Abstract
OBJECTIVE
To examine the role of area-level socioeconomic status (SES) on the development of abnormal glucose metabolism (AGM) using national, population-based data.
RESEARCH DESIGN AND METHODS
The Australian Diabetes, Obesity and Lifestyle (AusDiab) study is a national, population-based, longitudinal study of adults aged ≥25 years. A sample of 4,572 people provided complete baseline (1999 to 2000) and 5-year follow-up (2004 to 2005) data relevant for these analyses. Incident AGM was assessed using fasting plasma glucose and 2-h plasma glucose from oral glucose tolerance tests, and demographic, socioeconomic, and behavioral data were collected by interview and questionnaire. Area SES was defined using the Index of Relative Socioeconomic Disadvantage. Generalized linear mixed models were used to examine the relationship between area SES and incident AGM, with adjustment for covariates and correction for cluster design effects.
RESULTS
Area SES predicted the development of AGM, after adjustment for age, sex, and individual SES. People living in areas with the most disadvantage were significantly more likely to develop AGM, compared with those living in the least deprived areas (odds ratio 1.53; 95% CI 1.07–2.18). Health behaviors (in particular, physical activity) and central adiposity appeared to partially mediate this relationship.
CONCLUSIONS
Our findings suggest that characteristics of the physical, social, and economic aspects of local areas influence diabetes risk. Future research should focus on identifying the aspects of local environment that are associated with diabetes risk and how they might be modified.
The prevalence of type 2 diabetes and dysglycemia is rapidly increasing in Australia (1) and worldwide (2), and it has been shown that people in the lower socioeconomic groups experience disproportionately high levels of abnormal glucose metabolism (AGM) (including impaired fasting glucose [IFG], impaired glucose tolerance [IGT], and type 2 diabetes) (3–5). Area-level disadvantage as a risk factor for diabetes, however, has received little attention. Yet, if shown to independently influence one’s risk of development of AGM, it would have important implications for policy interventions that target local populations and communities, in addition to the current focus on more individually based behavioral interventions.
National survey monitoring data from Australia shows that rates of self-reported diabetes are greater in areas with greatest disadvantage (6). Work from the U.S. and Scotland also demonstrates the relationship between neighborhood socioeconomic characteristics and diabetes incidence (7–9). These previous studies, however, comprised significant limitations, using select populations without objective measures of incident diabetes. The reliance on self-report for diabetes diagnosis is a particularly important limitation, as rates of both clinical diagnosis and recall of diagnoses may vary by socioeconomic status (SES) (10) and availability of local healthcare resources. Furthermore, the relationship between area SES and diabetes risk is likely to be confounded by individual SES (11); therefore, it is essential to account for individual socioeconomic characteristics in analyses.
The pathways that might explain the relationship between area SES and AGM have not previously been explored. Various models of how the social determinants of health have been proposed. In this study, we draw on the model proposed by Turrell and Mathers (12), which illustrates the stages at which different socioeconomic determinants exert their influence on health. It divides the causes of health inequalities into upstream (such as SES at an individual and environmental level), midstream (individual health behaviors and psychosocial adversity), or downstream (biological risk factors such as obesity) determinants of disease.
The current study aims to examine the prospective relationship between area-level SES and objective measurement of incident AGM using Australian national, population-based data. This dataset also allows us to investigate the potential moderating role of individual SES, another upstream factor, as well as the possible mediation of midstream (health behaviors and psychosocial adversity) and downstream determinants (adiposity).
RESEARCH DESIGN AND METHODS
Study population
The Australian Diabetes, Obesity and Lifestyle (AusDiab) study baseline methods and response rates have been described in detail elsewhere (13). In brief, it is a national, population-based survey of 11,247 adults aged ≥25 years in 1999 to 2000. A stratified cluster sample was drawn from 42 randomly selected census collector district clusters across Australia. Information was collected using a brief household interview, followed by a biomedical examination. Of the eligible adults, 70% completed the household interview, and 55% (n = 11,247) of these completed the baseline biomedical examination (14). All participants provided written consent. In 2004 to 2005, all living eligible participants were invited to attend follow-up. Those who were considered ineligible included those who refused further contact (n = 128), were deceased (n = 310), had moved overseas or into a nursing facility classified for high care, or had a terminal illness (n = 21). Among those eligible, 6,537 returned for the 5-year follow-up during which baseline assessment was repeated; the response rate was 60.6%. Compared with nonresponders, attendees were significantly less likely to be hypertensive, to have a lower level of education attainment, or to be smokers, and they had lower 2-h plasma glucose (2hPG), glycated hemoglobin, and smaller waist circumferences at baseline.
