Abstract
OBJECTIVE
Elevated plasma interleukin-18 (IL-18) has been linked to onset of diabetes mellitus (DM) and its complications. However, so far this association has been shown only in predominantly white populations. We examined IL-18 levels and their association with incident DM in a racially heterogeneous population.
RESEARCH DESIGN AND METHODS
In a nested case-cohort design representing a 9-year follow-up of 9,740 middle-aged, initially healthy, nondiabetic white and African American participants of the Atherosclerosis Risk in Communities Study, we selected and measured analytes on race-stratified (50% white, 50% African American) random samples of both cases of incident diabetes (n = 548) and eligible members of the full cohort (n = 536).
RESULTS
Baseline IL-18 levels were significantly higher in white participants compared with African American participants (P < 0.001). Although white participants in the fourth (versus first) quartile of IL-18 levels had a significant hazard ratio (HR) for developing DM (HR: 2.1, 95% CI: 1.3–3.4), after adjustment for age, sex, and study center, no difference was seen among African Americans (HR: 1.0, 95% CI: 0.6–1.7). Unlike those in African Americans, IL-18 levels in whites had a significant correlation with age (P < 0.01); anthropometric characteristics such as waist circumference (P < 0.001), height (P = 0.04), waist-to-hip ratio (P < 0.001), and BMI (P < 0.01); and total (P < 0.001) and high-molecular-weight (P < 0.001) adiponectin.
CONCLUSIONS
There are racial differences in levels of IL-18 and the association of IL-18 with risk factors and incident type 2 DM. In addition, there seems to be a complex interplay of inflammation and adiposity in the development of DM.
Diabetes and its complications lead to an enormous disease burden. It has been estimated that 366 million people worldwide will develop diabetes by the year 2030 (1). Studies have shown that parental history of diabetes, anthropometric measures, and systolic blood pressure (SBP), alone or combined with simple laboratory measures including fasting glucose and lipid parameters, can characterize the risk for diabetes in middle-aged adults (2). In addition, there is growing evidence to suggest that insulin resistance and diabetes are associated with chronic inflammation (3,4). Several proinflammatory cytokines are found to be elevated in diabetes and its complications (5). In contrast, anti-inflammatory molecules like adipocytokines have been consistently lower in individuals with diabetes and metabolic syndrome (6,7), a finding that indicates their protective role in the pathogenesis of diabetes. However, the exact pathological mechanisms underlying these inflammatory and anti-inflammatory pathways are largely unknown.
Interleukin-18 (IL-18) is a cytokine that is produced constitutively in several different cells in the body including macrophages, endothelial cells, vascular smooth muscle cells, dendritic cells, and Kupffer cells, as well as adipocytes (8). It is expressed as an inactive precursor molecule that is cleaved by the enzyme caspase-1 (9). IL-18 is proinflammatory and enhances maturation of T cells and natural killer cells and promotes production and release of other cytokines, chemokines, and adhesion molecules (9). IL-18 induces the production of tumor necrosis factor (TNF)-α, which in turn promotes the synthesis and release of IL-6 and C-reactive protein (CRP) (10).
Elevated levels of IL-18 have been associated with obesity, metabolic syndrome, insulin resistance, dyslipidemia, and atherosclerosis (11,12). Recently, two studies have shown that elevated levels of IL-18 are associated with higher risk of diabetes (13,14). Both of these studies looked at populations of European descent. It is unknown if a similar association exists for other ethnicities. In a study involving the Atherosclerosis Risk in Communities Study (ARIC) cohort, Duncan et al. (4) showed that low-grade inflammation was associated with onset of diabetes. However, the authors noted that although an inflammation score was robustly associated with onset of diabetes in whites, no association was found in the African American population. They postulated that not all sources of low-grade systemic inflammatory states increase the risk of developing diabetes.
With this background, we aimed to determine whether elevated levels of IL-18 were associated with incident diabetes, independent of traditional risk factors, in a heterogeneous population such as the ARIC cohort and whether racial differences exist with this association.
