Abstract
At the forefront of cognitive neuroscience research in normal humans are the new techniques of functional brain imaging: positron emission tomography and magnetic resonance imaging. The signal used by positron emission tomography is based on the fact that changes in the cellular activity of the brain of normal, awake humans and laboratory animals are accompanied almost invariably by changes in local blood flow. This robust, empirical relationship has fascinated scientists for well over a hundred years. Because the changes in blood flow are accompanied by lesser changes in oxygen consumption, local changes in brain oxygen content occur at the sites of activation and provide the basis for the signal used by magnetic resonance imaging. The biological basis for these signals is now an area of intense research stimulated by the interest in these tools for cognitive neuroscience research.
Over the past 10 years the field of cognitive neuroscience has emerged as a very important growth area in neuroscience. Cognitive neuroscience combines the experimental strategies of cognitive psychology with various techniques to actually examine how brain function supports mental activities. Leading this research in normal humans are the new techniques of functional brain imaging: positron emission tomography (PET) and magnetic resonance imaging (MRI) along with event-related potentials obtained from electroencephalography or magnetoencephalography.
The signal used by PET is based on the fact that changes in the cellular activity of the brain of normal, awake humans and unanesthetized laboratory animals are invariably accompanied by changes in local blood flow (for a review, see ref. 1). This robust, empirical relationship has fascinated scientists for well over a hundred years, but its cellular basis remains largely unexplained despite considerable research.
More recently it has been appreciated that these changes in blood flow are accompanied by much smaller changes in oxygen consumption (2, 3). This leads to changes in the actual amount of oxygen remaining in blood vessels at the site of brain activation (i.e., the supply of oxygen is not matched precisely with the demand). Because MRI signal intensity is sensitive to the amount of oxygen carried by hemoglobin (4), this change in blood oxygen content at the site of brain activation can be detected with MRI (5–8).
Studies with PET and MRI and magnetic resonance spectroscopy (MRS) have brought to light the fact that metabolic changes accompanying brain activation do not appear to follow exactly the time-honored notion of a close coupling between blood flow and the oxidative metabolism of glucose (9, 10). Changes in blood flow appear to be accompanied by changes in glucose utilization that exceed the increase in oxygen consumption (11, 12), suggesting that the oxidative metabolism of glucose may not supply all of the energy demands encountered transiently during brain activation. Rather, glycolysis alone may provide the energy needed for the transient changes in brain activity associated with cognition and emotion.
Because of the prominent role of PET and MRI in the study of human brain function in health and disease, it is important to understand what we currently know about the biological basis of the signals they monitor. Individuals using these tools or considering the results of studies employing them should have a working knowledge of their biological basis. This paper reviews that information which is, at times, conflicting and incomplete.
Although it is easy to conclude that much of this work transpired over the past decade or so because of its recent prominence in the neuroscience literature, in truth work on these relationships and the tools to exploit them have been developing for more than a century. To place present work in its proper perspective, a brief historical review of work on the relationships between brain function, blood flow, and metabolism is included.
Historical Background
The quest for an understanding of the functional organization of the normal human brain, using techniques to assess changes in brain circulation, has occupied mankind for more than a century. One has only to consult William James’ monumental two-volume text Principles of Psychology (13) on page 97 of the first volume to find reference to changes in brain blood flow during mental activities. He references primarily the work of the Italian physiologist Angelo Mosso (14) who recorded the pulsation of the human cortex in patients with skull defects following neurosurgical procedures. Mosso showed that these pulsation increased regionally during mental activity and concluded, correctly we now know, that brain circulation changes selectively with neuronal activity.
No less a figure than Paul Broca was also interested in the circulatory changes associated with mental activities as manifest by changes in brain temperature (15). Although best known for his seminal observations on the effect of lesions of the left frontal operculum on language function (16), Broca also studied the effect of various mental activities, especially language, on the localized temperature of the scalp of medical students (15). Although such measurements might seem unlikely to yield any useful information, the reported observations, unbiased by preconceived notions of the functional anatomy of the cortex, were remarkably perceptive. Also active in the study of brain temperature and brain function in normal humans were Mosso (17) and Hans Berger (18). Berger later abandoned his efforts in this area in favor of the development of the electroencephalogram.
Despite a promising beginning, including the seminal animal experimental observations of Roy and Sherrington (9), which suggested a link between brain circulation and metabolism, interest in this research virtually ceased during the first quarter of the twentieth century. Undoubtedly, this was due in part to a lack of tools sufficiently sophisticated to pursue this line of research. In addition, the work of Leonard Hill, Hunterian Professor of the Royal College of Surgeons in England, was very influential (19). His eminence as a physiologist overshadowed the inadequacy of his own experiments that led him to conclude that no relationship existed between brain function and brain circulation.
There was no serious challenge to Leonard Hill’s views until a remarkable clinical study was reported by John Fulton in the 1928 issue of the journal Brain (86). At the time of the report Fulton was a neurosurgery resident under Harvey Cushing at the Peter Bent Brigham Hospital in Boston. A patient presented to Cushing’s service with gradually decreasing vision caused by an arteriovenous malformation of the occipital cortex. Surgical removal of the malformation was attempted but unsuccessful, leaving the patient with a bony defect over the primary visual cortex. Fulton elicited a history of a cranial bruit audible to the patient whenever he engaged in a visual task. Based on this history Fulton pursued a detailed investigation of the behavior of the bruit that he could auscultate and record over occipital cortex. Remarkably consistent changes in the character of the bruit could be appreciated depending upon the visual activities of the patient. Although opening the eyes produced only modest increases in the intensity of the bruit, reading produced striking increases. The changes in cortical blood flow related to the complexity of the visual task and the attention of the subject to that task anticipated findings and concepts that have only recently been addressed with modern functional imaging techniques (20).