Measurements
Abnormal glucose metabolism.
All participants, except for those currently receiving treatment for diabetes or who were pregnant, underwent a standard 75-g oral glucose tolerance test (14). Only those people with normoglycemia at baseline were included in these analyses (1,575 with baseline AGM and an additional 143 with missing data were excluded). The outcome category of incident AGM comprised the pooled categories of IFG, IGT, and diabetes at 5-year follow-up. Glucose tolerance status at baseline and follow-up was based on plasma glucose results, using the 1999 World Health Organization diabetes classification (15). Diabetes was classified on the basis of fasting plasma glucose (FPG) 7.0 mmol/L or 2hPG 11.1 mmol/L or current treatment with insulin or oral hypoglycemic agents. For those not reporting treatment for diabetes, FPG <7.0 mmol/L and 2hPG 7.8 mmol/L but <11.1 mmol/L indicated IGT, FPG 6.1–6.9 mmol/L and 2hPG <7.8 mmol/L indicated IFG, and both FPG <6.1 mmol/L and 2hPG <7.8 mmol/L indicated normal glycemia.
Area-level SES.
Area SES was measured using an aggregate measure of individual-level data called the Index of Relative Socioeconomic Disadvantage (IRSD). The IRSD, one component of the Socioeconomic Indexes for Areas (16), characterizes the general SES of census collection districts (the smallest geographic area defined in the Australian Standard Geographical Classification, comprising an average of 225 dwellings) in Australia. It is developed by the Australian Bureau of Statistics (16) to create a summary measure from a group of 20 variables (related to education, income, employment, family composition, housing benefits, car ownership, ethnicity, English language proficiency, and residential overcrowding) that display dimensions of social disadvantage. Principal components analysis determined the importance of each of the 20 characteristics depending on their interrelationships, and these relationships are used to create a weight for each characteristic (see Supplementary Tables 1 and 2 for further details about Socioeconomic Indexes for Areas, as well as full list of IRSD variables included in the index and their individual weighting). The IRSD has been shown to have excellent construct validity, tested against other large health and economic datasets and through stringent examination by an external expert panel (more information about the IRSD construction and validation process can be found elsewhere [17]). For these analyses, the IRSD scores were based on the 2001 census, and the data were divided into quartiles.
Individual-level variables.
At baseline and follow-up, the physical examination included blood samples, anthropometric measurements, and questionnaires. Details on the glucose and lipid assays used between studies can be found in detail elsewhere (13). Height, weight, and waist were measured as described previously (14). BMI was calculated as weight (kg) divided by the square of the height (m2). Blood pressure was measured using Dinamap or a standard mercury sphygmomanometer with appropriate adjustments made as previously described (13). Individual-level SES data (education and total household income) were collected by an interviewer-administered questionnaire, as was information on health behaviors. Education was classified into four categories, based on the highest educational qualifications received: 1) secondary school education; 2) trade/technical certificates; 3) associate degree, undergraduate diplomas, and nursing/teaching qualifications; and 4) bachelor's degree and postgraduate qualifications. Total household income per week was assessed by a question asking the participant to choose the closest income band for their total household income before tax. The responses were categorized into tertiles.
Total leisure-time physical activity was measured using the Active Australia questionnaire (18). Sedentary behavior was measured using self-reported television viewing as used previously (19); this determined the total time spent watching television or videos in the preceding week. Smoking history (current smoker, past smoker, or never smoked) was collected using a previously validated instrument (20). Diet was measured using the self-administered Anti-Cancer Council of Victoria food frequency questionnaire (21), and an overall index of diet quality was created using a scale of 1–100 (with 100 indicating high diet quality) (22). Alcohol data were also obtained from the Anti-Cancer Council of Victoria food frequency questionnaire, as a continuous measure expressed in grams of ethanol per day. Perceived stress was measured at baseline using the Perceived Stress Questionnaire (23). The total score ranged from 0 to 1; these scores were then categorized into quartiles.
Statistical analysis
Baseline demographic, socioeconomic, behavioral, and biochemical characteristics across the four categories of area SES were analyzed using complex survey methods to allow for the clustered design in the survey. Differences in proportions presented in Table 1 were analyzed with Pearson χ2 tests, whereas differences in means were tested using analyses of variance.