RESEARCH DESIGN AND METHODS
Materials
ARIC is a population-based study involving both whites and African Americans aged 45–64 years in four U.S. communities in North Carolina, Mississippi, Minnesota, and Maryland (15). Between 1987 and 1989, 15,792 men and women were recruited and attended a baseline exam (visit 1). Cohort members attended three subsequent clinic visits at ∼3-year intervals (visits 2–4). Human subject research review committees at the involved institutions approved the study, and all participants gave written informed consent. A stratified, nested case-cohort design was used in an ancillary study investigating inflammatory precursors of diabetes. This design has been used to investigate the role of total and high-molecular-weight (HMW) adiponectin, leptin, ferritin, free fatty acids, and other plasma biomarkers in the development of diabetes in the ARIC study (4,6,15–17).
From the baseline cohort, we excluded 2,018 participants with prevalent diabetes, 95 members of ethnic groups other than African Americans or whites, 853 participants not returning for any follow-up visit, 26 with no valid diabetes determination at follow-up, 6 with restrictions on stored plasma use, 12 with missing baseline anthropometric data, 2,514 participants in previous ARIC case-control and case-cohort studies involving cardiovascular disease for whom stored plasma was either previously exhausted or held in reserve, 212 for incomplete fasting (<8 h), and 316 with missing information for hypertension and inflammation markers. A final sampling frame of 9,740 individuals (71% of those in the full cohort without diabetes at baseline) was established after exclusions. Of these, 1,105 participants (11.3% of the final sample) were ascertained to have incident diabetes during follow-up. From the eligible members of this baseline cohort, we selected and measured analytes on ethnicity-stratified (50% white, 50% African American) random samples of both cases of incident diabetes and eligible members of the full cohort (1,095 individuals in total). Some participants within the cohort random sample developed diabetes during follow-up and were part of the case random sample as well. We excluded an additional 11 participants who had missing values for IL-18, leaving a total of 1,084 subjects for analysis.
Data collection
Baseline clinical and demographic data, including parental history of diabetes, were recorded. We defined parental history of diabetes as a report of diabetes in either parent. The definitions and methods for baseline measurements (height, weight, SBP, hypertension, triglycerides [TGs], HDL cholesterol [HDL-C], inflammation markers, and adipocytokines) have been previously described (4,6). A score was computed to indicate low-grade systemic inflammation, ranging from 0 to 6; one point was attributed to a value greater than the median of the cohort random sample for each of the six markers of systemic inflammation: IL-6, CRP, orosomucoid, sialic acid, white blood cell (WBC) count, and fibrinogen (4). Glucose was measured at baseline and at follow-up visits by standard hexokinase method.
Anthropometric measures were obtained at baseline visit. BMI was computed using the formula weight/height2. Smoking was assessed as a binary variable with 0 = never or former smokers and 1 = current smokers. Sport and physical activity was assessed with semicontinuous indices created from individual component questions described in detail elsewhere (18). The index ranged from 1 (low) to 5 (high). For education, we used a four-level categorical variable: 1, no high school diploma or less; 2, high school or some college; 3, beyond bachelors; and 4, missing data.
Diagnosis of incident diabetes
Cases of incident diabetes were defined on the basis of: 1) a reported physician diagnosis, 2) reported use of antidiabetic medications during the 2 weeks prior to the visit, or 3) a fasting (≥8 h) glucose value of at least 7.0 mmol/L. The date of diabetes incidence was estimated by linear interpolation using glucose values at the ascertaining visit and the previous visit (4,6).
Measurement of IL-18 levels
Circulating levels of IL-18 were measured in a centralized laboratory in plasma samples that were continuously stored at −70°C prior to analysis. All measurements were performed in duplicate using a sandwich ELISA according to manufacturer’s protocol (R&D Systems, Minneapolis, MN). The intra- and interassay coefficients of variance were 5.4 and 12.5%, respectively. The reliability coefficient was 0.98 based on 39 blinded quality control split samples.
