45 kg/m2, serum creatinine >1.5 mg/dL (132.6 μmol/L), or other serious illness. The trial included 77 clinical sites in the U.S. and Canada, grouped into seven clinical center networks.
ACCORD was a double two-by-two factorial, parallel-treatment trial. All eligible participants (N = 10,251) were randomly assigned to the intensive or standard glycemia therapy group. Participants were also assigned to either the blood pressure or lipid trial based on eligibility. In the ACCORD blood pressure trial, 4,733 participants were randomly assigned to receive intensive or standard blood pressure control. In the ACCORD lipid trial, 5,518 participants were randomly assigned to receive simvastatin plus fenofibrate or simvastatin plus placebo. Randomization of 1,174 participants occurred during the vanguard phase of the trial in 2001–2002. The remaining 9,077 participants were randomized from 1 January 2003 to 29 October 2005. During the trial, ACCORD achieved a median A1C of 6.4 and 7.5% in the intensive and standard glycemia therapy groups, respectively. The intensive glycemia intervention protocol was stopped in February 2008 because of higher all-cause mortality in those assigned to this treatment strategy (12).
BONE ancillary study design
Five of the seven clinical center networks agreed to participate in the BONE ancillary study, including 54 of 77 clinical sites and 7,287 participants. The BONE ancillary study was initiated during the main recruitment for the ACCORD trial. Beginning in January 2006, at the next annual visit participants were asked about the occurrence of any nonspine fractures since randomization. After the annual visit in 2006, participants were asked if they had suffered a fracture since their last annual visit. Fractures of the lumbar and thoracic spine were excluded because of the additional burden and difficulty of adjudicating such fractures. Reported fracture events were centrally adjudicated, based on radiology records, at the University of California, San Francisco (UCSF), with the adjudicators blinded to treatment assignment. A sample of reported events was independently adjudicated by a panel of experts at two time points during the trial for quality assurance. Only confirmed nonspine fractures were included in these analyses. As per protocol, pathological fractures, confirmed as occurring secondary to neoplasm, necrosis, or sepsis, and periprosthetic fractures were excluded (N = 7). These analyses are limited to confirmed fractures that occurred on or before 5 February 2008, when the intensive glycemia intervention was ended. The main outcome was all nonspine fractures. Hip, proximal humerus, distal forearm, ankle, and foot fractures were analyzed individually as secondary outcomes.
Standing height was measured according to a standard protocol at baseline and annual visits on all ACCORD participants. Among participants in the BONE ancillary study, 6,979 (3,482 intensive and 3,497 standard glycemia) participants had at least one height measurement during follow-up and were included in these analyses.
At each annual visit starting in January 2006, participants were also asked about falling: “In the last 12 months have you fallen and landed on the floor or ground, or fallen and hit an object like a table or stair?” Those who answered “yes” were also asked how many times they had fallen in the previous 12 months. These analyses include results from the annual visits that occurred before 5 February 2008, the close of the intensive glycemia arm. Of those in the BONE ancillary study, 6,782 participants answered at least one question about falls.
At five clinical sites in the Minneapolis, Minnesota, area and five sites in the Winston-Salem, North Carolina, area, participants were asked to participate in the BMD substudy. Recruitment started in May 2005 and closed in October 2005, when the main trial recruitment was complete. Dual X-ray absorptiometry (DXA) scans of the hip, spine, and whole body were obtained at baseline on 130 (63 intensive and 67 standard glycemia) participants. Follow-up DXA scans were obtained on 107 (48 intensive and 59 standard glycemia) participants at a visit ~2 years after randomization, and these participants are included in analyses of changes in BMD. DXA scans were obtained on Hologic fan-beam densitometers at the University of Minnesota (4500A) and Wake Forest University (Delphi), using software versions 9.8 and 12.3, respectively. Centralized quality assurance was provided by UCSF. Spine and whole-body phantoms were scanned regularly at both sites to assess longitudinal performance.
Institutional review boards at all participating institutions approved the protocol for the ACCORD BONE ancillary study, and written informed consent was obtained from all participants.