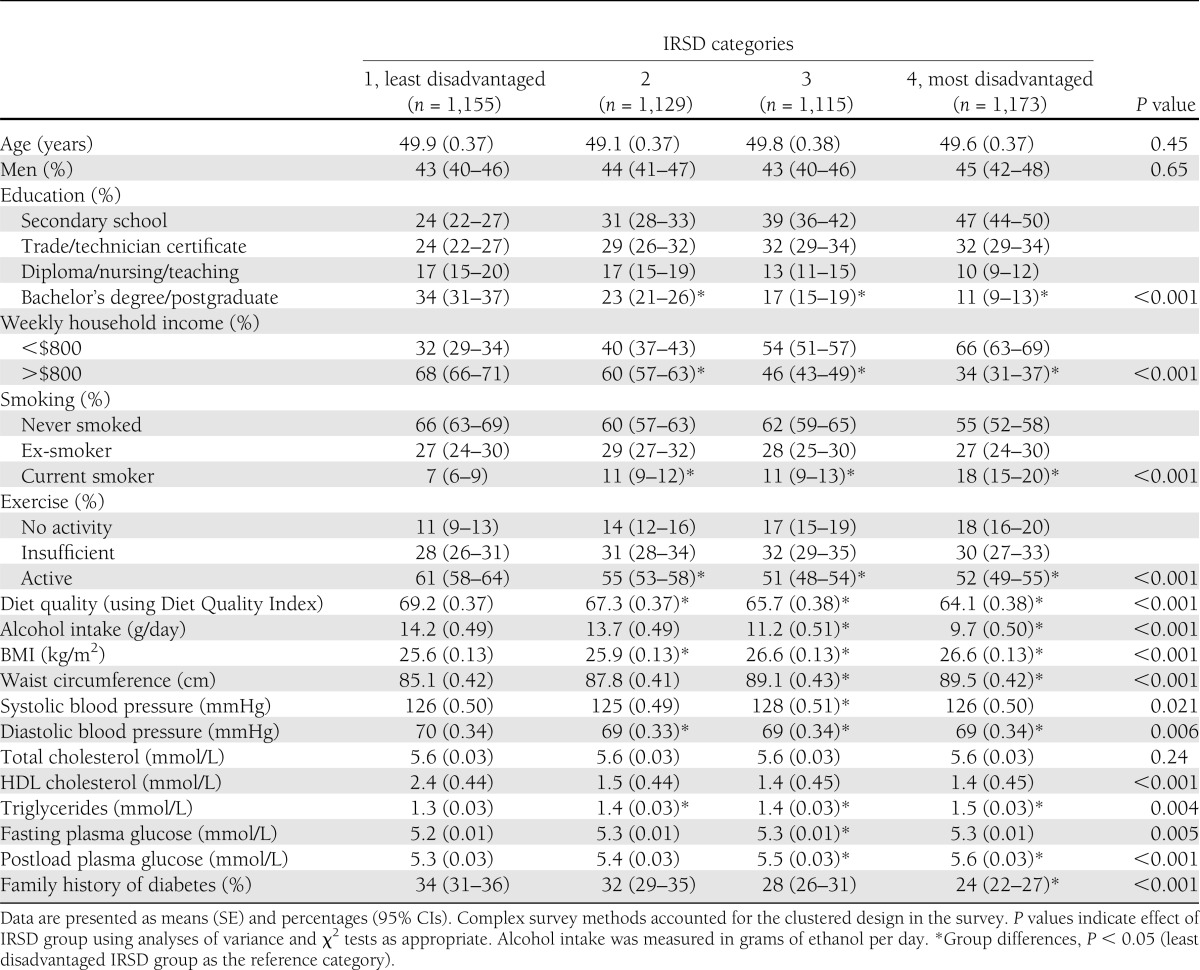
Table 1.
Baseline characteristics (means and percentages) of the sample according to area SES categories
To allow for the fact that individuals living within clusters were more likely to be similar to each other compared with others from different clusters and to accommodate the collinearity between the clusters and area disadvantage, generalized linear mixed models (using the binomial distribution with the logit link function) were employed to examine the relationship between area disadvantage and AGM. Because area SES is defined at the collection district level, all members of a cluster had the same area SES level; therefore, collection district was included as the random effects term to correct for the design effects. Collinearity between fixed-effects variables was assessed using bivariate correlations; none were observed. The fixed effects for Model 1 were age, sex, and area SES (the area with the lowest area disadvantage was used as the reference category). Interaction terms were included to detect any interactions among age and sex and area SES. No interaction effects were identified, and therefore, these terms were omitted from the analyses. Age- and sex-stratified analyses were performed, and similar relationships were observed across sex and age categories (results not shown). Model 2 included the other upstream variables, individual markers of SES, education, and household income as potential confounding variables, with Model 3 incorporating the midstream health behaviors of physical activity, sedentary behavior, and smoking as possible mediators. Missing data for diet quality (n = 145) and alcohol consumption (n = 289) meant these variables could not be included in main models; however, sensitivity analyses tested their inclusion, and the same results for Model 3 were observed. Models 4 and 5 assessed the roles of stress (perceived stress) and central adiposity (waist circumference), respectively, as possible mid- and downstream mediators of the relationship between area SES and AGM (respectively). The final fully adjusted model (Model 6) included all previous covariates, as well as the baseline metabolic syndrome components of systolic blood pressure (SBP; defined according to 10-mmHg intervals), triglycerides, and HDL cholesterol. Potential mediation by mid- and downstream variables was inferred by attenuation of the odds ratio (OR) for area SES on AGM in relevant models. To test for trend, the models were reanalyzed, with area SES (IRSD) included as a continuous variable in separate generalized linear mixed models. Analyses were performed using SPSS version 19 (IBM), with a significance level of P < 0.05.
