Abstract
OBJECTIVE
Frequent episodes of severe hypoglycemia may increase the risk of cardiovascular disease (CVD) in people with diabetes. Our aim was to study the relationship between severe hypoglycemic episodes and CVD incidence in subjects with type 1 diabetes, and further, to assess if markers of inflammation/endothelial injury were enhanced in individuals who experienced hypoglycemic episodes.
RESEARCH DESIGN AND METHODS
The prospective study included 2,181 type 1 diabetic patients from the EURODIAB Prospective Complications Study. At baseline, frequency of self-reported severe hypoglycemia, defined as episodes serious enough to require the help of another person, was assessed based on responses to a patient questionnaire. Both fatal/nonfatal CVD was assessed 7.3 years after baseline examination. At the follow-up visit, data on both severe and nonsevere hypoglycemic episodes in the previous year were collected through a questionnaire and markers of inflammation/stress response/endothelial injury measured by enzyme-linked immunosorbent assays in the 531 subjects of the nested case-control study, including 363 case subjects with one or more complications of diabetes and 168 control subjects with no evidence of any complication.
RESULTS
During the follow-up period, 176 patients had incident CVD. Logistic regression analysis showed that severe hypoglycemia at the baseline examination was not associated with incidence of CVD (adjusted odds ratios [95% CI]: one to two episodes, 0.87 [0.55–1.37]; three or more episodes, 1.09 [0.68–1.75]). Furthermore, follow-up serum levels of markers of endothelial damage/inflammation were not cross-sectionally associated with the frequency of hypoglycemic episodes.
CONCLUSIONS
Taken together our data do not support the hypothesis that in type 1 diabetes, severe hypoglycemia increases the risk of CVD.
Hypoglycemia is the most common side effect of glucose-lowering therapies in patients with diabetes, and intensive glucose control invariably increases the risk of severe hypoglycemia. Recent large randomized trials, looking at the effect of intensive glycemic control on macrovascular complications in type 2 diabetic patients, have individually shown either no benefit or increased mortality (1). Although the reason for this increased mortality is unclear and hypoglycemia has not been proven to be involved, these studies have fueled longstanding concerns that hypoglycemia itself may increase the risk of cardiovascular disease (CVD).
Evidence linking hypoglycemia to CVD comes predominantly from studies in type 2 diabetic patients (2–8). However, the risk of hypoglycemia is even greater in type 1 diabetic patients, and in the Diabetes Control and Complications Trial (DCCT), severe hypoglycemic episodes requiring assistance affected nearly one-third of the intensively treated patients (9). The DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) study showed a reduction in CVD at later follow-up (10,11); however, the risk of cardiovascular (CV) events among the people who experienced severe hypoglycemia was not reported, and this issue has been scarcely explored in clinical and epidemiological studies.
Recently, additional support for the hypothesis of a role of hypoglycemia in CVD in type 1 diabetic patients was offered by indirect evidence. First, repeated hypoglycemic episodes were associated with preclinical atherosclerosis (12). Second, physiological studies have shown that acute provoked hypoglycemia induces a rise in the circulating levels of markers of both inflammation and endothelial dysfunction (1,13–16). Most of these studies, however, are acute observations and the long-term effects of hypoglycemia on markers of inflammation/endothelial injury are largely unknown.
The aim of the current study was to examine in a large, 7-year prospective cohort study of patients with type 1 diabetes if the frequency of severe hypoglycemic episodes predicts incident CVD at follow-up. Moreover, we tested if both severe and nonsevere hypoglycemic episodes were cross-sectionally associated with serum levels of markers of both inflammation and endothelial dysfunction.
RESEARCH DESIGN AND METHODS
The EURODIAB Prospective Complications Study (PCS) is a follow-up of the EURODIAB IDDM Complications Study (17). Full details of the design, methods, and recruitment to the EURODIAB cohort have been published elsewhere (17,18). Baseline investigations were performed on 3,250 men and women with type 1 diabetes aged between 15 and 60 years and recruited from 31 centers in 16 European countries. The sampling frame contained all type 1 diabetic patients attending at least once in the last year for each center. Sample selection was stratified by age, sex, and duration of diabetes to ensure sufficient representation in all categories (17). Type 1 diabetes was defined as a diagnosis made before the age of 36 years, with a need for continuous insulin therapy within a year of diagnosis. Of those invited, 85% participated. Patients with a duration of diabetes <1 year and pregnant women were excluded. The local ethics committees approved this study at each center, and all people provided written informed consent.
Severe hypoglycemic episodes
Definition of severe hypoglycemia was based on information obtained from questionnaires completed by subjects at both the baseline and the follow-up visit. All patients were asked, “Over the past year, how many hypoglycemic attacks have you had, serious enough to require the help of another person?” The questionnaire also provided information on physical activity, smoking habits, frequency of insulin injections, and number of daily insulin units injected per kilogram body weight (17,19). At the follow-up examination, information on numbers of nonsevere hypoglycemic episodes, defined as hypoglycemic episodes over the past year not requiring the help of another person, was also collected.