At the close of World War II, Seymour Kety and his colleagues opened the next chapter in studies of brain circulation and metabolism. Working with Lou Sokoloff and others, Kety developed the first quantitative methods for measuring whole brain blood flow and metabolism in humans. The introduction of an in vivo tissue autoradiographic measurement of regional blood flow in laboratory animals by Kety’s group (21, 22) provided the first glimpse of quantitative changes in blood flow in the brain related directly to brain function. Given the later importance of derivatives of this technique to functional brain imaging with both PET and functional MRI (fMRI) it is interesting to note the (dis)regard the developers had for this technique as a means of assessing brain functional organization. Quoting from the comments of William Landau to the members of the American Neurological Association meeting in Atlantic City (21): “Of course we recognize that this is a very secondhand way of determining physiological activity; it is rather like trying to measure what a factory does by measuring the intake of water and the output of sewage. This is only a problem of plumbing and only secondary inferences can be made about function. We would not suggest that this is a substitute for electrical recording in terms of easy evaluation of what is going on.” With the introduction of the deoxyglucose technique for the regional measurement of glucose metabolism in laboratory animals (23) and its later adaptation for PET (24), enthusiasm was much greater for the potential of such measurements to enhance our knowledge of brain function (1).
Soon after Kety and his colleagues introduced their quantitative methods for measuring whole brain blood flow and metabolism in humans, David Ingvar, Neils Lassen and their Scandinavian colleagues introduced methods applicable to humans that permitted regional blood flow measurements to be made by using scintillation detectors arrayed like a helmet over the head (25). They demonstrated directly in normal human subjects that blood flow changed regionally during changes in brain functional activity. The first study of functionally induced regional changes in blood flow by using these techniques in normal humans was actually reported by Ingvar and Risberg (26) at an early meeting on brain blood and metabolism and was greeted with cautious enthusiasm and a clear sense of its potential importance for studies of human brain function by Seymour Kety (27). However, despite many studies of functionally induced changes in regional cerebral blood that followed (1, 28), this approach was not embraced by most neuroscientists or cognitive scientists. It is interesting to note that this indifference was to disappear almost completely in the 1980s, a subject to which we will return shortly.
In 1973 Godfrey Hounsfield (29) introduced x-ray computed tomography, a technique based on principles presented in 1963 by Alan Cormack (30, 31). Overnight the way in which we looked at the human brain changed. Immediately, researchers envisioned another type of tomography, PET, which created in vivo autoradioagrams of brain function (32, 33). A new era of functional brain mapping began. The autoradiographic techniques for the measurement of blood flow (21, 22) and glucose metabolism (23) in laboratory animals could now be performed safely in humans (24, 34). In addition, quantitative techniques were developed (35, 36) and, importantly, validated (36, 37) for the measurement of oxygen consumption.
Soon it was realized that highly accurate measurements of brain function in humans could be performed with PET (38). Although this could be accomplished with either measurements of blood flow or metabolism (1), blood flow became the favored technique because it could be measured quickly (<1 min) by using an easily produced radiopharmaceutical (H215O) with a short half life (123 sec) that allowed many repeat measurements in the same subject.
The study of human cognition with PET was aided greatly by the involvement of cognitive psychologists in the 1980s whose experimental designs for dissecting human behaviors by using information-processing theory fit extremely well with the emerging functional brain imaging strategies (38). It may well have been the combination of cognitive science and systems neuroscience with brain imaging that lifted this work from a state of indifference and obscurity in the neuroscience community in the 1970s to its current role of prominence in cognitive neuroscience.
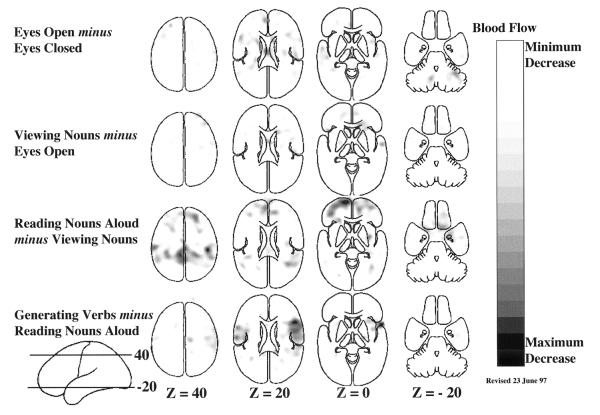
As a result of collaboration among neuroscientists, imaging scientists, and cognitive psychologists, a distinct behavioral strategy for the functional mapping of neuronal activity emerged. This strategy was based on a concept introduced by the Dutch physiologist Franciscus C. Donders in 1868 (reprinted in ref. 39). Donders proposed a general method to measure thought processes based on a simple logic. He subtracted the time needed to respond to a light (say, by pressing a key) from the time needed to respond to a particular color of light. He found that discriminating color required about 50 msec. In this way, Donders isolated and measured a mental process for the first time by subtracting a control state (i.e., responding to a light) from a task state (i.e., discriminating the color of the light). An example of the manner in which this strategy has been adopted for functional imaging is illustrated in Fig. 1.
Figure 1.
Four different hierarchically organized conditions are represented in these mean blood flow difference images obtained with PET. All of the changes shown in these images represent increases over the control state for each task. A group of normal subjects performed these tasks involving common English nouns (40, 83, 84) to demonstrate the spatially distributed nature of the processing by task elements going on in the normal human brain during a simple language task. Task complexity was increased from simply opening the eyes (row 1) through passive viewing of nouns on a television monitor (row 2); reading aloud the nouns as they appear on the screen (row 3); and saying aloud an appropriate verb for each noun as it appeared on the screen (row 4). These horizontal images are oriented with the front of the brain on top and the left side to the reader’s left. The markings “Z = 40” indicate millimeters above and below a horizontal plane through the brain marked “Z = 0”.
One criticism of this approach has been that the time necessary to press a key after a decision to do so has been made is affected by the nature of the decision process itself. By implication, the nature of the processes underlying key press, in this example, may have been altered. Although this issue (known in cognitive science jargon as the assumption of pure insertion) has been the subject of continuing discussion in cognitive psychology, it finds its resolution in functional brain imaging, where changes in any process are directly signaled by changes in observable brain states. Events occurring in the brain are not hidden from the investigator as in the purely cognitive experiments. Careful analysis of the changes in the functional images reveals whether processes (e.g., specific cognitive decisions) can be added or removed without affecting ongoing processes (e.g., motor processes). Processing areas of the brain that become inactive during the course of a particular cognitive paradigm are illustrated in Fig. 2. By examining the images in Figs. 1 and 2 together, a more complete picture emerges of the changes taking place in the cognitive paradigm illustrated together in these two figures. Clearly, some areas of the brain active at one stage in a hierarchically designed paradigm can become inactive as task complexity is increased. Although changes of this sort are hidden from the view of the cognitive scientist they become obvious when brain imaging is employed.