RESULTS
Baseline characteristics
Table 1 presents the unadjusted baseline characteristics of the sample, divided according to the four categories of area socioeconomic disadvantage. People living in the more disadvantaged areas reported having lower educational achievement and receiving significantly lower weekly incomes compared with the people in the least disadvantaged area. In general, there were significant behavioral and biological differences between the areas, with people living in the more socially disadvantaged areas more likely to report smoking, physical inactivity, and lower quality of diet, as well as having unhealthier cardiovascular disease risk profiles (according to anthropometric, lipid, and glucose levels) at baseline compared with people in the area with the least disadvantage.
Area SES as a predictor of AGM
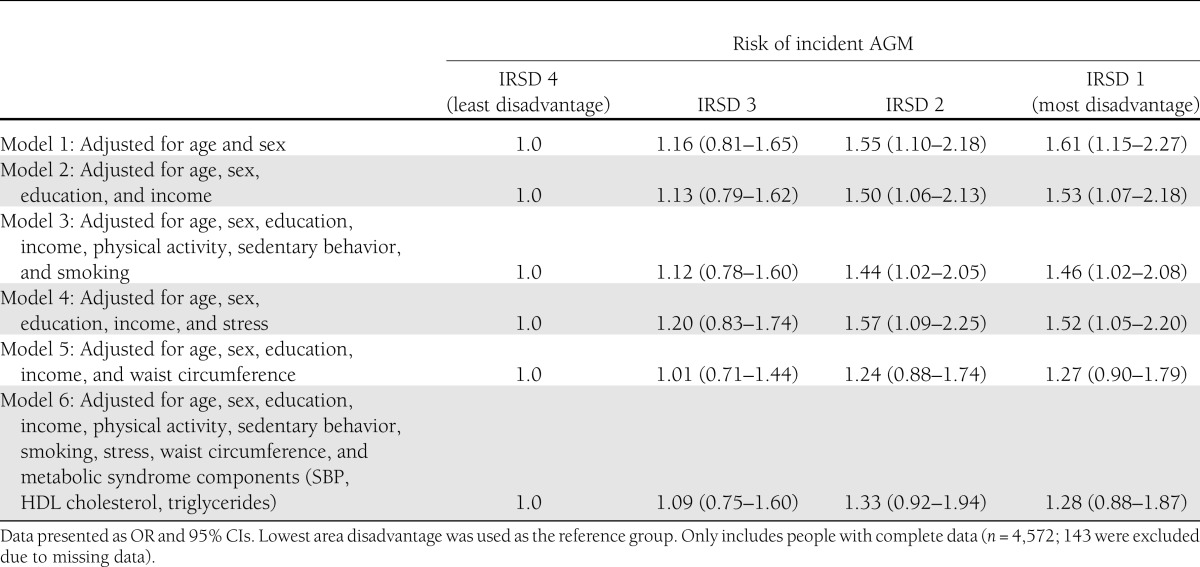
A total of 478 participants had developed AGM after 5 years (143 with IFG, 277 with IGT, and 58 with type 2 diabetes). As shown in Table 2, area SES was a significant predictor of AGM at follow-up in this sample, after controlling for age and sex (P = 0.002). This was a graded relationship with people living in the areas with greater social disadvantage being more likely to develop an impairment of glucose metabolism compared with those living in areas with lesser disadvantage. The test for trend showed that SES, as a continuous variable, was significantly related to AGM (P = 0.006). Adjustment for individual SES did not substantially attenuate the associations between area SES and AGM (Model 2), with area-level disadvantage remaining a significant predictor of incident AGM (OR 1.53; 95% CI 1.07–2.18). With the inclusion of physical activity, sedentary behavior, and smoking in Model 3, physical inactivity was associated with increased AGM (P = 0.004). This adjustment for health behaviors mildly attenuated the estimates of the relationship between area SES and AGM (1.46; 1.02–2.08). Adjustment for perceived stress in Model 4 did not affect the association between area SES and AGM (1.52; 1.05–2.20)
Table 2.