Statistical analysis
Weighted analyses were done based on the case-cohort sampling design to allow for inference to the entire cohort. Weights were defined as the inverse of the ethnicity-specific sampling fractions. Spearman correlations were calculated to describe unadjusted associations between IL-18 and baseline covariates. Total and HMW adiponectin were logarithmically transformed to approximate normality. Quartiles of IL-18 were created from IL-18 distribution in the cohort random sample. Quartile values were used in the models, and test of trend was generated across the quartiles of IL-18. Cox proportional hazards models were fitted to investigate the relationship between IL-18 and time to onset of diabetes. Wald tests of interaction terms were used to test heterogeneity in the association between race and IL-18 quartile. All statistical testing was performed using two-sided tests with P = 0.05 as the cutoff for statistical significance. Statistical analyses were performed using the SAS (SAS Institute Inc., Cary, NC) and SUDAAN (Research Triangle Institute, Research Triangle Park, NC) statistical software packages.
RESULTS
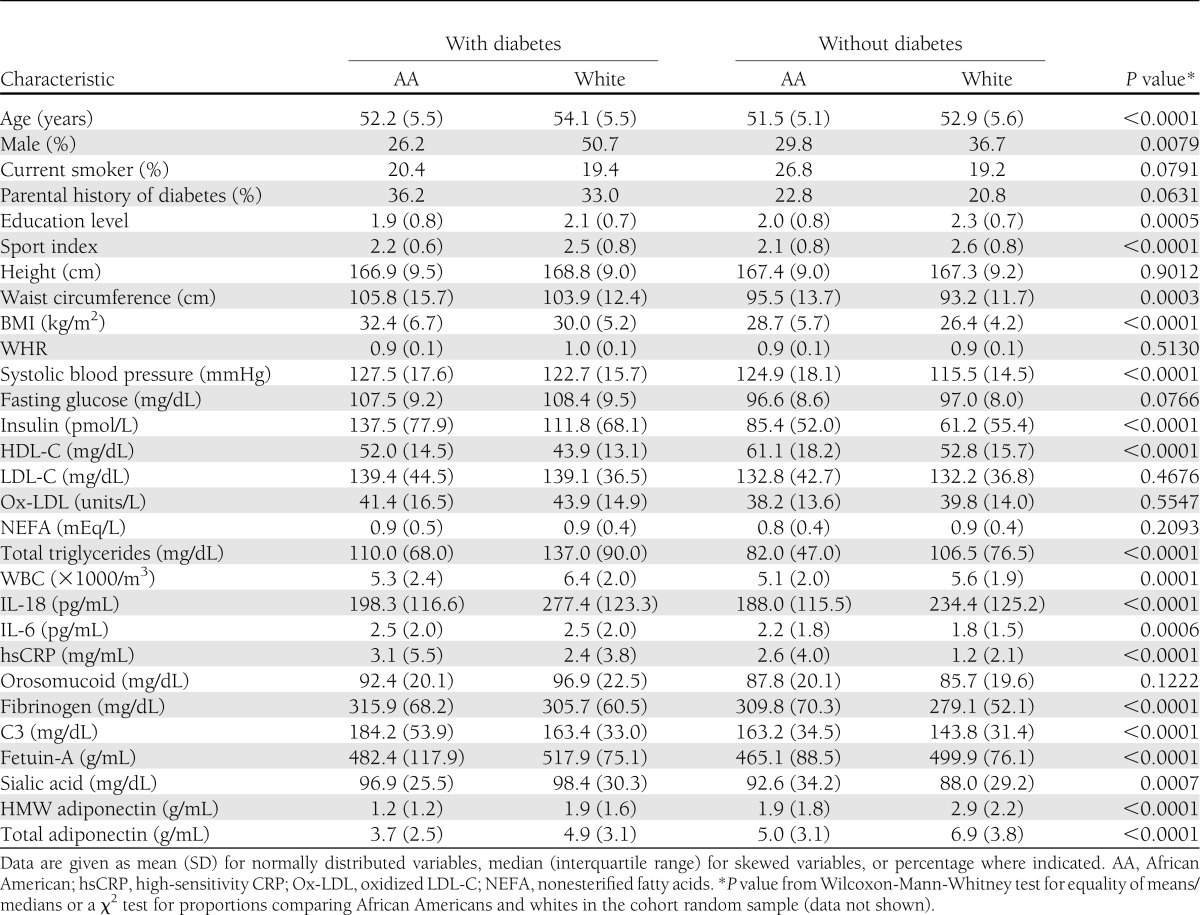
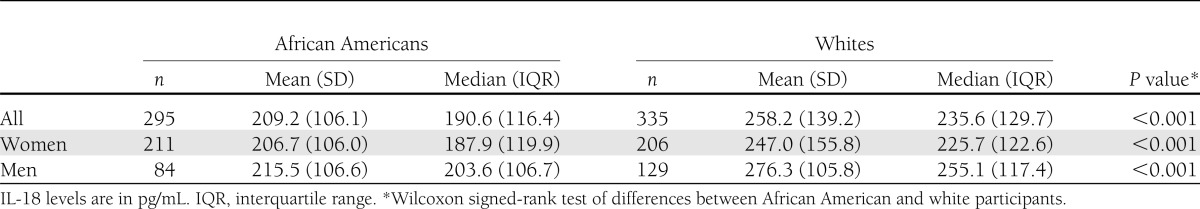
Our case-cohort sample of 1,084 ARIC study participants included 488 (45%) African American and 596 (55%) white individuals. The cohort random sample comprised 630 individuals, and the incident diabetes random sample included 548 individuals. Of the participants selected in the cohort random sample, 94 developed diabetes during follow-up, and 71 of them were included in the incident diabetes random sample. Thus, there were 23 incident diabetes cases in addition to those in the incident diabetes sample. Among cases, 146 were white men, 142 were white women, 68 were African American men, and 192 were African American women. Among noncases, the corresponding numbers were 113, 195, 68, and 160, respectively. Table 1 shows overall and ethnicity-specific distribution of the baseline demographic, clinical, and biomarker characteristics in this cohort