Figure 2.
Hierarchically organized subtractions involving the same task conditions as shown in Fig. 1 with the difference being that these images represent areas of decreased activity in the task condition as compared with the control condition. Note that the major decreases occurred when subjects read the visually presented nouns aloud as compared with viewing them passively as they appeared on the television monitor (row 3); and when they said aloud an appropriate verb for each noun as it appeared on the television monitor as compared with reading the noun aloud (row 4). Combining the information available in Figs. 1 and 2 provides a fairly complete picture of the interactions between tasks and brain systems in hierarchically organized cognitive tasks when studied with functional brain imaging.
A final caveat with regard to certain cognitive paradigms is that the brain systems involved do not necessarily remain constant through many repetitions of the task. Although simple habituation might be suspected when a task is tedious, this is not the issue referred to here. Rather, when a task is novel and, more importantly, conflicts with a more habitual response to the presented stimulus, major changes can occur in the systems allocated to the task. A good example relates to the task shown in Figs. 1 and 2 (row 4) where subjects are asked to generate an appropriate verb for visually presented nouns rather than simply read the noun aloud as they had been doing (40). In this task, regions uniquely active when the task is first performed (Fig. 1, row 4 and Fig. 3, row 1) are replaced by regions active when the task has become well practiced (Fig. 3, row 2). Such changes have both practical and theoretical implications when it comes to the design and interpretation of cognitive activation experiments. Functional brain imaging obviously provides a unique perspective that is unavailable in the purely cognitive experiment.
Figure 3.
Practice-induced changes in brain systems involve both the disappearance of activity in systems initially supporting task performance (row 1) and the appearance of activity in other systems concerned with practiced performance (row 2). In this example, generating verbs aloud for a visually presented nouns (see also row 4 of Figs. 1 and 2 for changes during the naïve performance of the task), subjects acquired proficiency on the task after 10 min of practice. This improved performance was associated with a disappearance of activity in areas of frontal and temporal cortex and the right cerebellum (row 1) and the appearance of activity in Sylvian-insular and occipital cortex (row 2). These images were created by subtracting the naïve performance of verb generation from the practiced performance of the task. More details on these changes can be obtained from Raichle et al. (40).
Finally, another technology emerged contemporaneously with PET and computed tomography. This was MRI. MRI is based on yet another set of physical principles that have to do with the behavior of hydrogen atoms or protons in a magnetic field. These principles were discovered independently by Felix Block (41) and Edward Purcell and his colleagues in 1946 (42) and expanded to imaging by Paul Lauterbur in 1973 (43). Initially MRI provided superb anatomical information but inherent in the data also was important metabolic and physiological information. An opening for MRI in the area of functional brain imaging emerged when it was discovered that during changes in neuronal activity there are local changes in the amount of oxygen in the tissue (2, 3). By combining this observation with a much earlier observation by Pauling and Coryell (44) that changing the amount of oxygen carried by hemoglobin changes the degree to which hemoglobin disturbs a magnetic field, Ogawa et al. (4) were able to demonstrate that in vivo changes blood oxygenation could be detected with MRI. The MRI signal (technically known as T2* or “tee-two-star”) arising from this unique combination of brain physiology (2) and nuclear magnetic resonance physics (44, 45) became known as the blood-oxygen-level-dependent or BOLD signal (4). There quickly followed several demonstrations of BOLD signal changes in normal humans during functional brain activation (5–8) giving birth to the rapidly developing field of fMRI.
In the discussion to follow it is important to keep in mind that when a BOLD signal is detected blood flow to a region of brain has changed out of proportion to the change in oxygen consumption (46). When blood flow changes more than oxygen consumption, in either direction, there is a reciprocal change in the amount of deoxyhemoglobin present locally in the tissue changing the local magnetic field properties. As you will see, both increases and decreases occur in the BOLD signal in the normal human brain.
Metabolic Requirements of Cognition
Although many had assumed that behaviorally induced increases in local blood flow would be reflected in local increases in the oxidative metabolism of glucose (10), evidence from brain imaging studies with PET (2, 3) and fMRI (46) have indicated otherwise. Fox and his colleagues (2, 3) demonstrated that in normal, awake adult humans, stimulation of the visual or somatosensory cortex results in dramatic increases in blood flow but minimal increases in oxygen consumption. Increases in glucose utilization occur in parallel with blood flow (3, 12), an observation fully anticipated by the work of others (23, 47). However, changes in blood flow and glucose utilization were much in excess of the changes in oxygen consumption, an observation contrary to most popularly held notions of brain energy metabolism (10). These results suggested that the additional metabolic requirements associated with increased neuronal activity might be supplied largely through glycolysis alone.
Another element of the relationship between brain circulation and brain function that was not appreciated before the advent of functional brain imaging was that regional blood flow and the fMRI BOLD signal not only increase in some areas of the brain appropriate to task performance but also decrease from a resting baseline in other areas (48) as shown in Fig. 2. An appreciation of how these decreases arise in the context of an imaging experiment is diagrammatically represented in Fig. 4. The possible physiological implications of these changes are discussed below.
Figure 4.
Functional images obtained with PET and fMRI represent comparisons between two conditions usually referred to as a control state and a task state. The task state is designed to contain specific mental operations of interest. Because the task state invariably contains additional mental operations not of interest, a control state is selected which contains those operations to be ignored yet does not contain the operations of interest in the task state. Depending on the actual changes in brain activity in each state and the comparison made between states, the resulting changes depicted in the functional image will have either a positive (Fig. 1) or negative (Fig. 2) sign. This figure is designed to illustrate how the sign (i.e., positive or negative change) arises from the primary image data. Absolute changes (Absolute Magnitudes) are represented on the left for a hypothetical area in the brain as monitored by either PET or fMRI. The horizontal axis on the left represents four states studied in the course of a hypothetical imaging experiment. An Absolute Magnitude above the horizontal axis (A) represents an increase over the other states studied whereas an Absolute Magnitude below this axis (B) represents a decrease. The comparisons (i.e. 2-1, 3-2, and 4-3) leading to the functional images themselves are shown on the right (Difference Magnitudes). It should be appreciated from this figure that the sign of the change in the functional image is dependent on both the change in activity within an area during a particular task (Absolute Magnitudes) and the particular comparison subsequently made between states (Difference Magnitudes). These general principles should be kept in mind when evaluating data of the type shown in Figs. 1–3.