Statistical methods
The BONE ancillary study was designed to have 80% power to detect a relative reduction in risk of all nonspine clinical fractures of 22–29%. This was based on an estimated total number of fractures between 259 and 494, calculated with the following assumptions: a rate of clinical nonspine fracture among women in the standard glycemia therapy group between 15 and 25 per 1,000 person-years, fracture rates for men between 35 and 40% of the rates for women of the same age, and an average follow-up time of 4.6 years.
All analyses were by intention to treat without regard to adherence. Following standard ACCORD procedures, all analyses adjusted for presence of cardiovascular disease at baseline, assignment to either the blood pressure trial or the lipid trial, assignment to the intensive blood pressure intervention in the blood pressure trial, and assignment to receive fibrate in the lipid trial. ACCORD procedures also specified tests for interaction between treatment assignment in the glycemic control trial and assignment in the lipid or blood pressure trial. Analyses were performed at UCSF using SAS version 9.1 (SAS Institute) and, for the outcome of falls, Stata version 11 (Stata).
Cox proportional hazards models were used to compare nonspine fracture risk in the intensive and standard groups; only first nonpathologic fractures were considered in this analysis. We also compared treatment effects across strata defined by prespecified baseline covariates: sex, age, race, BMI, A1C, duration of diabetes, insulin use, thiazolidinedione use, metformin use, sulfonylurea use, and serum creatinine. Continuous variables were dichotomized at the mean value with the exception of age, at 65 years, and serum creatinine, at 1.4 mg/dL, the threshold for renal insufficiency.
Height loss was compared by treatment assignment using linear mixed models with random intercepts and slopes. Treatment effects were captured by the interaction between treatment assignment and time. The proportions losing >2 cm of height during follow-up were compared using logistic models. This degree of height loss is associated with incident vertebral fracture with 94% specificity but only 28% sensitivity (14).