random sample. Supplementary Table 1 compares the baseline characteristics of the participants selected in the final sample with those of the entire cohort, stratified by ethnicity. Baseline IL-18 levels were significantly higher in white compared with African American participants (median [interquartile range]; 235.6 [129.7] vs. 190.6 [116.4] pg/mL; P < 0.001). This difference was seen in both men (P < 0.001) and women (P < 0.001) (Table 2). A statistically significant interaction between race and IL-18 quartile for incident diabetes was found in the study population after adjusting for age, sex, and race (P = 0.01). However, after additional adjustment for parental history of diabetes, BMI, SBP, and waist-to-hip ratio (WHR), the interaction between race and IL-18 quartile lost significance (P = 0.15).
Table 1.
Baseline characteristics in participants who developed diabetes and those who did not stratified by race/ethnicity
Table 2.
Distribution of baseline IL-18 levels in the cohort random sample by race and sex
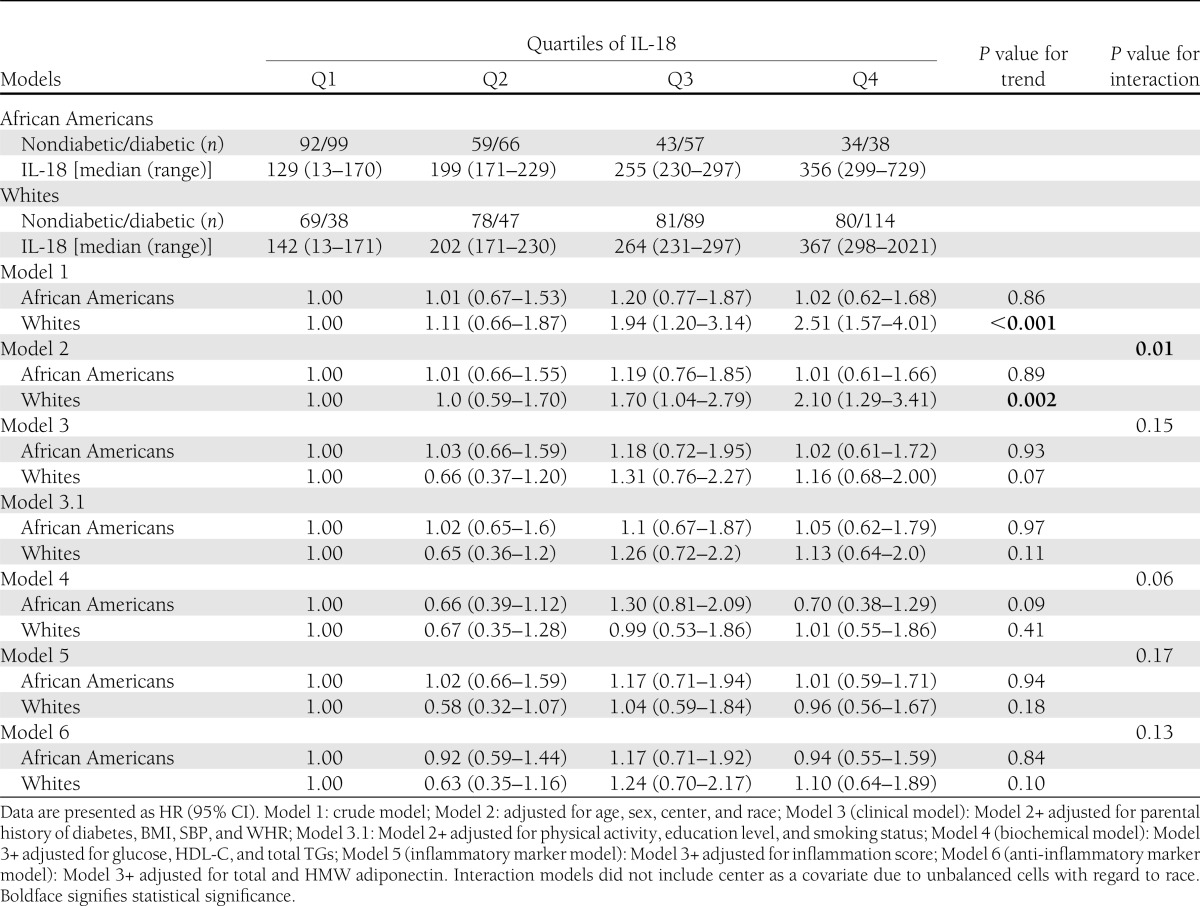
HRs for developing diabetes by quartiles of IL-18 are shown in Supplementary Table 2. A statistically significant HR for developing diabetes was seen for study participants in the fourth (versus first) quartile of IL-18 levels (HR: 1.5; 95% CI: 1.1–2.1) in the crude analysis. The HRs for fourth versus first quartile remained significant even after adjusting for age, sex, study center, and race (1.7; 1.2–2.3). The effects were attenuated, and the HR were no longer statistically significant after sequential additional adjustment for parental history of diabetes, BMI, SBP, and WHR (1.2; 0.8–1.7); sport index, education level, and smoking status (0.9; 0.7–1.7); glucose, HDL-C, and TGs (0.9, 95% CI: 0.6–1.4); inflammation score (1.0; 0.7–1.5); or total and HMW adiponectin (1.1; 0.8–1.6) (Supplementary Table 2).