Physiologists have long recognized that individual neurons in the cerebral cortex can both increase or decrease their activities from a resting, baseline firing pattern depending on task conditions. Examples abound in the neurophysiological literature (49). A parsimonious view of these decreases in neuronal activity is that they reflect the activity of inhibitory interneurons acting within local neuronal circuits of the cerebral cortex. Because inhibition is energy requiring (50), it is impossible to distinguish inhibitory from excitatory cellular activity on the basis of changes in either blood flow or metabolism. Thus, on this view a local increase in inhibitory activity would be as likely to increase blood flow and the fMRI BOLD signal as would a local increase in excitatory activity. How, then, might decreases in blood flow or the fMRI BOLD signal arise?
To understand the possible significance of the decreases in blood flow in functional imaging studies it is important to distinguish two separate conditions in which they might arise‡. The less interesting and more usually referred to circumstance arises when two images are compared in which one contains a regional increase in blood flow caused by some type of task activity (e.g., let us consider hand movement that produces increases in contralateral motor cortex blood flow) and a control image that does not (i.e., in this example, no hand movement). In our example, subtracting the image associated with no hand movement from the image associated with hand movement reveals the expected increase in blood flow in motor cortex. Simply reversing the subtraction produces an image with a decrease in the same area. Although this example may seem trivial and obvious, such subtraction reversals are often presented in the analysis of very complex tasks and in such a manner as to be quite confusing even to those working the field. A diagrammatic representation of how this occurs is presented in Fig. 4.
The second circumstance (Fig. 4) in which decreases in blood flow and the fMRI BOLD signal appear is not caused by the above type of data manipulations (i.e., an active task image subtracted from a passive state image). Rather, blood flow and the fMRI BOLD signal actually decrease from the passive baseline state (i.e., the activity in a region of brain has not been first elevated by a task). The usual baseline conditions from which this occurs consist of lying quietly but fully awake in an MRI or PET scanner with eyes closed or passively viewing a television monitor and its contents, be it a fixation point or even a more complex stimulus (Fig. 2, row 3). In the examples discussed by Shulman et al. (48), areas of the medial orbital frontal cortex, the posterior cingulate cortex, and precuneus consistently showed decreased blood flow when subjects actively processed a wide variety of visual stimuli as compared with a passive baseline condition (compare with the example shown in Fig. 2).
The hypothesis one is led to consider, regarding these rather large area reductions in blood flow, is that a large number of neurons reduce their activity together (for one of the few neurophysiological references to such a phenomenon, see ref. 52). Such group reductions could not be mediated by a local increase in the activity of inhibitory interneurons as this would be seen as an increase in activity by PET and fMRI. Rather, such reductions are likely mediated through the action of diffuse projecting systems like dopamine, norepinephrine, and serotonin or a reduction in thalamic inputs to the cortex. The recognition of such changes probably represents an important contribution of functional brain imaging to our understanding of cortical function and should stimulate increased interest in the manner in which brain resources are allocated on a large systems level during task performance.
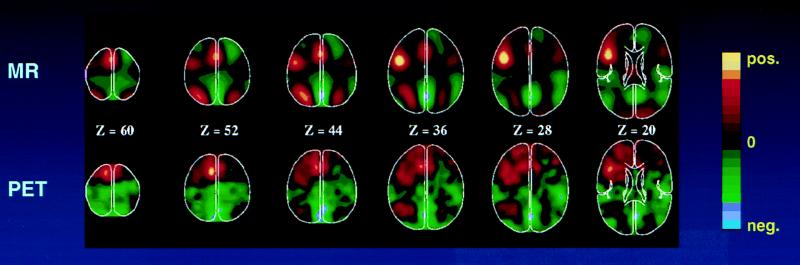
The metabolic accompaniments of these functionally induced decreases in blood flow from a passive baseline condition were not initially explored, and it was tacitly assumed that such reductions would probably be accompanied by coupled reductions in oxygen consumption. Therefore, it came as a surprise that the fMRI BOLD signal, based on tissue oxygen availability, detected both increases and decreases during functional activation (Fig. 5). Decreases in the BOLD signal during a task state as compared with a passive, resting state have been widely appreciated by investigators using fMRI although, surprisingly, no formal publications on the subject have yet to appear.
Figure 5.
fMRI (Upper) of the BOLD signal (4) and PET (Lower) images of blood flow change. These images were obtained during the performance of a task in which subjects viewed three letter word stems and were asked to speak aloud (PET) or think silently (fMRI) the first word to come to mind whose first three letters corresponded to the stems [e.g., see cou, say or think couple (85)]. The color scale employed in these images shows activity increases in reds and yellows and activity decreases in greens and blues. Note that both PET and fMRI show similar increases as well as decreases. The fMRI images were blurred to the resolution of the PET images (18 mm full width at half maximum) to facilitate comparison.
Complementing these observations from functional brain imaging on the relationship between oxygen consumption and blood flow during decreases are earlier quantitative metabolic studies of a phenomenon known as cerebellar diaschisis (53, 54). In this condition, there is a reduction in blood flow and metabolism in the hemisphere of the cerebellum contralateral to an injury to the cerebral cortex, usually a stroke. Of particular interest is the fact that blood flow is reduced significantly more than oxygen consumption (53, 54). The changes in the cerebellum are thought to reflect a reduction in neuronal activity within the cerebellum due to reduced input from the cerebral cortex. One can reasonably hypothesize that similar, large-scale reduction in systems level activity are occurring during the course of normal functional brain activity (48).