Numbers of falls reported at each annual visit were compared by treatment assignment using a repeated-measures negative binomial model, with robust SEs to account for clustering of the repeated outcomes within participants; the log of the length of the reporting period varied slightly and was included as an offset. We also compared treatment effects across strata defined by prespecified baseline covariates (listed above). In addition, we compared the proportions reporting at least one fall in each reporting period using a logistic model, again with robust SEs to account for clustering. A similar approach was used to compare those reporting two or more falls with those reporting one or none during each year. In these models, we flexibly modeled the falling rate among controls using a three-knot restricted cubic spline.
A linear model was used to compare percent changes in BMD in the intensive and standard glycemia groups. These models were adjusted for baseline BMD, age, sex, race, DXA site (Minneapolis or Wake Forest), and baseline thiazolidinedione use, in addition to the standard ACCORD adjustment variables identified above (15).
RESULTS
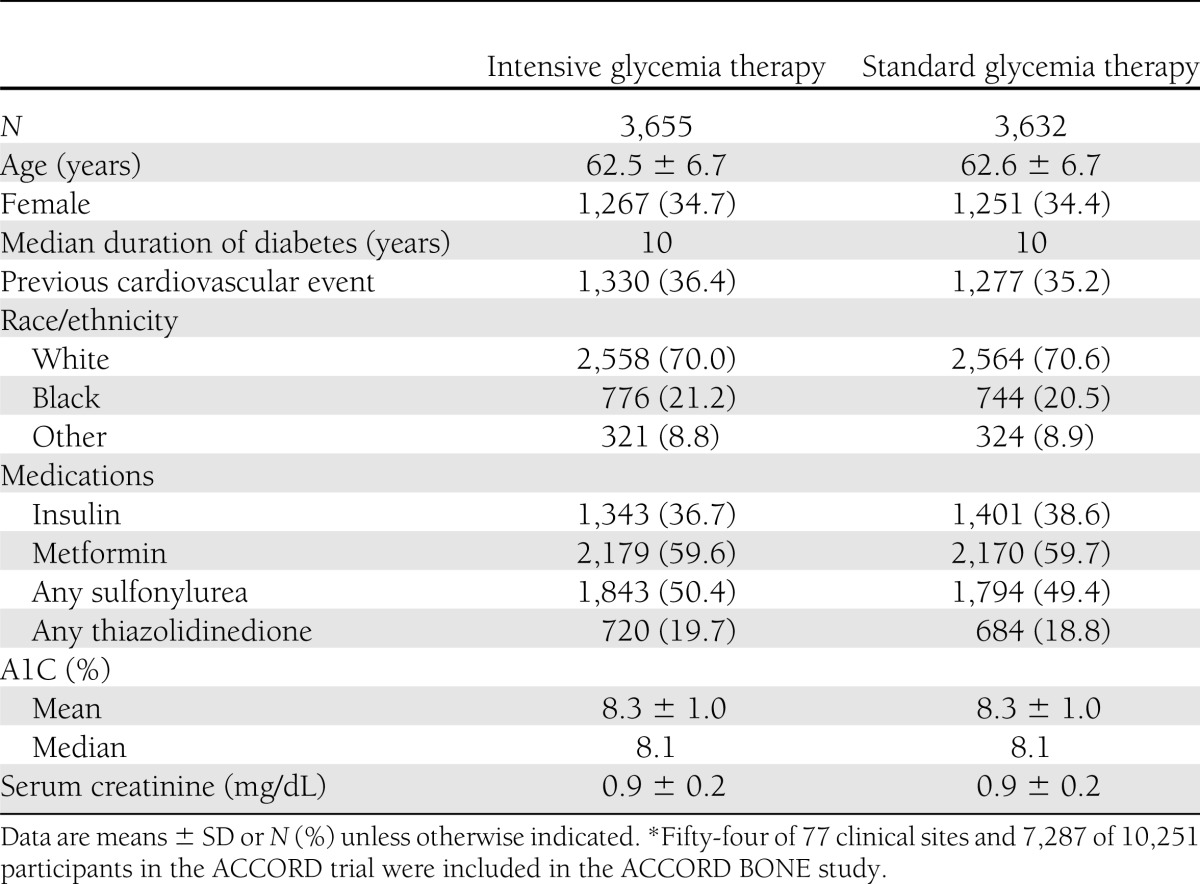
Table 1 provides baseline demographic and clinical data for ACCORD BONE participants. Mean (SD) age was 62.5 (6.7) years, and median duration of diabetes was 10 years. A total of 3,655 participants were assigned to intensive glycemia therapy, and 3,632 were assigned to standard glycemia therapy.
Table 1.
Baseline characteristics of participants in the ACCORD BONE ancillary study*
Nonspine fractures
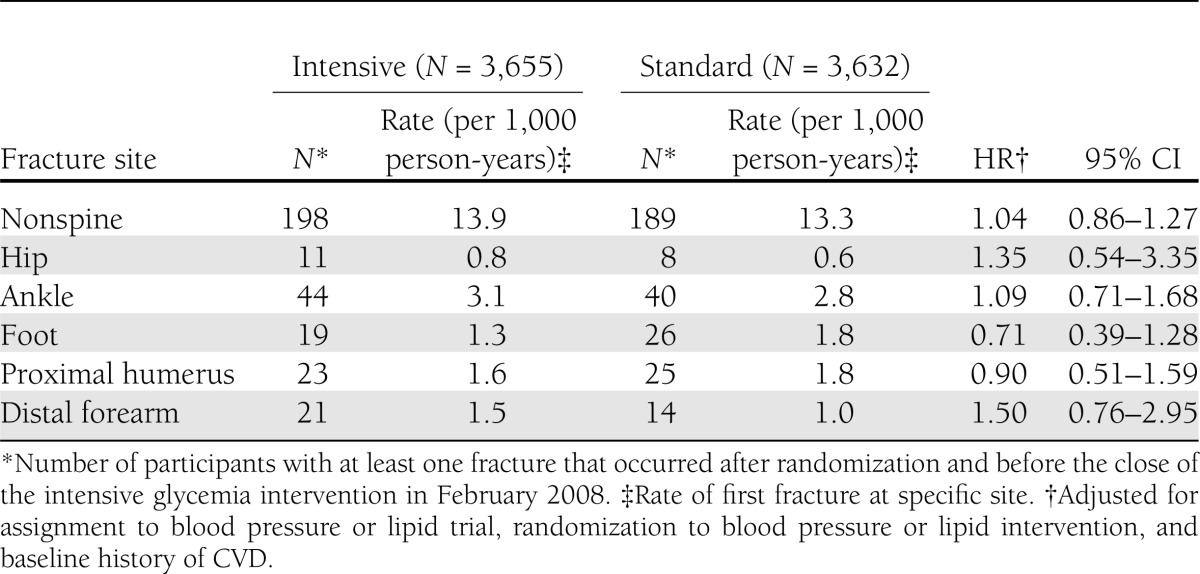
During an average follow-up of 3.8 (SD 1.3) years, 198 participants in the intensive glycemia and 189 participants in the standard glycemia group experienced at least one nonspine fracture. Of these, 25 and 24 participants experienced two or more fractures in the intensive and standard glycemia groups, respectively. The rates of first nonspine fracture were 13.9 per 1,000 person-years in the intensive glycemia and 13.3 per 1,000 person-years in the standard glycemia group (Table 2). The most common fracture sites were ankle (N = 84 participants), rib (N = 51), proximal humerus (N = 48), foot (N = 45), and distal forearm (N = 35). Other sites included toe (N = 32 participants), proximal tibia/fibula (N = 20), hip (N = 19), elbow (N = 19), finger (N = 18), hand (N = 15), pelvis (N = 15), and patella (N = 15), with smaller numbers at the upper leg, clavicle, chest/sternum, tailbone, face, and heel.