Risk of incident abnormal glucose metabolism, according to level of socioeconomic disadvantage
The inclusion of waist circumference in Model 5 attenuated the associations, such that elevated risk of AGM in individuals living in the most disadvantaged, compared with the least disadvantaged, areas were no longer statistically significant, although there remained a trend toward increased risk of AGM in the most deprived area (OR 1.27, 95% CI 0.90–1.79). Waist circumference was a significant predictor of AGM (1.05, 1.04–1.06). Model 6 made adjustments for the potential confounders of education and income, the possible mediators of physical activity, sedentary behavior, smoking, stress, and waist circumference, as well as the metabolic syndrome components of SBP, triglycerides, and HDL cholesterol. In this model, only age (1.02; 1.01–1.03), waist circumference (1.03; 1.02–1.04), SBP (1.13; 1.05–1.21), and triglycerides (1.28; 1.16–1.42) were shown to predict incident AGM. Area-level SES was no longer significantly associated with an increased risk of developing glucose metabolism dysfunction (1.28; 0.88–1.87).
CONCLUSIONS
Using population-based data, we have shown that people living in areas with greater socioeconomic disadvantage were significantly more likely to develop AGM compared with those in areas with less disadvantage. This relationship persisted despite the inclusion of measures of individual SES. This finding indicates that area-level factors are relevant to the development of diabetes, beyond their relationship with individual socioeconomic characteristics. Although one’s choice of residential neighborhood is clearly patterned by individual factors, such as income and ethnicity, it has been demonstrated that measurement of individual SES does not capture a person’s complete socioeconomic experience (11). Understanding the different levels of factors that influence the progression of diabetes is essential if we are to successfully reduce the escalating rates. Most importantly, the findings from this study identify people’s environmental circumstances as an important focus for interventions to prevent diabetes and its progression, in addition to the current focus on interventions directed primarily at individual level factors.
To our knowledge, this is the first study to use objective markers of glucose tolerance to explore the relationship between area-level socioeconomic characteristics and the development of AGM. Previous studies have included select populations with self-report diabetes outcomes (7–9). There is some evidence that undiagnosed diabetes is associated with lower SES (10), a finding supported by unpublished AusDiab data. Therefore, the investigation of socioeconomic predictors of diabetes using self-report doctor-diagnosed diabetes may bias the association between area SES and incident diabetes to the null.
This is also the first study, of which we are aware, to investigate the role of mid- and downstream mediators of the association between area SES and AGM. The inclusion of the health behaviors, in particular physical activity, in these analyses caused partial attenuation of the impact of area disadvantage on AGM (missing data for diet quality meant that this behavior was not included in the analyses; however, separate analyses indicate that its inclusion would not have affected the relationships observed), suggesting that aspects of the environment patterned by area SES may influence health behavior patterns and thus impact the risk of diabetes. This partial mediation of health behaviors on the relationship between area SES and diabetes has been observed previously (7). The Multi-Ethnic Study of Atherosclerosis has found that neighborhoods characterized by greater physical activity resources and availability of healthy food had a 38% lower incidence of diabetes (24). Other work has observed a socioeconomic gradient in the density of fast food options (25) and food-purchasing behavior, with people living in low SES areas in Australia significantly less likely to buy healthy food options (26). These findings were independent of individual socioeconomic variables. Although other evidence supporting such findings is equivocal (27,28), these results suggest there may be area-level differences in the availability and accessibility of these resources and that the differences are not solely due to individual level factors such as choice and affordability.
The inclusion of perceived stress in our analyses as a potential midstream-mediating variable did not influence the relationship between area SES and AGM. Previous research has not explicitly examined this relationship, although greater levels of psychosocial adversity, such as stress and depression, have been reported in areas of low SES (29,30). It is possible that the single time-point assessment in this study may not have been sensitive enough to measure the cumulative impact of long-term exposure to psychosocial disadvantage (31). Other psychosocial factors, such as the safety of an area, are also crucial to encourage people to participate in exercise, and people living in areas with lower SES are more likely to report feeling unsafe (32). Furthermore, other perceptions of one’s environment, such as perceived access to attractive public areas, may affect residents’ physical activity behavior (33).
We have shown that the downstream variable of adiposity, determined in these analyses by the central adiposity measure of waist circumference, explained a large proportion of the relationship between area SES and AGM. This supports findings from the U.S. Black Women’s Health Study, which found that the association between neighborhood SES and incidence of self-reported diabetes was attenuated by the inclusion of BMI in the model and therefore likely to be a key mechanism in the pathway linking SES and diabetes (7).