In a similar subanalysis by race (Table 3), risk for developing diabetes in the fourth (versus first) quartile of IL-18 levels was statistically significant among whites in the crude analysis (HR: 2.5; 95% CI: 1.6–4.0). This association remained significant after adjustment for age, sex, and study center (2.1; 1.3–3.4). However, statistical significance was lost after additional adjustment for clinical variables (1.2; 0.7–2.0) and when adjustments were made for biochemical and inflammatory markers and adipocytokines. None of the models showed a significant association among African Americans.
Table 3.
HRs for developing diabetes by quartiles of IL-18 stratified by race
Correlations between IL-18 levels and various baseline demographic, clinical, biochemical, inflammatory, and anti-inflammatory variables markedly varied among the whites and the African Americans in the cohort random sample (Supplementary Table 3). Unlike those in African Americans, IL-18 levels in whites had a significant correlation with age (P < 0.01) and anthropometric characteristics such as waist circumference (P < 0.001), height (P = 0.04), WHR (P < 0.001), and BMI (P < 0.01). Similarly, IL-18 levels among whites were significantly correlated with SBP (P < 0.01), fasting glucose (P < 0.001), and insulin (P < 0.001). IL-18 levels among whites showed a highly significant correlation with HDL-C (P < 0.001), LDL cholesterol (LDL-C; P < 0.001), and TGs (P < 0.001) and moderate correlation with oxidized LDL (P = 0.04), but no correlation with nonesterified fatty acids (P = 0.7). In contrast, IL-18 levels among African Americans had the strongest correlation with nonesterified fatty acids (P < 0.001) and a weaker correlation with HDL-C (P = 0.05), oxidized LDL (P = 0.04), and TGs (P = 0.02). For inflammatory markers, IL-18 had a highly statistically significant correlation with IL-6 (P < 0.001), CRP (P < 0.001), intercellular adhesion molecule–1 (P < 0.001), and complement 3 (C3; P < 0.001) among whites and a highly significant correlation only with intercellular adhesion molecule–1 (P < 0.001) among African Americans. Although IL-18 levels were significantly correlated with total (P < 0.001) and HMW (P < 0.001) adiponectin among whites, no correlation was seen in African Americans.