Table 2.
Effect of intensive compared with standard glycemia therapy on fracture risk by fracture site in the ACCORD BONE study (2001–2008)
There was no significant effect of intensive, compared with standard, glycemia therapy on the rate of nonspine fractures (hazard ratio [HR] 1.04 [95% CI 0.86–1.27]) (Supplementary Figure 1S). Prespecified subgroup analyses revealed significant heterogeneity in the effect of intensive glycemia compared with standard control across the four subgroups defined by the lipid and blood pressure trial treatments (P = 0.003). This was due to interaction in the lipid trial between intensive glycemia and the lipid trial intervention (P = 0.002). Intensive glycemia, compared with standard glycemia, reduced nonspine fractures in those assigned to simvastatin plus placebo in the lipid trial (0.50 [0.32–0.77]) but had no effect on fractures in the simvastatin plus fenofibrate group (1.25 [0.85–1.84]). In the blood pressure trial, there were no significant interactions between glycemic arm assignment and assignment to intensive blood pressure control (P = 0.700).
Intensive, compared with standard, glycemia therapy did not affect the rates of hip, proximal humerus, distal forearm, ankle, or foot fractures (Table 2). The effect of intensive glycemia, compared with standard glycemia, on nonspine fractures did not differ across prespecified baseline characteristics (Supplementary Figure 2S), including sex (P for interaction = 0.325), race (white and nonwhite, P = 0.716), and age (≥65 and <65 years, P = 0.719).
Height loss
Mean annual change in height was −0.17 and −0.15 cm per year in the intensive and standard glycemia groups, respectively. The rate of height loss did not differ between groups (P = 0.573). Height loss of >2 cm during ACCORD was experienced by 678 (19.5%) participants in the intensive and 686 (19.6%) participants in the standard glycemia group (odds ratio [OR] 0.99 [95% CI 0.88–1.11]).
Falls
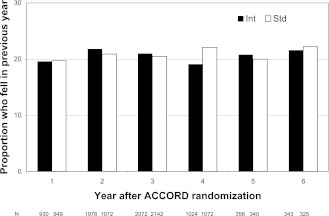
The average follow-up for falls was 2.0 years. In the intensive group, 1,122 of 3,364 participants and in the standard group 1,133 of 3,418 participants reported at least one fall during follow-up. The average proportion reporting one or more falls in any year was 20.8 and 20.9% in the intensive and standard control groups, respectively (Fig. 1). The average proportion reporting two or more falls in any year was 7.9 and 7.7%. In those aged ≥65 years, the average proportion reporting one or more falls was 19.4 and 18.5% among men and 24.1 and 25.5% among women in the intensive and standard control group, respectively. The OR for experiencing one or more falls in a year, with no falls as the reference group, was 0.99 (95% CI 0.91–1.09), comparing intensive and standard glycemia. For experiencing two or more falls, with one or no falls as the reference group, the OR was 1.02 (0.89–1.18).
Figure 1.