Follow-up and outcomes
All patients were recalled for follow-up assessment 6–8 years later. At the time of the follow-up study, data on mortality and morbidity forms were collected from available hospital case notes or other sources of clinical data in every participating center, detailing cause of death or the presence or absence of severe complications at their most recent visit. When death certificates could not be obtained, information considering the cause of death was reported by the physician or extracted from the hospital record. In the whole EURODIAB cohort, cause of death could not be obtained for 35 of 102 deceased subjects because of legal restrictions. Outcome variables were both fatal and nonfatal CVD incidence. Causes of death were coded according to the ICD-9 classification and assigned to different categories, such as coronary heart disease, stroke, other CVD, non-CVD, and unknown. Two observers (N.C. and J.H.F.) separately allocated cause of death with 100% agreement. CVD was defined as a positive medical history of a CV event, including myocardial infarction, angina pectoris, coronary artery bypass graft and/or stroke, and/or ischemic changes on a conventional 12-lead electrocardiogram (ECG) (18). Two observers classified the ECG abnormalities according to the Minnesota Code. Any discrepancies between the two observers were adjudicated by a third. ECG abnormalities suggestive of probable ischemia consist of major Q/QS waves and complete left bundle branch block. Possible ischemia consists of minor Q waves, ST segment abnormalities, and T wave abnormalities (18,20).
Definitions and measurements
Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and/or the current use of blood pressure–lowering drugs (21). Retinopathy was assessed by centrally graded retinal photographs, and each patient’s level of retinopathy (absent, nonproliferative, or proliferative) was defined by the level of the worst eye (20). Nephropathy was assessed using the albumin excretion rate (AER) calculated centrally from a single, timed, 24-h urine collection (22). Distal symmetrical polyneuropathy (DSP) was assessed on the basis of neuropathic symptoms and signs, including measurement of vibration perception threshold. The AER value was defined as normal (<20 μg/min), microalbuminuria (≥20 and <200 μg/min), or macroalbuminuria (≥200 μg/min). Glomerular filtration rate (GFR) was estimated using the four-component abbreviated equation from the Modification of Diet in Renal Disease Study Group (23). Subjects with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 were defined as having chronic kidney disease. Additional measurements included triglycerides, total cholesterol, HDL cholesterol, and HbA1c.
Nested case-control study
A cross-sectional, nested case-control study was designed at the follow-up examination (24–30). Case subjects were selected to have the greatest complication burden as possible to provide sufficient numbers for subgroup analyses. Thus, case subjects were all those with CVD, proliferative retinopathy, or micro/macroalbuminuria at follow-up. Control subjects were selected to be completely free of complications. Applying these criteria, this yielded 363 case subjects and 168 control subjects with full data on complications. Blood samples from both case subjects and control subjects were collected at the follow-up examination, and markers of both endothelial damage and inflammation were measured. Soluble vascular cell adhesion molecule 1 (sVCAM-1) (25), soluble E-selectin (s-E-selectin) (25), interleukin 6 (IL-6) (25,27), tumor necrosis factor-α (TNF-α) (25,27), heat shock protein 27 (HSP27) (28), and anti-HSP60 and anti-HSP70 antibodies (29) were measured by commercially available ELISA (R&D Systems, Oxon, U.K.; HSP27, Calbiochem San Diego, CA; anti-HSP60 and anti-HSP70 antibodies: anti–EKS-650 and –EKS-750, Stressgen Biotechnologies Corporation). Plasma levels of C-reactive protein (CRP) were measured by an in-house ELISA (26,27).
Statistical analysis
Data were expressed as the mean and SD. Variables with skewed distributions were logarithmically transformed for statistical analysis and expressed as geometric mean (25th–75th percentile). Overall group differences in continuous variables were tested with ANOVA. Pearson χ2 test was used for differences in categorical measures. Follow-up duration for fatal and nonfatal CVD incidence was calculated as the time between the baseline examination and date of first event (myocardial infarction, angina pectoris, coronary artery bypass graft, or stroke), date of abnormal ECG finding suggestive of ischemia, date of loss to follow-up, or date of follow-up examination. As there was little variation in follow-up time among individuals, logistic regression was used to analyze the association of severe hypoglycemic episodes at baseline with incidence of fatal and nonfatal CVD. Odds ratios (ORs) were adjusted for age, sex, diabetes duration, and DSP at baseline. In addition, in models 2 and 3, adjustment was also performed for nonsevere and severe hypoglycemic episodes at the follow-up examination, respectively.