Taken together the data we have at hand suggest that blood flow changes more than oxygen consumption in the face of increases as well as decreases in local neuronal activity (Fig. 6). Glucose utilization also changes more than oxygen consumption during increases in brain activity (we presently have no data on decreases in glucose utilization) and may equal the changes in blood flow in both magnitude and spatial extent (3, 12) Although surprising to many, these results were not entirely unanticipated.
Figure 6.
A summary of currently available data on the relationship of blood flow, glucose utilization, and oxygen consumption to the cellular activity of the brain during changes in functional activity is shown in this figure. The changes occurring in blood flow and glucose utilization exceed changes in oxygen consumption. The degree to which oxygen consumption actually changes, if at all, remains to be determined. PET measures the changes in blood flow. fMRI measures a BOLD (4) signal or contrast that arises when changes in blood flow exceed changes in tissue oxygen consumption.
Experimental studies of epilepsy in well-oxygenated, passively ventilated experimental animals§ (56) had indicated that blood flow increased in excess of the oxygen requirements of the tissue. During the increased neuronal activity of a seizure, discharge increase in the brain venous oxygen content was routinely observed (56). Because of the increase in blood pressure associated with the seizure discharge, the fact that blood flow exceeded the oxygen requirements of the tissue was attributed to a loss of cerebral autoregulation (56). A similar concern was expressed about equally prescient experiments involving brain blood flow changes during sciatic nerve stimulation in rodents (57, 58). However, experiments by Ray Cooper and his colleagues largely circumvented that concern (59, 60). They demonstrated that oxygen availability measured locally in the cerebral cortex of awake patients undergoing surgery for the treatment of intractable epilepsy increased during changes in behavioral activity (e.g., looking at pictures, manual dexterity, reading). These changes in oxygen availability occurred in the absence of any change in blood pressure and were observed during normal brain function in humans. Surprisingly, these observations were largely ignored until the work of Fox and his colleagues called attention to the phenomenon in normal human subjects with PET (2, 3).
Interpretation of these blood flow–metabolism relationships during changes in functional brain activity are presently controversial. Several schools of thought have emerged. One hypothesis that addresses the role of glycolysis in brain functional activation is most eloquently articulated by Pierre Magistretti and colleagues based on their work with cultured astrocytes (61, 62). In this theory, increases in neuronal activity stimulated by the excitatory amino acid transmitter glutamate result in relatively large increases in glycolytic metabolism in astrocytes. The energy supplied through glycolysis in the astrocyte is used to metabolize glutamate to glutamine before being recycled to neurons. Coupled with estimates that increased firing rates of neurons require little additional energy over and above that required for the normal maintenance of ionic gradients (63) leads to the hypothesis that the primary metabolic change associated with changes (at least increases) in neuronal activity are glycolytic and occur in astrocytes.
In somewhat greater detail, neuronal activation results in sodium ion influx and potassium efflux. This is accompanied by an influx of protons into neurons, initially alkalinizing the extracellular space, which results in alkalinization of the astrocyte (64). Alkalinization of the astrocyte results in stimulation of glycolysis (65) with the breakdown of glycogen (66) and the production of both pyruvate and lactate in excess of astrocyte metabolic needs and despite normal tissue oxygenation. The lactate can then leave the astrocyte and be taken up by neurons to be oxidatively metabolized by neurons (67). Because glucose metabolism exceeds oxygen consumption during increases in neuronal activity (3) another fate for lactate must also be sought. This might possibly occur through enhanced removal from the brain by flowing blood, a hypothesis for which we presently have only indirect evidence (68, 69), or re-incorporation into astrocytic glycogen (70).
Additional support for this hypothesis comes from in vivo observations that increases in neuronal activity are associated with glycogenolysis in astrocytes (71), a convenient source of readily available energy for such a process, located in a cell uniquely equipped enzymatically for the process (62, 71). Finally, measurements of tissue lactate with MRS in humans (72) and with substrate-induced bioluminescence in laboratory animals (73) has shown localized increases in tissue lactate during physiologically induced increases in neuronal activity.
Not surprisingly, the above hypothesis has been challenged and alternatives offered to explain the observed discrepancy between changes in blood flow and glucose utilization, which appear to change in parallel, and oxygen consumption, which changes much less than either. One suggestion is that the observed discrepancy is transient (74). Measuring brain glucose and lactate concentrations and blood oxygenation with MRI and MRS in normal human volunteers, Frahm et al. (74) observed a rise in visual cortex lactate concentration that peaked after 3 min of visual stimulation and returned to baseline after 6 min of continuous stimulation. During this same period of time blood oxygen concentration was initially elevated but also returned to baseline by the end of the stimulation period. In a complementary study Hyder et al. (75) similarly suggest, on the basis of MRS studies of anesthetized rats during forepaw stimulation that “oxidative CMRGlu supplies the majority of energy during sustained brain activation.” However, in a very careful study of this question by Bandettini et al. (76) in awake humans, they conclude from their own data and a careful analysis of the literature that BOLD signal changes and blood flow remain elevated during prolonged periods of brain activation provided that there is no habituation to the presented stimulus. This conclusion is entirely consistent with the original observations of Fox and Raichle (2).
Another popular hypothesis is based on optical imaging work of physiologically stimulated visual cortex by Malonek and Grinvald (77). In their work they measure changes in reflected light from the surface of visual cortex in anesthetized cats. By using wavelengths of light sensitive to deoxyhemoglobin and oxyhemoglobin they note an almost immediate increase in deoxyhemoglobin concentration followed, after a brief interval, by an increase in oxyhemoglobin which, although centered at the same location as the change in deoxyhemoglobin, is greater in magnitude and extends over a much larger area of the cortex than do the changes in deoxyhemoglobin (77). They interpret these results to mean that increases in neuronal activity are associated with highly localized increases in oxygen consumption which stimulate a vascular response, delayed by several seconds, that is large in relation to both the magnitude of the increase in oxygen consumption and the area of cerebral cortex that is actually active. In other words, by their theory increases in neuronal activity in the cerebral cortex are, actually, associated with increased oxidative metabolism of glucose. Because the blood flow response to the change in neuronal activity is relatively slow, oxygen reserves in the area of activation are temporarily depleted. When the blood flow response does occur, after a delay of 1–3 sec, it exceeds the needs of the tissue, delivering to the active area of cortex and its surroundings oxygen in excess of metabolic needs. This hypothesis has stimulated interest in the use of high field strength MRI systems to detect the initial oxygen depletion predicted by the small increases in deoxyhemoglobin (78). The hope would be that both spatial and temporal resolution of fMRI would be improved by focusing on this postulated early and spatially confined event.