CONCLUSIONS
This is the first study to look at the association of IL-18 levels with incident diabetes in a racially heterogeneous population. Two previous studies have looked at this association in predominantly white populations (13,14). In our study, higher IL-18 levels showed a significant association with incident diabetes in the entire study cohort and in whites, but not in African Americans. Upon adjustment for clinical risk factors associated with diabetes, including age, parental history of diabetes, BMI, SBP, and WHR, this association almost reached statistical significance. Further addition of fasting glucose and inflammation score to the models weakened the independent association of IL-18 levels with incident diabetes in our study. The exact role of IL-18 in causation of diabetes is not clear. The findings of our study support the hypothesis that elevated fasting glucose may itself be a regulator of IL-18 levels. This is consistent with the other studies showing that persistent hyperglycemia upregulates the synthesis of IL-18 and other proinflammatory cytokines (19). Hyperglycemia can lead to oxidative stress and membrane lipid peroxidation. It has been shown that the degree of lipid peroxidation is directly proportional to blood glucose concentrations (20). Persistent oxidative stress induces stress-sensitive signaling pathways, such as nuclear factor of κ light polypeptide gene enhancer in B cells (NF-κB). NF-κB is a transcription factor that plays a major role in mediating inflammatory responses and in the production of proinflammatory cytokines like IL-6, TNF-α, and IL-18. The inflammatory cytokines in turn lead to insulin resistance and overt diabetes (21), further increasing the plasma glucose levels. This leads to a vicious cascade phenomenon with a positive-feedback loop, causing difficulty in determining whether fasting glucose is upstream or downstream of IL-18 in the pathway for onset of diabetes.
It is plausible that both impaired fasting glucose and elevated IL-18 levels are drivers of the proinflammatory pathway culminating in diabetes. This hypothesis is further strengthened by our observation that elevated IL-18 levels were not associated with incident diabetes independent of inflammatory markers including IL-6, CRP, sialic acid, and orosomucoid. This is consistent with previous reports showing that IL-18 induces production of TNF-α, IL-6, and CRP (22). In another model in our study, addition of HDL-C and TGs weakened the association of IL-18 with incident diabetes. Because IL-18 is a proinflammatory molecule, further adjustments with lipid parameters associated with inflammation may have resulted in overadjustment and weakening of the independent association of IL-18 with incident diabetes.
IL-18 levels showed a weak association with incident diabetes when adjusted for plasma adiponectin levels in our study. This is consistent with previous studies showing that future risk of diabetes was associated with lower levels of total and HMW adiponectin (13). It may imply that though a greater amount of IL-18 is produced in tissues outside the adipose tissue (23), adiposity may drive the IL-18 production in later stages. This is supported by a significant, but weak, correlation of IL-18 levels with total and HMW adiponectin levels as well as obesity in the white subgroup of our study. In their study in a white female population, Hivert et al. (13) showed an association of IL-18 levels with incident diabetes independent of HMW adiponectin and BMI. The authors, however, did not adjust the models for fasting glucose, inflammatory markers, and lipid parameters, all relevant factors in the occurrence of diabetes. The study also showed that the association of IL-18 with diabetes tends to be stronger in women with lower BMI, suggesting that increasing adiposity masks the association of IL-18 with diabetes (13).
Our second aim was to evaluate racial differences in IL-18 levels and their association with incident diabetes. We found significantly lower baseline levels of IL-18 in African Americans compared with whites. It is possible that the difference in mean IL-18 levels between races is attributable at least in part to genetic and anthropometric differences.