In the nested case-control study, logistic regression analysis was used to estimate the ORs of severe and nonsevere hypoglycemia for all complications and CVD, independently of age and sex. Analysis was hypothesis oriented and did not use stepwise regression (31). Variables were retained in the final model if they added significantly to the likelihood of models or to the estimated coefficients of predictors. The −2 log likelihood ratio test was used to assess the overall significance of models. All reported P values are two sided, and a P value of <0.05 was considered to indicate statistical significance. All analyses were performed with STATA (Stata Release 10.0; Stata Corporation, College Station, TX).
RESULTS
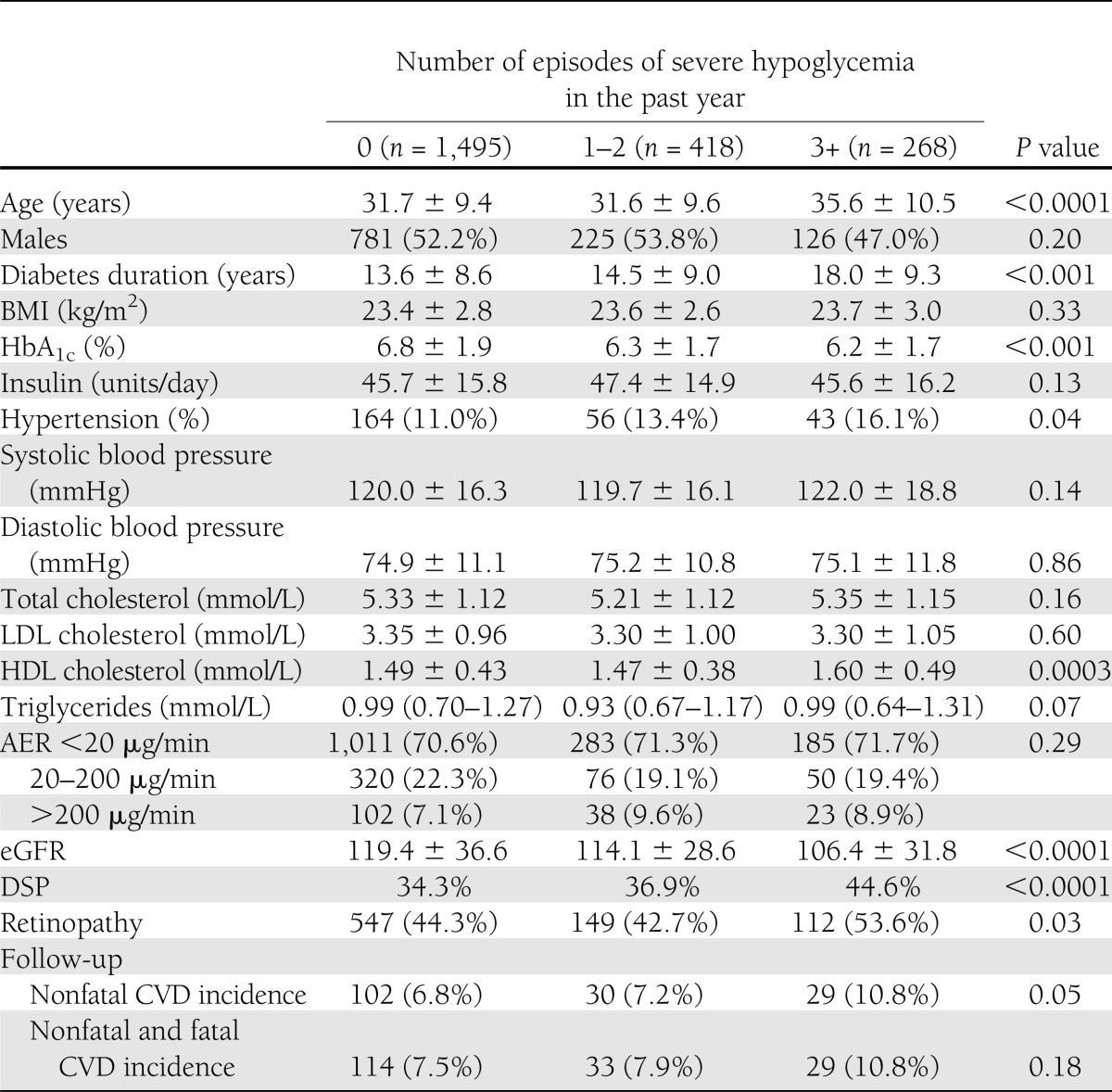
Of the 3,250 patients recruited at baseline, response data on severe hypoglycemic episodes were available for 3,248 (99.9%) subjects. Out of these subjects, 2,899 people had no evidence of CVD at the baseline examination. Out of these 2,899 subjects, vital status at follow-up was not obtained for 383 people because of the following reasons: four local centers did not participate in the prospective study (n = 358), 7 patients did not fulfill the inclusion criteria, and 18 patients had an unknown vital status. We also excluded 335 subjects with missing morbidity and/or mortality (22 of 74) data, resulting in 2,181 individuals with complete data. Baseline distribution of severe hypoglycemic episodes did not differ (P = 0.46) between patients remaining in the study (none, 1,495 [68.5%]; one to two, 418 [19.2%]; three or more, 268 [12.3%] severe hypoglycemic episodes) and those who were lost at follow-up (none, 477 [66.5%]; one to two, 141 [19.6%]; three or more, 100 [13.9%] severe hypoglycemic episodes), including subjects with unknown cause of death (none, 14 of 22 [63.6%]; one to two, 2 of 22 [9%]; three or more, 6 of 22 [27.2%]). Table 1 shows the baseline characteristics according to hypoglycemic categories. People who had experienced three or more recent severe hypoglycemic episodes had lower HbA1c values but longer duration of diabetes with higher prevalence of both retinopathy and DSP.