Proportion of participants in the ACCORD BONE study who fell in the previous year at each visit (2006–2008) by glycemia therapy group (intensive [Int] or standard [Std]).
The average rate of falls was 60.8 and 55.3 per 100 person-years in the intensive and standard control groups, respectively. The rate ratio for falling was 1.10 (95% CI 0.84–1.43) for intensive versus standard glycemic control. The rate ratio for falling for intensive compared with standard glycemic control did not differ by subtrial (blood pressure trial or lipid trial) assignment or subtrial intervention. There was evidence of interaction by baseline age (P = 0.018) (Supplementary Figure 3S). In those aged <65 years, the rate ratio for falls was 1.27 (0.93–1.73), comparing intensive and standard glycemia, while in those aged ≥65 years, the rate ratio was 0.75 (0.55–1.01). There was also evidence of interaction with baseline insulin use (P = 0.028). In those who did not use insulin at baseline, the rate ratio for falls was 0.84 (0.66–1.06), comparing those in the intensive and standard glycemia groups. In those using insulin at baseline, the rate ratio for falls was 1.47 (0.97–2.23). There was no evidence of interaction by sex (P = 0.452) or other baseline factors considered.
Changes in BMD
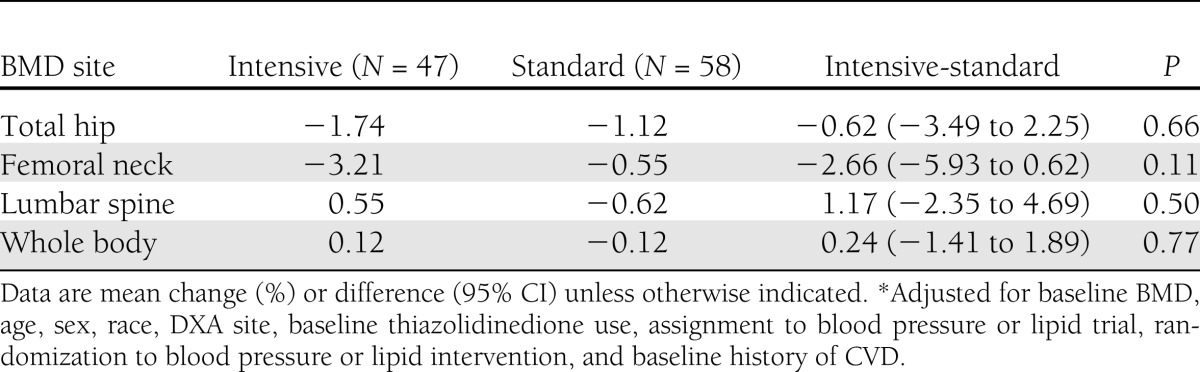
Baseline and follow-up DXA scans were obtained on 48 intensive and 59 standard glycemia therapy participants at follow-up, with mean time interval between DXA scans of 2.2 years (range 1.9–2.5). Eighty-two participants were scanned before the close of the intensive glycemia intervention on 5 February 2008, and 25 participants were scanned shortly afterward. The median time from close of the intensive glycemia intervention to the second DXA scan was 14 days (maximum 43 days). In adjusted models, there were no statistically significant differences in BMD change for the total hip, femoral neck, lumbar spine, or whole body comparing the intensive and standard glycemia groups, but 95% CIs were wide (Table 3).
Table 3.
Two-year percent change in BMD by glycemia assignment in the ACCORD BONE study (2005–2008)*
CONCLUSIONS
In this first randomized trial assessing the effect of intensive glycemia therapy on fractures and falls, we found no statistically significant net effect of glycemia treatment strategy on occurrence of nonspine fractures, height loss, falls, or change in bone density. These results provide evidence that intensive lowering of A1C for several years neither increases nor reduces fracture or fall risk. Of particular importance, fracture and fall risks with intensive glycemia were not increased in those aged ≥65 years. However, modest adverse as well as protective effects cannot be ruled out with 95% confidence.