For these analyses, we used the category of AGM as the incident outcome measure instead of diabetes alone. For this, the categories of IFG, IGT, and diabetes were combined, because they share the common pathophysiological mechanisms of insulin resistance and β-cell dysfunction (34,35). Given the short follow-up period of 5 years, we chose to use a binary classification system indicating a wide spread of hyperglycemia and disordered carbohydrate metabolism.
The national, population-based nature of this sample and the quality of the glucose tolerance data are significant strengths of our study, and therefore, the results may be generalizable to other populations of similar ethnicity and socioeconomic range. However, there are also some limitations to be considered. This study was not able to assess whether these results are generalizable across all ethnic groups; the AusDiab sample included limited ethnic group variation (97% spoke English as a first language, and only 0.7% were of Aboriginal or Torres Strait Island origin). The inclusion of language and ethnicity in the analyses did not influence the results presented (data not shown). Differences observed between responders and nonresponders mean that the sample may be subject to selection bias, the effects of which cannot be fully ascertained. However, lower SES recorded in nonresponders (according to education levels) could indicate that our results have underestimated the strength of the relationship between SES and AGM. The single time-point assessment of area disadvantage also limits the measurement of social mobility across time. The origins of socioeconomic and behavioral risk factors track back to the early years of life, even in utero (36). Although education was the earliest indicator of SES available, we did adjust for birth weight as a marker of possible early life exposure (results not shown). This did not affect the predictive impact of area SES on incident AGM, and therefore, the socioenvironmental factors that influence early development and promote disease later in life did not appear to moderate the relationships observed. The self-reported behavioral data in this study means that the contributions of these variables may not be accurate, and this may partly explain why the inclusion of behavioral variables had a small effect on the socioeconomic gradient in AGM. It is possible that the adjustment for individual SES in the analyses may introduce methodological issues since the measurement of area SES comprised an aggregation of the socioeconomic characteristics of its residents (11). This is, however, an accepted method of determining the effects attributable to the individual- and area-level contexts (7,24,26). Although we adjusted for education and income, it is possible that residual confounding remained, and we were not able to take into account the full estimate of these individual effects. Conversely, as described by King et al. (37), adjustment for the individual characteristics may represent an overadjustment, because these variables may act as mediators in the relationship between area SES and AGM. More work should focus on the extent to which individual characteristics moderate or mediate the relationship between area-level disadvantage and health (38). We acknowledge that the inclusion of upstream and downstream factors in our final model makes the parameter estimates difficult to interpret.
Our findings confirm the socioeconomic gradient in the development of AGM and extend our understanding of the influence of area-level socioeconomic characteristics. The results indicate that mid- and downstream variables of health behaviors and central adiposity partially mediate this relationship. As discussed by Turrell (39), to reduce socioeconomic disparities in society, policies are needed that focus on upstream determinants. However, interventions at each level of the disease process are likely to have the greatest impact (39). Therefore, designing community-level programs that target these individual risk factors may help address the socioeconomic gradient in chronic disease risk. Furthermore, our findings also have significant policy implications for neighborhood and area planning (40) and the allocation of resources to lower socioeconomic areas for the prevention of diabetes.
ACKNOWLEDGMENTS
E.D.W. is supported by a Diabetes UK Fellowship (09/0003833). D.J.M. is supported by the Victorian Cancer Agency Fellowship for 2011. C.E.S. is supported by National Health and Medical Research Council (NHMRC) Health Services Research Grant 456130. J.E.S. is supported by NHMRC Fellowship 586623. The AusDiab study, co-coordinated by the Baker IDI Heart and Diabetes Institute, gratefully acknowledges the generous support given by NHMRC Grant 233200, Australian Government Department of Health and Ageing, Abbott Australasia Pty Ltd, Alphapharm Pty Ltd, AstraZeneca, Bristol-Myers Squibb, City Health Centre-Diabetes Service-Canberra, Department of Health and Community Services–Northern Territory, Department of Health and Human Services–Tasmania, Department of Health–New South Wales, Department of Health–Western Australia, Department of Health–South Australia, Department of Human Services–Victoria, Diabetes Australia, Diabetes Australia Northern Territory, Eli Lilly Australia, Estate of the Late Edward Wilson, GlaxoSmithKline, Jack Brockhoff Foundation, Janssen-Cilag, Kidney Health Australia, Marian and E.H. Flack Trust, Menzies Research Institute, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk, Pfizer Pty Ltd, Pratt Foundation, Queensland Health, Roche Diagnostics Australia, Royal Prince Alfred Hospital, Sydney, sanofi-aventis, and Sanofi Synthelabo. The AusDiab study was also supported in part by the Victorian Government’s Operational Infrastructure Support Program. No other potential conflicts of interest relevant to this article were reported.