Support for the hypothesis of Malonek and Grinvald (77) comes from theoretical work by Buxton and Frank (79). In their modeling work they show that in an idealized capillary tissue cylinder in the brain, an increase in blood flow in excess of the increased oxygen metabolic demands of the tissue is needed to maintain proper oxygenation of the tissue. This finding results from the poor diffusivity and solubility of oxygen in brain tissue. In this theory, blood flow remains coupled to oxidative metabolism but in a nonlinear fashion designed to overcome the diffusion and solubility limitations of oxygen in brain tissue to maintain adequate tissue oxygenation.
Although the hypothesis that reactive hyperemia is a normal and necessary consequence of increased neuronal activity merits careful consideration, several observations remain unexplained. First, it does not account for the increased glucose utilization that parallels the change in blood flow observed in normal humans (3, 12) and laboratory animals (73, 80, 81). Second, it does not agree with the observations of Woolsey et al. (80) as well as others (81) who have demonstrated a remarkably tight spatial relationship between changes in neuronal activity within a single, rat whisker barrel and the response of the vascular supply as well as glucose metabolism to that barrel. There is little evidence in these studies for spatially diffuse reactive hyperemia surrounding the stimulated area of cortex. Third, in the paper by Malonek and Grinvald (77), the initial rise in deoxyhemoglobin seen with activation is not accompanied by a fall in oxyhemoglobin as would be expected with a sudden rise in local oxygen consumption that precedes the onset of increased oxygen delivery to the tissue. In the presence of somewhat conflicting evidence on capillary recruitment in brain (80–82), which could explain this observation, we should exercise caution in accepting uncritically the data of Malonek and Grinvald (77) until an explanation for this particular discrepancy is found and better concordance is achieved with other experiments. Clearly, more information is needed on the exact nature of the microvascular events surrounding functional brain activation. Finally, we are left without an explanation for the observation that when blood flow decreases below a resting baseline during changes in the functional activity of a region of the brain (Fig. 2), a negative BOLD signal arises due to the fact that blood flow decreases more than the oxygen consumption (Figs. 5 and 6).
One final caveat should be mentioned. From the perspective of this review it would be easy to assume that because blood flow and glucose utilization appear to increase together and more than oxygen utilization during increases in neuronal activity, the increase in blood flow serves to deliver needed glucose. Recent data from Powers et al. (82) suggest otherwise. They noted no change in the magnitude of the normalized regional blood flow response to physiological stimulation of the human brain during stepped hypoglycemia. They concluded that the increase in blood flow associated with physiological brain activation was not regulated by a mechanism that matched local cerebral glucose supply to local cerebral glucose demand (82).
So what are we to conclude at this point in time? Any theory designed to explain functional brain imaging signals must accommodate three observations (Fig. 6). First, local increases and decreases in brain activity are reliably accompanied by changes in blood flow. Second, these blood flow changes exceed any accompanying change in the oxygen consumption. If this were not the case, fMRI based on the BOLD signal changes could not exist. Third, while paired data on glucose metabolism and blood flow are limited, they suggest that blood flow changes are accompanied by changes in glucose metabolism of approximately equal magnitude and spatial extent.
Several additional factors must be kept in mind in the evaluation of extant data and the design of future experiments. Anesthesia, a factor present in many of the animal experiments discussed in this review, may well have a significant effect on the relationships among blood flow, metabolism, and cellular activity during brain activation. Also, habituation of cellular activity to certain types of stimuli (74, 76) as well as rapid, practice-induced shifts in the neuronal circuitry used for the performance of a task (Fig. 3) may well complicate the interpretation of resulting data if overlooked in experiments designed in investigate these relationships.
Presently we do not know why blood flow changes so dramatically and reliably during changes in brain activity or how these vascular responses are so beautifully orchestrated. These questions have confronted us for more than a century and remain incompletely answered. At no time have answers been more important or intriguing than presently because of the immense interest focused on them by the use of functional brain imaging with PET and fMRI. We have at hand tools with the potential to provide unparalleled insights into some of the most important scientific, medical, and social questions facing mankind. Understanding those tools is clearly a high priority.
Acknowledgments
I would like to acknowledge many years of generous support from the National Institute of Neurological Disorders and Stroke; the National Heart, Lung, and Blood Institute; The McDonnell Center for Studies of Higher Brain Function at Washington University; as well as The John D. and Katherine T. MacArthur Foundation and The Charles A. Dana Foundation.
ABBREVIATIONS
- PET
positron emission tomography
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- fMRI
functional MRI
- BOLD
blood-oxygen-level-dependent
Footnotes
Some have wondered whether these reductions in blood flow are merely the hemodynamic consequence of increases elsewhere (i.e., an intracerebral steal phenomenon). Such a hypothesis is very unlikely to be correct because of the tremendous hemodynamic reserve of the brain (51) and also because there is no one to one spatial or temporal correlation between increases and decreases (e.g., see Fig. 1 and 2).
Wilder Penfield is frequently given credit for the observation that venous oxygenation increases during a seizure discharge (i.e., so-called “red veins on the cortex”). Careful reading of his many descriptions of the cortical surface of the human brain during a seizure fail to disclose such a description. Rather, he describes quite clearly the infrequent appearance of arterial blood locally in pial veins after a focal cortical seizure (55): “… the almost invariable objective alteration in the exposed hemisphere coincident with the onset of the fit is a cessation of pulsation in the brain” (page 607).