Results from smaller observational studies of glycemic control and fracture have been mixed. A recent study of Japanese men with type 2 diabetes found an association between prevalent vertebral fracture, identified with spine films, and high A1C (≥ 9%) levels among men who were overweight or obese (16), but previous studies reported no association between baseline A1C or fasting glucose and fracture risk (17–20). A 1-year clinical trial assessed the effect of improved glycemic control on bone density in 50 patients with type 2 diabetes, aged 60–75 years, who presented with high A1C (mean 11.6%) levels. Good metabolic control was achieved for all patients within 15 days. In both groups, bone density, measured as bone mineral content, increased at the femoral neck, and osteocalcin, a marker of bone turnover, was reduced after 1 year of treatment (21).
Observational studies of glycemic control and falls have also reported mixed results. In one study of older adults with diabetes, good control (A1C ≤7%) was associated with increased fall risk (22), but other studies have reported no association between A1C level and falls (23,24). Another study among older adults with diabetes found that A1C ≤6% was associated with increased risk of falls but only in those using insulin therapy (25). However, in ACCORD we found that the intensive intervention aimed at an A1C <6% did not result in more frequent falls compared with the standard intervention aimed at an A1C of 7.0–7.9%. The age-stratified results in ACCORD indicated that, if anything, those aged ≥65 years were less susceptible to increased fall risk with intensive glycemia than those aged <65 years.
Because intensive glycemic control increases hypoglycemic episodes, there has been concern that intensive control of A1C could contribute to fracture risk through increased falls in older adults (25). In ACCORD, intensive glycemia was associated with increased frequency of hypoglycemic episodes (16.2 vs. 5.1%) (12,26,27). In spite of this increase, we did not observe a higher risk of falls or fractures with intensive therapy.
Previous studies have reported associations in diabetic and broader populations between fractures and poor renal function (28), peripheral neuropathy (18,19,29), and poor vision (20,30). In ACCORD, the intensive glycemia therapy slowed the progression of diabetic retinopathy (8). In addition, although two composite measures of advanced microvascular complication scores were not significantly improved by intensive glycemia therapy, this treatment strategy did reduce the onset or progression of some early microvascular complications, including progression to microalbuminuria and macroalbuminuria, worsening of visual acuity, and loss of light touch sensation (9). However, the favorable effects of the intensive glycemic strategy on these complications did not translate into a short-term reduction in fracture or fall risk in ACCORD.
Weight gain is associated with decreased fracture risk in broader populations, although the association is stronger at lower body size (31). In ACCORD, 28% of participants in the intensive group gained >10 kg during the trial compared with 14% in the standard group (12). However, this weight gain did not result in a reduced fracture risk in the intensive glycemia group.
The intensive glycemia group had greater exposure to all classes of diabetes drugs than the standard group (12). Of particular interest for the fracture outcome, this included thiazolidinediones, mainly rosiglitazone. In the intensive glycemia group, 92% of participants were prescribed a thiazolidinedione compared with 58% in the standard glycemia group, and among those on thiazolidinediones, daily doses were higher in the intensive than the standard glycemic treatment group (12). Evidence from other clinical trials indicates that thiazolidinedione use doubles the risk of fracture in women, although trials have found no effect in men (32). Given the frequency of thiazolidinedione use in the two arms, we would have expected to observe an increased fracture risk in women of ~20%, or an HR of 1.2, comparing intensive and standard glycemia, due to thiazolidinedione use alone if risk is indeed doubled with thiazolidinedione use. ACCORD BONE was not adequately powered to detect a 20% increase in the relative rate of fracture, especially in one subgroup. In women, the HR for the effect of intensive glycemia on nonspine fracture was 1.14 (95% CI 0.87–1.50), with a 95% CI that is compatible with no effect or with an HR of 1.2. The high thiazolidinedione exposure in the standard group makes it difficult to draw conclusions about thiazolidinedione use and fracture risk from the comparison of the glycemia groups.
The interaction identified between intensive glycemia therapy and assignment to the placebo group in the lipid trial for the outcome of nonspine fracture was unexpected. Plausible reasons for this interaction are not evident, and it may be due to chance.