Table 1.
Baseline characteristics and 7.3-year CVD incidence of type 1 diabetic patients according to hypoglycemic categories
Hypoglycemia and incident CVD
After a median follow-up of 7.3 years (interquartile range 6.9–7.9), 176 patients had incident CVD (either fatal or nonfatal). The percentages of case subjects with incident CVD were similar among patients with none (7.6%; n = 114; 12 fatal and 102 nonfatal), one to two (7.9%; n = 33; 3 fatal and 30 nonfatal), and three or more severe hypoglycemic episodes (10.8%; n = 29; 0 fatal and 29 nonfatal). The proportion of patients who had fatal/nonfatal CV events was comparable (P = 0.22) in patients reporting (9.0%) and not reporting (7.5%) severe hypoglycemia at baseline as well as at follow-up (nonfatal only, 9.1 and 7.8%; P = 0.40). Numbers of severe hypoglycemic events at baseline and at follow-up showed a statistically significant correlation (R = 0.29; P = <0.0001). Moreover, a significant correlation between numbers of severe and total hypoglycemic episodes (R = 0.57; P < 0.0001) was found at the follow-up examination.
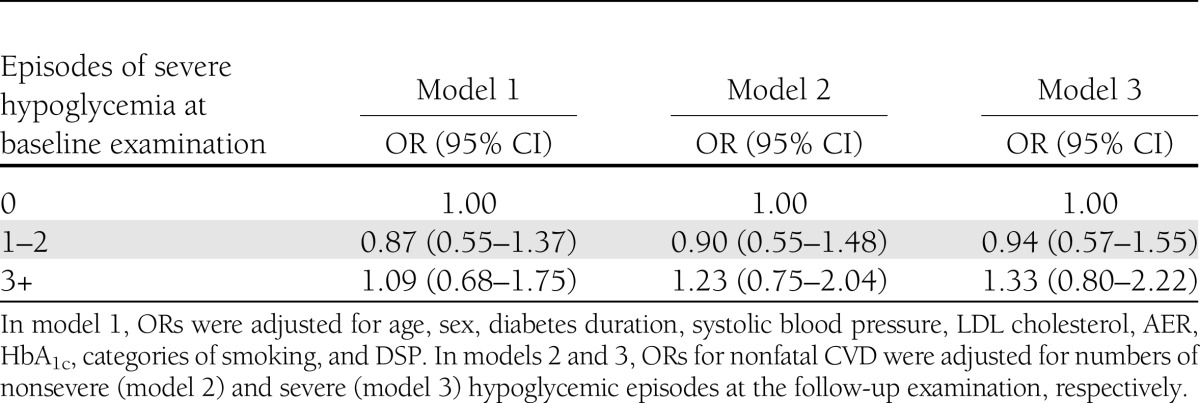
Logistic regression analysis showed that severe hypoglycemia at the baseline examination was not associated with incident fatal and nonfatal CVD (Table 2). Further adjustment for numbers of both severe and nonsevere hypoglycemic events at follow-up did not modify ORs for nonfatal CVD.
Table 2.
