Abstract
OBJECTIVE
Epidemiological evidence of diabetes as a lung cancer risk factor is limited and conflicting. Therefore, we assessed associations among diabetes, diabetes therapy, and lung cancer risk in postmenopausal women participating in the Women’s Health Initiative (WHI) study.
RESEARCH DESIGN AND METHODS
Postmenopausal women (n = 145,765), ages 50–79 years, including 8,154 women with diabetes at study entry were followed for a mean of 11 years with 2,257 lung cancers diagnosed. Information on diabetes therapy was collected via two methods (self-reported information on treatment history collected on a questionnaire at baseline and a face-to-face review of current medication containers that participants brought to the baseline visit). Lung cancers were confirmed by central medical record and pathology report review. Cox proportional hazards regression models adjusted for lung cancer risk factors were used to estimate hazard ratios (HRs) (95% CI) for diagnosis of diabetes and treatment of disease as risk factors for lung cancer.
RESULTS
Compared with women without diabetes, women with self-reported treated diabetes had a significantly higher risk of lung cancer (HR 1.27 [95% CI 1.02–1.59]), with risks increasing for women with diabetes requiring insulin treatment (1.71 [1.15–2.53]). However, we did not observe a significant association between lung cancer risk and diabetes not treated with medication or with duration of diabetes.
CONCLUSIONS
Postmenopausal women with treated diabetes, especially those using insulin, have a significantly higher risk of lung cancer. The influence of diabetes severity and specific classes of therapy for diabetes on lung cancer risk require future study.
The prevalence of diabetes has been rapidly growing worldwide and has become a major public health concern. Epidemiological studies have shown that diabetes is associated with increased risk of several types of cancer, notably liver, pancreatic, endometrial, and colorectal cancers (1).
Lung cancer is the leading cause of cancer-related death globally and in the U.S. (2). Preclinical studies support a role for diabetes and/or hyperglycemia in lung cancer development and growth (3–8). However, epidemiological evidence on the association of diabetes with lung cancer is limited and conflicting (1). Whereas some studies have reported significant (9) or nonsignificant higher risks of lung cancer associated with diabetes (10–13), especially among women (12,13), others have reported an inverse association between diabetes and lung cancer (14–17). These inconsistent results could stem from a number of factors including small sample size, different study design, or potential misclassification of exposures or confounding.
The primary barrier to a more clear understanding of the association has been the lack of prospective studies of sufficient size and duration. The Women’s Health Initiative (WHI) is well positioned to overcome this barrier: it is a large prospective cohort study of postmenopausal women in which detailed information on diabetes and potential risk factors was collected at baseline, with 2,257 lung cancer cases adjudicated by centrally trained physicians through September 2010. In this study, we assessed associations among diabetes, diabetes therapy, and lung cancer risk in women participating in the WHI.
RESEARCH DESIGN AND METHODS
Women’s Health Initiative
The WHI was designed to address the major causes of morbidity and mortality in postmenopausal women (18), including both multicenter clinical trials and an observational study. Details of the scientific rationale, eligibility requirements, and baseline characteristics of the participants in the WHI have previously been published (19–23). Briefly, a total of 161,808 women ages 50–79 years were recruited at 40 clinical centers throughout the U.S. between 1 September 1993 and 31 December 1998. The WHI clinical trial includes four overlapping components: two hormone therapy trials (27,347 women), a dietary modification trial (48,835 women), and a calcium/vitamin D supplementation trial (36,282 women). Participants in the observational study included 93,676 women who were screened for the clinical trials but proved to be ineligible or unwilling to participate or were recruited through a direct invitation for the observational study. The study was overseen by institutional review boards at all 40 clinical centers and at the coordinating center, as well as by a study-wide data- and safety-monitoring board. All WHI participants gave informed signed consent.
The following participants were excluded from the original cohort of 161,808 for this analysis: 14,849 women who had a history of cancer (except nonmelanoma skin cancer) at baseline, 783 women who enrolled in WHI but provided no follow-up information, 217 women who were diagnosed with diabetes before age 20 years and/or who were ever hospitalized for diabetic coma (these were deemed likely to have type 1 diabetes diagnosis and not comparable), and 194 women who had missing values of the main exposures (including diagnosis of diabetes, age at diagnosis, and diabetes treatment). After exclusions, 145,765 women remained for further analysis.
Measurement of exposures, outcome, and confounders
Exposures.