Strengths of this study include the randomized design, the central adjudication of fractures, and the large sample size for assessment of fractures and falls. However, important limitations must also be recognized. The initial identification of a possible fracture event relied on self-report at annual visits with the possibility of under- or overreporting of fractures. Completion of annual visits was similar between the two glycemia groups, so it is unlikely that recall varied by intervention. With central adjudication, our specificity was likely close to 100%. In this situation, nondifferential lack of sensitivity would result in reduced power but would not bias the effect estimate (33). Our fracture outcome did not include fractures of the lumbar or thoracic spine by design. As a surrogate, we used change in height to indirectly assess the effect of intensive glycemia on vertebral fracture, but this method has low sensitivity. Resulting misclassification would tend to attenuate any real association.
The measurement of falls was based on self-report at annual visits. Participants may have failed to recall and report falls. Participants may have had varying interpretations of what constituted a fall, with possible over- and underreporting of falls. In broader populations, ~30% of older adults fall each year, which is somewhat higher than the proportion of older participants who reported falling each year in ACCORD (19% for men and 25% for women) (34). It is possible that inconsistencies in recall and reporting differed by glycemia group. Those who experienced a hypoglycemic episode may not have accurately recalled what happened during the episode. This would have led to underreporting of falls in the intensive glycemia group and an underestimate of the effect of intensive, compared with standard, glycemia. In addition, we did not measure the consequences of the reported falls and therefore do not know whether the rate of injurious falls differed by glycemia groups. However, one of the most significant injuries that can result from a fall is a fracture, and these were not increased in the intensive group.
Our study lacked sufficient power to identify differences in risk for specific fractures, particularly hip fractures. Power to detect differences was also low for the BMD substudy. ACCORD enrolled few participants aged >79 years, so our results may not apply to older age-groups that might be more sensitive to the adverse effects of hypoglycemia.
The ACCORD trial was designed to compare a regimen of intensive glycemia with one of standard glycemia. In addition to different A1C goals, the two regimens differed in other factors that may affect fracture risk. This comparison of the net effect of intensive glycemia therapy on fracture risk answers key questions about the safety and efficacy of glycemic control, as achieved in ACCORD, with respect to the risk of fractures and falls. However, questions remain regarding the independent effects of achieved glycemic control, microvascular complications, diabetes medications, and hypoglycemia on these outcomes. Additional analyses will be undertaken in the future to help determine the separate effects of these factors.
Given the higher mortality associated with the intensive glycemia strategy, this regimen is not recommended for diabetic patients (12). However, improved glycemic control continues to be an important treatment goal. In this context, the results of ACCORD BONE provide reassurance that lower A1C can be achieved in older adults without increasing the risk of fractures or falls. Although the intensive therapy group experienced more hypoglycemic episodes, this did not result in an increased fracture or fall risk. On the other hand, these results indicate that achieving lower A1C for several years is not sufficient to reduce these risks in diabetic patients. As with older adults in general, fracture and fall risk assessment and implementation of specific measures for osteoporosis prevention and treatment are needed in this population (35–37). In conclusion, compared with standard glycemia, intensive glycemia treatment that achieved a median A1C of 6.4% had no net effect on risk of nonspine fractures or falls in older adults with long-standing type 2 diabetes.
Acknowledgments
The ACCORD BONE ancillary study was funded by a grant (R01DK069514) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The ACCORD study was supported by grants (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01- HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) from the National Heart, Lung, and Blood Institute; by other components of the National Institutes of Health, including the NIDDK, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers.
The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, sanofi-aventis, and Schering-Plough. J.B.G. has been a paid consultant for Medtronic; received grant funding from Medtronic, Merck, and Amylin; received speaking fees from Merck and Takeda; and received payment for educational presentations from Taking Control of Your Diabetes and the American Association of Clinical Endocrinology. No other potential conflicts of interest relevant to this article were reported.
A.V.S. and E.V. wrote the manuscript, researched data, contributed to discussion, and reviewed and edited the manuscript. K.L.M., B.P.H., J.B.G., and R.W.F. researched data, contributed to discussion, and reviewed and edited the manuscript. D.E.S., D.E.B., R.G.J., A.M.S., D.L.S., T.F.H., and D.C.B. contributed to discussion and reviewed and edited the manuscript. W.T.A. and L.P. researched data and reviewed and edited the manuscript. H.H.A. and P.J.O. reviewed and edited the manuscript. A.V.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Footnotes
Clinical trial reg. no. NCT00324350, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2184/-/DC1.
The entire list of the ACCORD investigators can be found in an appendix to ref. 12.