Role of severe hypoglycemia in incidence of CVD in the prospective study; results of logistic regression analyses
Nested case-control study at the follow-up examination
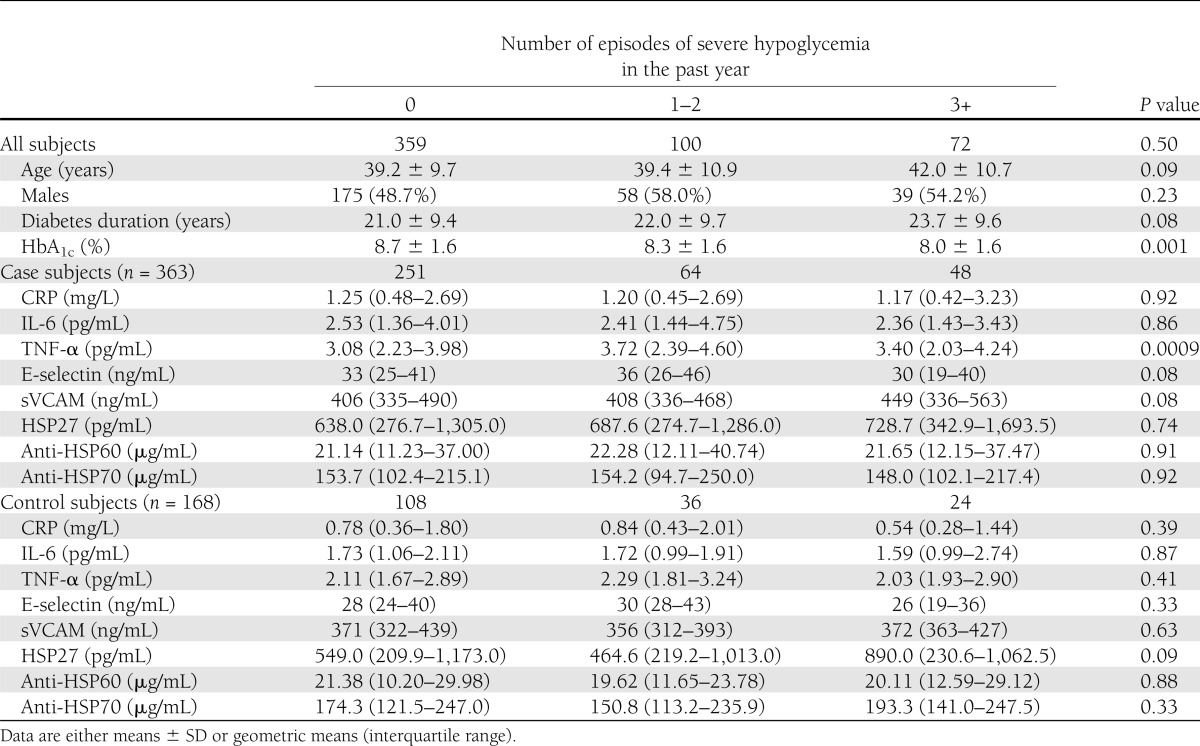
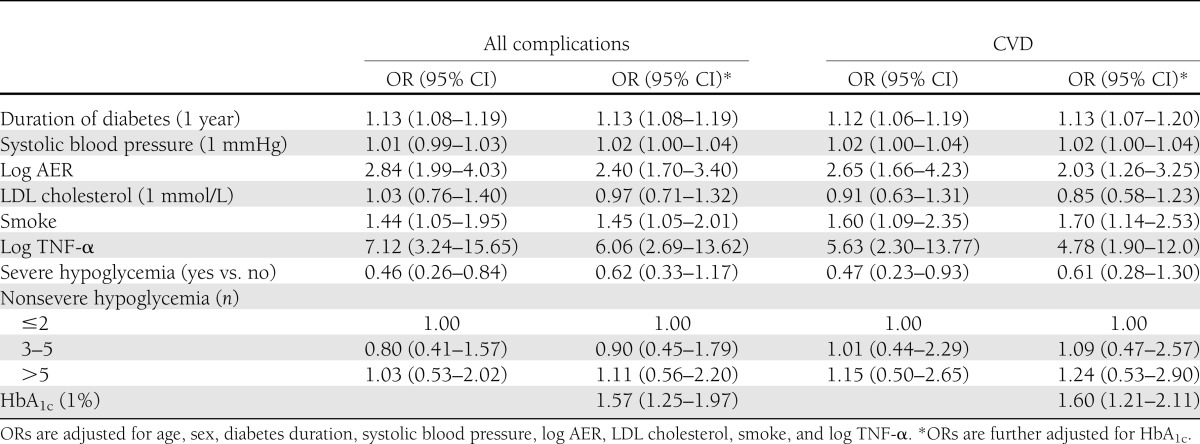
As shown in Table 3, almost one-third (33%) of the 531 subjects of the nested case-control study declared one or more severe hypoglycemic episodes over the past year. Nineteen percent of the patients had one to two episodes and 14% had three or more episodes. The proportions of case subjects and control subjects were similar through hypoglycemic categories. Those with vascular complications had a more adverse risk factor profile than control individuals, as we have previously reported (29–31). There were no significant differences in serum CRP, IL-6, sVCAM, HSP27, anti-HSP60, and anti-HSP70 among groups. Differences in E-selectin levels were clinically not relevant, though statistically significant. In case subjects, TNF-α values were lower in patients who did not experience any severe hypoglycemic episode than in patients with severe hypoglycemic episodes. However, after adjustment for eGFR, the difference was no longer significant (P = 0.84). Adjustment for treatment with beta blockers and lipid-lowering therapy did not modify observed associations. As shown in Table 4, in logistic regression analysis, people with severe hypoglycemic episodes at follow-up had a 53% (OR 0.47 [95% CI 0.23–0.93]) lower risk of CVD after adjustment for age and sex, and independently of main confounders (diabetes duration, systolic blood pressure, log AER, LDL cholesterol, smoke, and log TNF-α). However, after further adjustment for HbA1c, this association was no longer significant (0.61 [0.28–1.30]). Nonsevere hypoglycemic episodes in the past year were not associated with either all complications or CVD. Finally, further adjustments for treatment with beta blockers (all complications OR 0.62 [0.33–1.15]; CVD 0.58 [0.27–1.26]) and lipid-lowering drugs (all complications OR 0.62 [0.33–1.16]; CVD 0.59 [0.27–1.27]) did not modify observed associations.