E.D.W. wrote the manuscript. D.J.M. contributed to discussion, analyses, and manuscript writing and reviewed/edited the manuscript. P.Z.Z. reviewed/edited the manuscript. A.M.K., B.F.O., and J.E.S. contributed to discussion and reviewed/edited the manuscript. C.E.S. contributed to discussion and analyses and reviewed/edited the manuscript. E.D.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
For invaluable contributions to the setup and field activities of AusDiab, the authors thank the Baker IDI Heart and Diabetes Institute staff at the time of data collection: B. Atkins (also of Monash Medical Centre), A. Bonney, M. Dalton, M. de Courten (now of University of Copenhagen), D. Dunstan, H. Jahangir, A. Meehan, N. Meinig, S. Murray, A. Stewart, R. Tapp (now of National Vision Research Institute), T. Whalen, F. Wilson, and P. Zimmet, as well as A. Allman and C. Reid, HITECH Pathology; S. Bennett, Australian Institute of Health & Welfare; S. Chadban, University of Sydney; T. Dwyer, Menzies Centre for Population Research; D. Jolley, Monash University; D. McCarty, Centre for Eye Research Australia; K. O’Dea, Menzies School of Health Research; K. Polkinghorne, Monash Medical Centre; P. Phillips, Queen Elizabeth Hospital; and H. Taylor, Centre for Eye Research Australia.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1410/-/DC1.
References
- 1.Magliano DJ, Peeters A, Vos T, et al. Projecting the burden of diabetes in Australia—what is the size of the matter? Aust N Z J Public Health 2009;33:540–543 [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 3.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med 2004;164:1873–1880 [DOI] [PubMed] [Google Scholar]
- 4.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and diagnosed diabetes incidence. Diabetes Res Clin Pract 2005;68:230–236 [DOI] [PubMed] [Google Scholar]
- 5.Williams ED, Tapp RJ, Magliano DJ, Shaw JE, Zimmet PZ, Oldenburg BF. Health behaviours, socioeconomic status and diabetes incidence: the Australian Diabetes Obesity and Lifestyle Study (AusDiab). Diabetologia 2010;53:2538–2545 [DOI] [PubMed] [Google Scholar]
- 6.Turrell G, Stanley L, de Looper M, et al. Health Inequalities in Australia: Morbidity, health behaviours, risk factors and health service use, Vol 2 Canberra, Queensland University of Technology and the Australian Institute of Health and Welfare, 2006 [Google Scholar]
- 7.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women’s Health Study. Am J Epidemiol 2010;171:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schootman M, Andresen EM, Wolinsky FD, et al. The effect of adverse housing and neighborhood conditions on the development of diabetes mellitus among middle-aged African Americans. Am J Epidemiol 2007;166:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox M, Boyle PJ, Davey PG, Feng Z, Morris AD. Locality deprivation and Type 2 diabetes incidence: a local test of relative inequalities. Soc Sci Med 2007;65:1953–1964 [DOI] [PubMed] [Google Scholar]
- 10.Rathmann W, Haastert B, Icks A, et al. KORA Study Group Sex differences in the associations of socioeconomic status with undiagnosed diabetes mellitus and impaired glucose tolerance in the elderly population: the KORA Survey 2000. Eur J Public Health 2005;15:627–633 [DOI] [PubMed] [Google Scholar]
- 11.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010;1186:125–145 [DOI] [PubMed] [Google Scholar]
- 12.Turrell G, Mathers CD. Socioeconomic status and health in Australia. Med J Aust 2000;172:434–438 [DOI] [PubMed] [Google Scholar]
- 13.Magliano DJ, Barr EL, Zimmet PZ, et al. Glucose indices, health behaviors, and incidence of diabetes in Australia: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2008;31:267–272 [DOI] [PubMed] [Google Scholar]
- 14.Dunstan DW, Zimmet PZ, Welborn TA, et al. Australian Diabetes, Obesity and Lifestyle Study (AusDiab) The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)—methods and response rates. Diabetes Res Clin Pract 2002;57:119–129 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Report of a WHO Consultation. Geneva, Switzerland, World Health Organization, 1999 [Google Scholar]
- 16.Australian Bureau of Statistics. Information paper: Census of population and housing. Socio-economic indexes for areas, Australia 2001 [Internet], 2001. Available from http://www.ausstats.abs.gov.au/ausstats/free.nsf/0/AFF5E8542B58B94ECA256DD5007A3DF8/$File/20390_2001.pdf Accessed 16 March 2012