References
- 1.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 2007;18:427–444 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002;25:1749–1754 [DOI] [PubMed] [Google Scholar]
- 3.Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J Gerontol A Biol Sci Med Sci 2005;60:1157–1162 [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 6.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 8.Chew EY, Ambrosius WT, Davis MD, et al. ACCORD Study Group; ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail-Beigi F, Craven T, Banerji MA, et al. ACCORD trial group Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S, Hyer S, Tweed K, et al. Risk factors for fractures and falls in older women with type 2 diabetes mellitus. Calcif Tissue Int 2008;82:87–91 [DOI] [PubMed] [Google Scholar]
- 11.Brown JP, Josse RG, Scientific Advisory Council of the Osteoporosis Society of Canada 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ 2002;167(Suppl.):S1–S34 [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buse JB, Bigger JT, Byington RP, et al. ACCORD Study Group Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99(12A):21i–33i [DOI] [PubMed] [Google Scholar]
- 14.Siminoski K, Jiang G, Adachi JD, et al. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int 2005;16:403–410 [DOI] [PubMed] [Google Scholar]
- 15.Crager MR. Analysis of covariance in parallel-group clinical trials with pretreatment baselines. Biometrics 1987;43:895–901 [PubMed] [Google Scholar]
- 16.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Combination of obesity with hyperglycemia is a risk factor for the presence of vertebral fractures in type 2 diabetic men. Calcif Tissue Int 2008;83:324–331 [DOI] [PubMed] [Google Scholar]
- 17.Forsén L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia 1999;42:920–925 [DOI] [PubMed] [Google Scholar]
- 18.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 2005;165:1612–1617 [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res 2008;23:1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ, Blue Mountains Eye Study Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care 2001;24:1198–1203 [DOI] [PubMed] [Google Scholar]
- 21.Gregorio F, Cristallini S, Santeusanio F, Filipponi P, Fumelli P. Osteopenia associated with non-insulin-dependent diabetes mellitus: what are the causes? Diabetes Res Clin Pract 1994;23:43–54 [DOI] [PubMed] [Google Scholar]
- 22.Nelson JM, Dufraux K, Cook PF. The relationship between glycemic control and falls in older adults. J Am Geriatr Soc 2007;55:2041–2044 [DOI] [PubMed] [Google Scholar]
- 23.Miller DK, Lui LY, Perry HM, 3rd, Kaiser FE, Morley JE. Reported and measured physical functioning in older inner-city diabetic African Americans. J Gerontol A Biol Sci Med Sci 1999;54:M230–M236 [DOI] [PubMed] [Google Scholar]
- 24.Huang ES, Karter AJ, Danielson KK, Warton EM, Ahmed AT. The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the diabetes and aging study. J Gen Intern Med 2010;25:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Health, Aging, and Body Composition Study Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care 2008;31:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller ME, Bonds DE, Gerstein HC, et al. ACCORD Investigators The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaCroix AZ, Lee JS, Wu L, et al. Women’s Health Initiative Observational Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc 2008;56:1434–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Study of Osteoporotic Features Research Group Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 2001;86:32–38 [DOI] [PubMed] [Google Scholar]
- 30.de Boer MR, Pluijm SM, Lips P, et al. Different aspects of visual impairment as risk factors for falls and fractures in older men and women. J Bone Miner Res 2004;19:1539–1547 [DOI] [PubMed] [Google Scholar]
- 31.Reid IR. Relationships between fat and bone. Osteoporos Int 2008;19:595–606 [DOI] [PubMed] [Google Scholar]
- 32.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 2009;180:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White E. The effect of misclassification of disease status in follow-up studies: implications for selecting disease classification criteria. Am J Epidemiol 1986;124:816–825 [DOI] [PubMed] [Google Scholar]
- 34.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2009(2):CD007146. [DOI] [PubMed] [Google Scholar]
- 35.Kanis JA, Burlet N, Cooper C, et al. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2008;19:399–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson-Hughes B, Tosteson AN, Melton LJ, 3rd, et al. National Osteoporosis Foundation Guide Committee Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 2008;19:449–458 [DOI] [PubMed] [Google Scholar]
- 37.American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001;49:664–672 [PubMed] [Google Scholar]