Table 3.
Variables associated with severe hypoglycemia in the nested case-control study of the EURODIAB PCS
Table 4.
Result of logistic regression analysis of the case-control study within the EURODIAB PCS
CONCLUSIONS
In this study, we found that frequency of severe hypoglycemia at baseline was not a predictor of subsequent CV events. In addition, hypoglycemic episodes, both severe and nonsevere, were not cross-sectionally associated with a rise in the circulating levels of markers of both inflammation and endothelial injury. Therefore, our data do not support the hypothesis that in type 1 diabetic patients, severe hypoglycemia increases the risk of CVD.
In the prospective study, the percentages of case subjects with incident CVD were similar through baseline hypoglycemic categories. Furthermore, the proportion of patients who experienced CV events was comparable in patients reporting and not reporting severe hypoglycemic episodes at baseline. Finally, results of logistic regression analysis showed that severe hypoglycemia at the baseline examination was not associated with incident fatal and nonfatal CVD.
Results of intervention trials are consistent with our data. Indeed, in the DCCT study in type 1 diabetic patients, a glucose-lowering therapy that dramatically increased the risk of severe hypoglycemia did not cause long-term CV harm (9–11). Furthermore, a recent meta-analysis of the four major intervention trials in type 2 diabetic patients (UK Prospective Diabetes Study, Action to Control Cardiovascular Risk in Diabetes, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation, and Veterans Affairs Diabetes Trial) has shown that intensive therapy, carrying a 2.5-fold increased risk of severe hypoglycemic events, reduced, though modestly, the overall risk of first occurrence of major CV events (32). However, intervention trials do not explore the direct role of hypoglycemia, but only if an intervention, increasing the risk of hypoglycemia, enhances the risk of CV outcomes. Whether hypoglycemia is a risk factor for future CV events is best assessed in a prospective epidemiological study (33), and ours is the first large, clinically based, epidemiological study exploring the relationship between hypoglycemia and incident CVD in type 1 diabetic patients.
Some epidemiological data are available in type 2 diabetic patients. The epidemiological analysis of the Steno-2 trial has shown no association between hypoglycemic events and both total and CV mortality during the 13-year follow-up (34). In a recent epidemiological analysis of the Action to Control Cardiovascular Risk in Diabetes trial, participants with severe hypoglycemic episodes had an increased risk of all-cause mortality (35). Likewise, various prospective epidemiological studies have reported an increased all-cause mortality in diabetic patients with myocardial infarction who experienced in-hospital hypoglycemic episodes (6,8,36,37). However, data on CV events were either not available or not significant in these studies. This raises the hypothesis that people who are prone to hypoglycemia are at higher risk of death due to the coexistence of other risk factors, such as hepatic disease, renal disease, cognitive decline, cancer, and medications, rather than because of enhanced CV risk (33).
In the nested case-control study, we found no association between frequencies of severe hypoglycemic episodes over the past year and markers of both inflammation/stress response (CRP, IL-6, TNF-α, anti-HSP60, and anti-HSP70) and vascular injury (sVCAM and s-E-selectin). A significant increase in TNF-α levels was observed in case subjects, but it was no longer present after adjustment for renal function. Previous reports have shown that hypoglycemia induces an acute increase in markers of inflammation/endothelial injury (13–16). Our results are not in conflict with these findings, as hypoglycemia-induced changes are transient and our measurements were not performed immediately after a hypoglycemic episode. However, our data show that the cumulative and deleterious effects of repeated/severe hypoglycemic episodes over the previous year did not alter the circulating levels of markers, potentially linking hypoglycemia to diabetic vascular complications. Therefore, our data indicate that hypoglycemia does not induce sustained changes in markers of potential relevance in CVD and argues against the hypothesis that hypoglycemia-induced changes in nontraditional CV risk factors play a long-term role in CVD. We acknowledge that in the case subjects, who were selected to have the highest burden of complications, small hypoglycemia-induced differences in markers of inflammation/endothelial injury might have been masked by major changes due to concurrent micro/macrovascular disease; however, no differences were found in the control subjects, who were completely free of complications. Furthermore, a role for hypoglycemia-induced inflammation/vascular damage in CVD has been proposed particularly for people with pre-existing vascular disease (1).