References
- 1.Raichle M E. In: Handbook of Physiology: The Nervous System V: Higher Functions of the Brain. Plum F, editor. Bethesda, MD: Am. Physiol. Soc.; 1987. pp. 643–674. [Google Scholar]
- 2.Fox P T, Raichle M E. Proc Natl Acad Ssi USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox, P. T., Raichle, M. E., Mintun, M. A. & Dence, C. (1988) Science 241. [DOI] [PubMed]
- 4.Ogawa S, Lee T M, Kay A R, Tank D W. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa S, Tank D W, Menon R, Ellermann J M, Kim S-G, Merkle H, Ugurbil K. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwong K, Belliveau J W, Chesler D A, Goldberg I E, Weisskoff R M, Poncelet B P, Dennedy D N, Hoppel B E, Cohen M S, Turner R, Cheng H-M, Brady T J, Rosen B R. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandettini P A, Wong E C, Hinks R S, Tikofsky R S, Hyde J S. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 8.Frahm J, Bruhn H, Merboldt K-D, Hanicke W. J Magn Reson Imaging. 1992;2:501–505. doi: 10.1002/jmri.1880020505. [DOI] [PubMed] [Google Scholar]
- 9.Roy C S, Sherrington C S. J Physiol (London) 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siesjo B K. Brain Energy Metabolism. New York: Wiley; 1978. [Google Scholar]
- 11.Fox P T, Raichle M E. Proc Natl Acad Sci USA. 1988;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blomqvist G, Seitz R J, Sjogren I, Halldin C, Stone-Elander S, Widen L, Solin O, Haaparanta M. Acta Physiol Scand. 1994;151:29–43. doi: 10.1111/j.1748-1716.1994.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 13.James W. Principles of Psychology. New York: Henry Holt; 1890. pp. 97–99. [Google Scholar]
- 14.Mosso A. Ueber den Kreislauf des Blutes im Menschlichen Gehirn. Leipzig: von Veit; 1881. [Google Scholar]
- 15.Broca P. Bull Acad Med (Paris) 1879;2S:1331–1347. [Google Scholar]
- 16.Broca P. Bull Soc Anatomique (Paris) 1861;6:330–357. , 398–407. [Google Scholar]
- 17.Mosso A. La Temperature del Cervello. Milan; 1894. [Google Scholar]
- 18.Berger H. Zur Lehre von der Blutzirkulation in der Schandelhohle des Menschen. Fischer, Jena, Germany: von Gustav; 1901. [Google Scholar]
- 19.Hill L. The Physiology and Pathology of the Cerebral Circulation: An Experimental Research. London: Churchill; 1896. [Google Scholar]
- 20.Shulman G L, Corbetta M, Buckner R L, Raichle M E, Fiez J A, Miezin F M, Petersen S E. Cereb Cortex. 1997a;7:193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- 21.Landau W M, Freygang W H, Jr, Roland L P, Sokoloff L, Kety S. Trans Am Neurol Assoc. 1955;80:125–129. [PubMed] [Google Scholar]
- 22.Kety S. Methods Med Res. 1960;8:228–236. [Google Scholar]
- 23.Sokoloff L, Reivich M, Kennedy C, Des Rosiers M H, Patlak C S, Pettigrew K D, Sakurada O, Shinohara M. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 24.Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Hoffman E, Alavi A, Sokoloff L. Circ Res. 1979;44:127–137. doi: 10.1161/01.res.44.1.127. [DOI] [PubMed] [Google Scholar]
- 25.Lassen N A, Hoedt-Rasmussen K, Sorensen S C, Skinhoj E, Cronquist B, Bodforss E, Ingvar D H. Neurology. 1963;13:719–727. doi: 10.1212/wnl.13.9.719. [DOI] [PubMed] [Google Scholar]
- 26.Ingvar G H, Risberg J. Acta Neurol Scand Suppl. 1965;14:183–186. doi: 10.1111/j.1600-0404.1965.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 27.Kety S. Acta Neurol Scand Suppl. 1965;14:197. doi: 10.1111/j.1600-0404.1965.tb01947.x. (abstr.). [DOI] [PubMed] [Google Scholar]
- 28.Lassen N A, Ingvar D H, Skinhoj E. Sci Am. 1978;239:62–71. doi: 10.1038/scientificamerican1078-62. [DOI] [PubMed] [Google Scholar]
- 29.Hounsfield G N. Br J Radiol. 1973;46:1016–1022. doi: 10.1259/0007-1285-46-552-1016. [DOI] [PubMed] [Google Scholar]
- 30.Cormack A M. J Appl Phys. 1963;34:2722–2727. [Google Scholar]
- 31.Cormack A M. Phys Med Biol. 1973;18:195–207. doi: 10.1088/0031-9155/18/2/003. [DOI] [PubMed] [Google Scholar]
- 32.Ter-Pogossian M M, Phelps M E, Hoffman E J, Mullani N A. Radiology. 1975;114:89–98. doi: 10.1148/114.1.89. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman E J, Phelps M E, Mullani N A, Higgins C S, Ter-Pogossian M M. J Nucl Med. 1976;17:493–502. [PubMed] [Google Scholar]
- 34.Raichle M E, Martin W R W, Herscovitch P, Mintun M A, Markham J. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 35.Frackowiak R S J, G L, L, Jones T, Heather J D. J Comput Tomogr. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Mintun M A, Raichle M E, Martin W R W, Herscovitch P. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- 37.Altman D I, Lich L L, Powers W J. J Nucl Med. 1991;32:1738–1741. [PubMed] [Google Scholar]
- 38.Posner M I, Raichle M E. Images of Mind. New York: Freeman; 1994. [Google Scholar]
- 39.Donders F C. Acta Psychol. 1969;30:412–431. doi: 10.1016/0001-6918(69)90065-1. [DOI] [PubMed] [Google Scholar]
- 40.Raichle M E, Fiez J A, Videen T O, MacLeod A M, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 41.Block F. Physiol Rev. 1946;70:460–474. [Google Scholar]
- 42.Purcell E M, Torry H C, Pound R V. Physiol Rev. 1946;69:37. [Google Scholar]
- 43.Lauterbur P. Nature (London) 1973;242:190–191. [Google Scholar]