In addition, IL-18 levels were moderately associated with incident diabetes in whites but not at all in African Americans. There was a significant interaction between IL-18 and race in their associations with incident diabetes. However, this interaction was attenuated on adjustment for parental history of diabetes, BMI, SBP, and WHR. The reason for this interaction is not known. Duncan et al. (4) found an association of low-grade inflammation with diabetes in whites but not in African Americans in the ARIC cohort. It is well known that white cell counts differ in African Americans compared with whites (24). These findings suggest racial differences in inflammatory burden, triggering factors for inflammatory pathways, and the association of inflammation with diabetes.
Interestingly, in our study, IL-18 levels were correlated strongly with anthropometric measures related to obesity in whites but not in African Americans. IL-18 levels have been inconsistently associated with BMI and body fat in various studies, raising the question of whether the major source of IL-18 is not adipose tissue but muscle and liver. However, the absolute lack of correlation of IL-18 with either BMI or WHR in African Americans in our study is both a novel and interesting finding. Moreover, there was no correlation of adiponectin with IL-18 levels in African Americans, but there was a significant correlation in whites, again raising questions regarding the difference in the source of inflammatory markers and adipocytokines in the two races. Similarly, fasting glucose and insulin levels were correlated with IL-18 levels in whites but not in African Americans, whereas free fatty acids were correlated with IL-18 levels in African Americans and not in whites.
Although African Americans have long been recognized to have different characteristics of the metabolic syndrome compared with whites (low TG, high HDL-C, and high glucose) and increased frequency of incident diabetes, only more recently has there been an appreciation of the differences in inflammation markers associated with cardiometabolic risk. African Americans have lower WBC count, IL-18, fetuin-A, CC chemokine ligand–5, and regulated on activation, normal T-cell expressed, and secreted (RANTES) (25) than whites, but have higher levels of CRP, sialic acid, fibrinogen, C3, and IL-6 than whites in the ARIC cohort. There is growing evidence that markers of inflammation may have ethnic and genetic differences (24,26). Such differences are also seen in the association of these inflammatory markers with incident diabetes (4). One possible explanation for the racial differences in levels of various chemokines and inflammatory markers may be the polymorphism in the Duffy antigen receptor for chemokines (Darc) (27). Darc is a blood group alloantigen and serves as a vascular reservoir of proinflammatory cytokines. The difference in the WBC count in African Americans compared with whites is due to the common African-derived null variant of the Darc gene (28). By abolishing the expression of Darc on erythrocytes, the Duffy null variant may alter the concentration and distribution of chemokines in the blood (27). The null variant provides selective advantage against malaria in these populations. The relation of these inflammatory markers to the pathophysiology of diabetes is unknown. Evolutionary diversity along with associated social and environmental factors may contribute to the variable association of diabetes with insulin resistance, markers of inflammation, and anthropometric parameters in different races (29). However, these novel markers of inflammation and their association with incident diabetes need further validation.
Our study has several strengths. The study cohort is derived from a large well-studied population based in four different U.S. communities, suitably designed to study racial differences and provide a long prospective follow-up of incident cases of diabetes (15).
Limitations
Markers of inflammation are usually stable in frozen samples, but this has not been studied over a prolonged period of time. In this study, we used a single baseline measurement of IL-18. Whether obtaining multiple measurements over time would have strengthened the association seen in the study is unknown.
Summary
Elevated IL-18 levels are associated with incident diabetes mellitus (DM), but there is a racial heterogeneity in this association. This may imply racial differences in the pathogenetic pathways of development of DM. Further studies are needed to elucidate the details of this mechanism of DM and the potential interplay of racial differences, anthropometric characteristics, and inflammation.
ACKNOWLEDGMENTS
ARIC is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute Contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. The current study was also supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-56918.
No potential conflicts of interest relevant to this article were reported.