An important strength of our work is the prospective clinically based design, evaluating a large, multicenter, European cohort of type 1 diabetic patients. Type 2 diabetes is often associated with numerous other CV risk factors, so that a disentangled analysis may remain open to imprecision, and type 1 diabetes offers a better model. However, CV events are relatively rare among younger type 1 diabetic patients and a similar study could be carried out only in such a large population as the one studied prospectively by the EURODIAB PCS.
Limitations were also present in this study. First, we cannot exclude the possibility that in our work the consequences of hypoglycemia were underestimated because we relied on self-reports of spontaneous hypoglycemia and, thus, some patients were probably misclassified. Therefore, the null hypothesis should be accepted with caution. Validation of suspected hypoglycemic episodes through self-measurement of blood glucose levels would have improved the accuracy in identifying the patients with true severe hypoglycemia. However, patients may not experience hypoglycemic symptoms severe enough to prompt a measurement of finger stick glucose, even when blood glucose levels fall below 50 mg/dL because of hypoglycemia unawareness. We acknowledge that only studies based on continuous glucose monitoring would provide unbiased definitive data. However, the feasibility and/or practicality of recruiting large numbers of subjects to assess person-years using continuous glucose monitoring-based methods and/or observations for CV risk assessment is currently limited.
Second, the potential benefits for vascular complications of better glucose control, leading to increased risk of severe hypoglycemia, may have mitigated the deleterious consequences of hypoglycemia, thus obscuring any harm. Indeed, in the DCCT study, participants in the intensive therapy group had a 41% reduction in CV events at the end of the active treatment period despite a much higher risk of severe hypoglycemia. Furthermore, analyses suggested that the difference in HbA1c during the active treatment period accounted for the CV benefit, though the risk of severe hypoglycemia was strongly and inversely correlated to the achieved HbA1c (10,11). Consistently, in our case-control analysis, severe hypoglycemia was protective with respect to CVD, but this effect was merely due to HbA1c. On the other hand, potential deleterious effects of severe hypoglycemia on CV outcomes may be overestimated because patients experiencing severe hypoglycemia had a longer duration of diabetes and greater burden of microvascular complications. However, our results were unaltered by adjustment for both diabetes duration and microvascular complications.
Third, both prospective analysis on incidence of CVD and case-control analysis at the follow-up examination were based on severe hypoglycemic episodes occurring in the previous year and no data were available throughout the follow-up period. However, we found a correlation between baseline and follow-up of severe hypoglycemic events in keeping with the DCCT results, showing that prior episodes of severe hypoglycemia are the strongest predictor of risk of future episodes (9). Fourth, repeated mild/modest hypoglycemic episodes may have a greater impact on CVD than severe ones, and we had no baseline information on nonsevere hypoglycemic episodes. However, at follow-up, severe episodes correlated with total hypoglycemic episodes, and inclusion of nonsevere hypoglycemic episodes in the logistic analyses did not alter the results. Fifth, the release of the confidential cause of death data was difficult due to legal restrictions in a number of countries, which led to a relatively large number of unknown causes of death. However, based on the incidence of CV death in the EURODIAB cohort, the few missing cases of CV death would have had a negligible effect on the final results. In addition, the baseline distribution of severe hypoglycemic episodes did not differ between patients remaining in the study and those who were lost to follow-up, including subjects with unknown causes of death. Sixth, type 1 diabetes was defined based on age and insulin treatment; however, only patients with continuous insulin therapy initiated <1 year from diagnosis were selected, making misclassification of diabetes unlikely. Finally, in the nested case-control study, the number of control subjects was lower than the overall number of case subjects, thus reducing the power of analyses.
In conclusion, our data suggest that severe hypoglycemic episodes in type 1 diabetes are not associated with changes in either nontraditional CV risk factors or an increase in the incidence of CVD. Therefore, our data do not support the hypothesis that severe hypoglycemia in patients with type 1 diabetes increases the risk of CVD.
Acknowledgments
This work was supported by the Compagnia di San Paolo, the University of Turin, the European Community, and the Wellcome Trust.
No potential conflicts of interest relevant to this article were reported.
G.G. and G.B. researched data and wrote the manuscript. F.B. wrote the manuscript. N.C. researched data and reviewed and edited the manuscript. C.S. and D.R.W. researched data. C.D.S. contributed to the discussion and reviewed and edited the manuscript. J.H.F. and P.C.P. contributed to the discussion. G.G. and G.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all the investigators and patients who took part in this study.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1531/-/DC1.