- 17.Australian Bureau of Statistics. Technical paper: Census of population and housing. Socio-economic indexes for areas (SEIFA), Australia 2001 [Internet], 2001. Available from http://www.ausstats.abs.gov.au/ausstats/free.nsf/0/A5561C69BF600637CA256E20007B5DF1/$File/2039055001_2001.pdf Accessed 16 March 2012
- 18.Australian Institute of Health and Welfare The Active Australia Survey: A Guide and Manual for Implementation, Analysis and Reporting. Canberra, Australian Institute of Health and Welfare, 2003 [Google Scholar]
- 19.Dunstan DW, Salmon J, Owen N, et al. AusDiab Steering Committee Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia 2005;48:2254–2261 [DOI] [PubMed] [Google Scholar]
- 20.Australian Institute of Health and Welfare Standard questions on the use of tobacco among adults. Canberra, Australian Institute of Health and Welfare, 1998 [Google Scholar]
- 21.Ireland P, Jolley D, Giles G, et al. Development of the Melbourne FFQ: a food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac J Clin Nutr 1994;3:19–31 [PubMed] [Google Scholar]
- 22.McNaughton SA, Dunstan DW, Ball K, Shaw J, Crawford D. Dietary quality is associated with diabetes and cardio-metabolic risk factors. J Nutr 2009;139:734–742 [DOI] [PubMed] [Google Scholar]
- 23.Levenstein S, Prantera C, Varvo V, et al. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res 1993;37:19–32 [DOI] [PubMed] [Google Scholar]
- 24.Auchincloss AH, Diez Roux AV, Mujahid MS, Shen M, Bertoni AG, Carnethon MR. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the Multi-Ethnic study of Atherosclerosis. Arch Intern Med 2009;169:1698–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reidpath DD, Burns C, Garrard J, Mahoney M, Townsend M. An ecological study of the relationship between social and environmental determinants of obesity. Health Place 2002;8:141–145 [DOI] [PubMed] [Google Scholar]
- 26.Turrell G, Bentley R, Thomas LR, Jolley D, Subramanian S, Kavanagh AM. A multilevel study of area socio-economic status and food purchasing behaviour. Public Health Nutr 2009;12:2074–2083 [DOI] [PubMed] [Google Scholar]
- 27.Thornton LE, Bentley RJ, Kavanagh AM. Individual and area-level socioeconomic associations with fast food purchasing. J Epidemiol Community Health 2011;65:873–880 [DOI] [PubMed] [Google Scholar]
- 28.Macdonald L, Ellaway A, Macintyre S. The food retail environment and area deprivation in Glasgow City, UK. Int J Behav Nutr Phys Act 2009;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mair C, Diez Roux AV, Galea S. Are neighbourhood characteristics associated with depressive symptoms? A review of evidence. J Epidemiol Community Health 2008;62:940–946 [DOI] [PubMed] [Google Scholar]
- 30.Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann Behav Med 2001;23:177–185 [DOI] [PubMed] [Google Scholar]
- 31.Amick BC, 3rd, McDonough P, Chang H, Rogers WH, Pieper CF, Duncan G. Relationship between all-cause mortality and cumulative working life course psychosocial and physical exposures in the United States labor market from 1968 to 1992. Psychosom Med 2002;64:370–381 [DOI] [PubMed] [Google Scholar]
- 32.Wilson DK, Kirtland KA, Ainsworth BE, Addy CL. Socioeconomic status and perceptions of access and safety for physical activity. Ann Behav Med 2004;28:20–28 [DOI] [PubMed] [Google Scholar]
- 33.Giles-Corti B, Donovan RJ. Socioeconomic status differences in recreational physical activity levels and real and perceived access to a supportive physical environment. Prev Med 2002;35:601–611 [DOI] [PubMed] [Google Scholar]
- 34.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 35.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King T, Kavanagh AM, Jolley D, Turrell G, Crawford D. Weight and place: a multilevel cross-sectional survey of area-level social disadvantage and overweight/obesity in Australia. Int J Obes (Lond) 2006;30:281–287 [DOI] [PubMed] [Google Scholar]
- 38.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health 2001;91:1783–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turrell G. Reducing socioeconomic health inequalities: issues of relevance for policy. N S W Public Health Bull 2002;13:47–49 [DOI] [PubMed] [Google Scholar]
- 40.Oldenburg B, McGuffog ID, Turrell G. Socioeconomic determinants of health in Australia: policy responses and intervention options. Med J Aust 2000;172:489–492 [DOI] [PubMed] [Google Scholar]