S.I.N. prepared the manuscript and contributed to data analyses. J.S.P. contributed to the discussion and reviewed and edited the manuscript. K.F. performed data analyses. R.C.H., N.Z., D.C., M.I.S., and B.B.D. reviewed and edited the manuscript. C.M.B. conceptualized the hypothesis, contributed to the discussion, and reviewed and edited the manuscript. C.M.B. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented in part at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank Kerrie C. Jara, Baylor College of Medicine, Houston, TX, for editorial assistance and the staff and participants of ARIC for important contributions.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1957/-/DC1.
References
- 1.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001;24:1936–1940 [DOI] [PubMed] [Google Scholar]
- 2.Schmidt MI, Duncan BB, Bang H, et al. Atherosclerosis Risk in Communities Investigators Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care 2005;28:2013–2018 [DOI] [PubMed] [Google Scholar]
- 3.Kowalska I, Straczkowski M, Nikolajuk A, et al. Insulin resistance, serum adiponectin, and proinflammatory markers in young subjects with the metabolic syndrome. Metabolism 2008;57:1539–1544 [DOI] [PubMed] [Google Scholar]
- 4.Duncan BB, Schmidt MI, Pankow JS, et al. Atherosclerosis Risk in Communities Study Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 2003;52:1799–1805 [DOI] [PubMed] [Google Scholar]
- 5.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol 1999;36:67–72 [DOI] [PubMed] [Google Scholar]
- 6.Zhu N, Pankow JS, Ballantyne CM, et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab 2010;95:5097–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 8.García VE, Uyemura K, Sieling PA, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol 1999;162:6114–6121 [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 2006;83:447S–455S [DOI] [PubMed] [Google Scholar]
- 10.Bouwmeester T, Bauch A, Ruffner H, et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol 2004;6:97–105 [DOI] [PubMed] [Google Scholar]
- 11.Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol 2007;157:465–471 [DOI] [PubMed] [Google Scholar]
- 12.Zirlik A, Abdullah SM, Gerdes N, et al. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol 2007;27:2043–2049 [DOI] [PubMed] [Google Scholar]
- 13.Hivert MF, Sun Q, Shrader P, Mantzoros CS, Meigs JB, Hu FB. Circulating IL-18 and the risk of type 2 diabetes in women. Diabetologia 2009;52:2101–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorand B, Kolb H, Baumert J, et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984-2002. Diabetes 2005;54:2932–2938 [DOI] [PubMed] [Google Scholar]
- 15.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 16.Duncan BB, Schmidt MI, Pankow JS, et al. Adiponectin and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 2004;53:2473–2478 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 1999;353:1649–1652 [DOI] [PubMed] [Google Scholar]
- 18.Evenson KR, Rosamond WD, Cai J, et al. Physical activity and ischemic stroke risk: the Atherosclerosis Risk in Communities Study. Stroke 1999;30:1333–1339 [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106:2067–2072 [DOI] [PubMed] [Google Scholar]
- 20.Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989;38:1539–1543 [DOI] [PubMed] [Google Scholar]
- 21.Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal 2005;7:1553–1567 [DOI] [PubMed] [Google Scholar]
- 22.Stephens JM, Butts MD, Pekala PH. Regulation of transcription factor mRNA accumulation during 3T3-L1 preadipocyte differentiation by tumour necrosis factor-alpha. J Mol Endocrinol 1992;9:61–72 [DOI] [PubMed] [Google Scholar]
- 23.Fain JN, Tichansky DS, Madan AK. Most of the interleukin 1 receptor antagonist, cathepsin S, macrophage migration inhibitory factor, nerve growth factor, and interleukin 18 release by explants of human adipose tissue is by the non-fat cells, not by the adipocytes. Metabolism 2006;55:1113–1121 [DOI] [PubMed] [Google Scholar]
- 24.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med 1999;133:15–22 [DOI] [PubMed] [Google Scholar]
- 25.Virani SS, Nambi V, Hoogeveen R, et al. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur Heart J 2011;32:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol 2005;46:464–469 [DOI] [PubMed] [Google Scholar]
- 27.Schnabel RB, Baumert J, Barbalic M, et al. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood 2010;115:5289–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet 2009;5:e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Real JM. Genetic predispositions to low-grade inflammation and type 2 diabetes. Diabetes Technol Ther 2006;8:55–66 [DOI] [PubMed] [Google Scholar]