The complete list of investigators can be found in the Supplementary Data online.
References
- 1.Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010;33:1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 2011;34:1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003;26:1485–1489 [DOI] [PubMed] [Google Scholar]
- 4.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010;375:481–489 [DOI] [PubMed] [Google Scholar]
- 5.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009;301:1556–1564 [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation 2008;117:1018–1027 [DOI] [PubMed] [Google Scholar]
- 7.Pinto DS, Skolnick AH, Kirtane AJ, et al. TIMI Study Group U-shaped relationship of blood glucose with adverse outcomes among patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2005;46:178–180 [DOI] [PubMed] [Google Scholar]
- 8.Svensson AM, McGuire DK, Abrahamsson P, Dellborg M. Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J 2005;26:1255–1261 [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 11.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giménez M, Gilabert R, Monteagudo J, et al. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care 2011;34:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dandona P, Chaudhuri A, Dhindsa S. Proinflammatory and prothrombotic effects of hypoglycemia. Diabetes Care 2010;33:1686–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010;33:1529–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism 2009;58:443–448 [DOI] [PubMed] [Google Scholar]
- 16.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010;33:1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson J, Fuller JH. Microvascular and acute complications in IDDM patients: the EURODIAB IDDM Complications Study. Diabetologia 1994;37:278–285 [DOI] [PubMed] [Google Scholar]
- 18.Koivisto VA, Stevens LK, Mattock M, et al. EURODIAB IDDM Complications Study Group Cardiovascular disease and its risk factors in IDDM in Europe. Diabetes Care 1996;19:689–697 [DOI] [PubMed] [Google Scholar]
- 19.Stephenson JM, Kempler P, Perin PC, Fuller JH. Is autonomic neuropathy a risk factor for severe hypoglycaemia? The EURODIAB IDDM Complications Study. Diabetologia 1996;39:1372–1376 [DOI] [PubMed] [Google Scholar]
- 20.van Hecke MV, Dekker JM, Stehouwer CD, et al. EURODIAB prospective complications study Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005;28:1383–1389 [DOI] [PubMed] [Google Scholar]
- 21.Joint National Committee on Prevention The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997;157:2413–2446 [DOI] [PubMed] [Google Scholar]
- 22.Kearney EM, Mount JN, Watts GF, Slavin BM, Kind PRN. Simple immunoturbidimetric method for determining urinary albumin at low concentrations using Cobas-Bio centrifugal analyser. J Clin Pathol 1987;40:465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 24.Schram MT, Chaturvedi N, Schalkwijk C, et al. EURODIAB Prospective Complications Study Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2003;26:2165–2173 [DOI] [PubMed] [Google Scholar]
- 25.Schram MT, Schalkwijk CG, Bootsma AH, Fuller JH, Chaturvedi N, Stehouwer CD, EURODIAB Prospective Complications Study Group Advanced glycation end products are associated with pulse pressure in type 1 diabetes: the EURODIAB Prospective Complications Study. Hypertension 2005;46:232–237 [DOI] [PubMed] [Google Scholar]
- 26.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes—the EURODIAB Prospective Complications Study. Diabetologia 2005;48:370–378 [DOI] [PubMed] [Google Scholar]
- 27.Gruden G, Bruno G, Chaturvedi N, et al. EURODIAB Prospective Complications Study Group Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes 2008;57:1966–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruden G, Bruno G, Chaturvedi N, et al. EURODIAB Prospective Complications Study Group ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/ macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med 2009;266:527–536 [DOI] [PubMed] [Google Scholar]
- 29.Burt D, Bruno G, Chaturvedi N, et al. Anti-heat shock protein 27 antibody levels and diabetes complications in the EURODIAB study. Diabetes Care 2009;32:1269–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shipchandler MT, Moore EG. Rapid, fully automated measurement of plasma homocyst(e)ine with the Abbott IMx analyzer. Clin Chem 1995;41:991–994 [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 1998 [Google Scholar]
- 32.Turnbull FM, Abraira C, Anderson RJ, et al. Control Group Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 33.Yakubovich N, Gerstein HC. Serious cardiovascular outcomes in diabetes: the role of hypoglycemia. Circulation 2011;123:342–348 [DOI] [PubMed] [Google Scholar]
- 34.Schroeder SA. Multifactorial intervention and mortality in type 2 diabetes. N Engl J Med 2008;358:2292; author reply 2292–2293 [DOI] [PubMed] [Google Scholar]
- 35.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malmberg K, Rydén L, Wedel H, et al. DIGAMI 2 Investigators Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–661 [DOI] [PubMed] [Google Scholar]
- 37.Goyal A, Mehta SR, Díaz R, et al. Differential clinical outcomes associated with hypoglycemia and hyperglycemia in acute myocardial infarction. Circulation 2009;120:2429–2437 [DOI] [PubMed] [Google Scholar]