- 44.Pauling L, Coryell C D. Proc Natl Acad Sci USA. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thulborn K R, Waterton J C, Matthews P M, Radda G K. Biochim Biophys Acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 46.Kim S G, Ugurbil K. Magn Reson Med. 1997;38:59–65. doi: 10.1002/mrm.1910380110. [DOI] [PubMed] [Google Scholar]
- 47.Yarowsky P, Kadekaro M, Sokoloff L. Proc Natl Acad Sci USA. 1983;80:4179–4183. doi: 10.1073/pnas.80.13.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 49.Georgopoulos A P, Kalaska J F, Caminiti R, Massey J T. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ackerman R F, Finch D M, Babb T L, Engel J., Jr J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heistad D D, Kontos H A. In: Handbook of Physiology: The Cardiovascular System. Sheppard J T, Abboud F M, editors. Vol. 3. Bethesda, MD: Am. Physiol. Soc.; 1983. pp. 137–182. [Google Scholar]
- 52.Creutzfeldt O, Ojemann G, Lettich E. Exp Brain Res. 1989;77:451–475. doi: 10.1007/BF00249600. [DOI] [PubMed] [Google Scholar]
- 53.Martin W R, Raichle M E. Ann Neurol. 1983;14:168–176. doi: 10.1002/ana.410140203. [DOI] [PubMed] [Google Scholar]
- 54.Yamauchi H, Fukuyama H, Kimura J. Stroke. 1992;23:855–860. doi: 10.1161/01.str.23.6.855. [DOI] [PubMed] [Google Scholar]
- 55.Penfield W. Res Pub Assoc Res Nervous Mental Disorder. 1937;18:605–637. [Google Scholar]
- 56.Plum F, Posner J B, Troy B. Arch Neurol. 1968;18:1–3. doi: 10.1001/archneur.1968.00470310015001. [DOI] [PubMed] [Google Scholar]
- 57.Howse D C, Plum F, Duffy T E, Salford L G. Trans Am Neurol Assoc. 1973;98:153–155. [PubMed] [Google Scholar]
- 58.Salford L G, Duffy T E, Plum F. In: Cerebral Circulation and Metabolism. Langfitt T W, McHenry L C, Reivich M, Wollman H, editors. New York: Springer; 1975. pp. 380–382. [Google Scholar]
- 59.Cooper R, Crow H J, Walter W G, Winter A L. Brain Res. 1966;3:174–191. doi: 10.1016/0006-8993(66)90075-8. [DOI] [PubMed] [Google Scholar]
- 60.Cooper R, Papakostopoulos D, Crow H J. In: Blood Flow and Metabolism in the Brain. Harper A M, Jennett W B, Miller J D, Rowan J O, editors. New York: Churchill Livingstone; 1975. pp. 14.8–14.9. [Google Scholar]
- 61.Tsacopoulos M, Magistretti P J. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bittar P G, Charnay Y, Pellerin L, Bouras C, Magistretti P. J Cereb Blood Flow Metab. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Creutzfeldt O D. In: Brain Work: The Coupling of Function, Metabolism and Blood Flow in the Brain. Ingvar D H, Lassen N A, editors. Copenhagen: Munksgaard; 1975. pp. 21–46. [Google Scholar]
- 64.Chesler M, Kraig R P. Am J Psychol. 1987;253:R666–R670. doi: 10.1152/ajpregu.1987.253.4.R666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hochachka P W, Mommsen T P. Science. 1983;219:1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- 66.Swanson R A, Morton M M, Sagar S M, Sharp F R. Neuroscience. 1992;51:451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- 67.Dringen R, Wiesinger H, Hamprecht B. Neurosci Lett. 1993a;163:5–7. doi: 10.1016/0304-3940(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 68.Knudsen G M, Paulson O B, Hertz M M. J Cereb Blood Flow Metab. 1991;11:581–586. doi: 10.1038/jcbfm.1991.107. [DOI] [PubMed] [Google Scholar]
- 69.Lear J L, Kasliwal R K. J Cereb Blood Flow Metab. 1991;11:576–589. doi: 10.1038/jcbfm.1991.106. [DOI] [PubMed] [Google Scholar]
- 70.Dringen R, Schmoll D, Cesar M, Hamprecht B. Biol Chem Hoppe-Seyler. 1993;374:343–347. doi: 10.1515/bchm3.1993.374.1-6.343. [DOI] [PubMed] [Google Scholar]
- 71.Harley C A, Bielajew C H. J Comp Neurol. 1992;322:377–389. doi: 10.1002/cne.903220307. [DOI] [PubMed] [Google Scholar]
- 72.Prichard J W, Rothman D L, Novotny E, Hanstock C C, Shulman R G. Proc Natl Acad Sci USA. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ueki M, Linn F, Hossmann K-A. J Cereb Blood Flow Metab. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- 74.Frahm J, Kruger G, Merboldt K-D, Kleinschmidt A. Magn Reson Med. 1996;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- 75.Hyder F, Chase J R, Behar K L, Mason G F, Rothman D L, Shulman R G. Proc Natl Acad Sci USA. 1996;93:7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bandettini P A, Kwong K K, Davis T L, Tootell R B H, Wong E C, Fox P T, Belliveau J W, Weisskoff R M, Rosen B R. Hum Brain Mapping. 1997;5:93–109. [PubMed] [Google Scholar]
- 77.Malonek D, Grinvald A. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- 78. Hu X, Le T H, Ugurbil K. Magn Reson Med. 1997;37:877–884. doi: 10.1002/mrm.1910370612. [DOI] [PubMed] [Google Scholar]
- 79.Buxton R B, Frank L R. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 80.Woolsey T A, Rovainen C M, Cox S B, Henegar M H, Liang G E, Liu D, Moskalenko Y E, Sui J, Wei L. Cereb Cortex. 1996;6:647–660. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- 81.Greenberg J H, Sohn N W, Hand P J. J Cereb Blood Flow Metab. 1997;17:S561. (abstr.). [Google Scholar]
- 82.Powers W J, Hirsch I B, Cryer P E. Am J Physiol. 1996;270:H554–H559. doi: 10.1152/ajpheart.1996.270.2.H554. [DOI] [PubMed] [Google Scholar]
- 83.Petersen S E, Fox P T, Posner M I, Mintum M, Raichle M E. Nature (London) 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 84.Petersen S E, Fox P T, Posner M I, Mintun M, Raichle M E. J Cognit Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- 85.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fulton J F. Brain. 1928;51:310–320. [Google Scholar]