Prevalence of diabetes at enrollment was defined by a positive answer to the following question: “Did a doctor ever say that you had sugar diabetes or high blood sugar when you were not pregnant?”. Treated diabetes at enrollment was defined as the participant reporting ever having been treated for diabetes with pills or insulin shots. Information on diabetes drug therapy was collected at baseline, when women were instructed to bring all medications that they had used at least once in the previous 2 weeks for review. All medications were matched to the Master Drug DataBase (MDDB; Medi-span, Indianapolis, IN). Women with diabetes were categorized into four mutually exclusive groups based on the drug inventory information: 1) no diabetes medication, 2) metformin alone use, 3) other oral medication use alone (without insulin), and 4) insulin use (alone and with oral medication). Thus, for example, if the inventory listed both metformin and insulin, we grouped it into the insulin use group. The duration of diabetes at enrollment was based on the difference between age of the participant when first diagnosed with diabetes and age at enrollment. Self-reported type of treatment for diabetes at enrollment was also created by combining the self-reported variables at enrollment: diabetes status and treatment information for diabetes. It was categorized as 1) no diabetes, 2) diabetes not treated with medication, 3) diabetes treated with oral medications alone, and 4) diabetes treated with insulin (alone and with oral medication).
Incidence of medically treated diabetes was also determined during WHI follow-up. The definition of incident diabetes was a positive response to the question on either the semiannual or annual follow-up questionnaires: “Since the date given on this form has a doctor prescribed for the first time any of the following pills or treatments?”, and subsequent selection of any of the following responses: pills for diabetes, insulin shots for diabetes, or (after 2005) diet and/or physical activity for diabetes.
Diagnosis of diabetes based on participant self-report was previously evaluated and deemed reliable. A validation study using a randomly selected sample of baseline specimens from the entire WHI population has shown that fasting glucose levels ≥126 mg/dL were seen in 3.4% of 5,884 women without self-reported diabetes. In the clinical trials, 79% of women who self-reported treated diabetes at baseline had a diabetes medication in the baseline medication inventory. The corresponding figure for the observational study was 77% (24). In addition, a recent validation study using 715 medical record reviews confirmed 92% of self-reported prevalent diabetes and 82% of self-reported incident diabetes. Evidence of diabetes was found in only 5% of women who did not report diabetes (25).
Follow-up and ascertainment of cases.
Incident lung cancer cases were identified by self-administered questionnaires (administered every 6 months in the clinical trial through 2005 and annually in the clinical trial after 2005 and in observational study), with all cases confirmed by medical record review. All primary lung cancer cases were then coded centrally in accordance with the Surveillance Epidemiology and End Results coding guidelines (ICD-O code 34.0–34.9).
Confounders.
In the multivariable models, we considered a series of potential confounders based on literature, which were also similar to those considered in a previous publication (26), including age at enrollment (<55, 55–59, 60–64, 65–69, 70–74, and ≥75 years), ethnicity (American Indian or Alaska Native, Asian or Pacific Islander, Black or African American, Hispanic/Latino, non-Hispanic white, and other), education (high school or less, some college/technical training, college or some postcollege, and Master’s or higher), smoking status (never, former [including years since quitting: ≥30, 20–29, 10–19, and <10], and current [including cigarettes smoked per day: <5, 5–14, 15–24, and ≥ 25]), BMI (<18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, and ≥40 kg/m2), waist-to-hip ratio (in quintiles), recreational physical activity (total METs per week: <5, 5 to <10, 10 to <20, 20 to <30, and ≥30), alcohol intake (nondrinker, past drinker, <1 drink/month, and current drinker [including frequency: <1 drink/month, 1 drink/month to <1 drink/week, 1 to <7 drinks/week, and ≥7 drinks/week]), total energy intake (kilocalories in quintiles), percent calories from fat (in quintiles), total fruit intake (median portion, in quintiles), total vegetable intake (median portion, in quintiles), and history of hormone therapy use (none, estrogen alone, estrogen and progestin, and mixed).
Statistical analysis
For the distribution of demographic characteristics by diabetes status, χ2 tests were used to evaluate differences for categorical covariates, and t tests were used for continuous variables. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) (95% CI) for the association between diabetes and risk of lung cancer. The underlying time metric in the Cox model is follow-up time since enrollment to the following end points: first lung cancer diagnosis, date of death, loss to follow-up (including nonparticipation in the extension study), or end of clinical trial or observational study follow-up (30 September 2010)—whichever occurred first. In the multivariable models, we adjusted for age, ethnicity, education, smoking status, BMI, waist-to-hip ratio, alcohol consumption, physical activity, total energy intake, percent calories from fat, total fruit intake and total vegetable intake, history of hormone therapy use, and different treatment assignments in clinical trials (estrogen plus progestin vs. placebo, estrogen alone vs. placebo, or low-fat eating pattern vs. usual diet). Different study cohorts (participation in observational study or clinical trials and different treatment assignments for all three clinical trials) were treated as strata in the model in order to take into account possible different baseline hazards in different subgroups and treatment effects.
The effect of exposure was examined in different ways. The primary analysis was focused on prevalent diabetes only as an exposure, including diabetes status, treatment of diabetes, and duration of prevalent diabetes at enrollment. In the secondary analysis, we considered all diabetes as an exposure, including incident diabetes newly occurring during WHI follow-up. In all analyses including incident diabetes, a time-dependent covariate was generated by taking into account changes in diabetes status during follow-up. That is, we considered women in the nondiabetic group until they were identified as having new-onset diabetes. Two sensitivity analyses were performed: one analyzed self-reported type of treatment for diabetes; the other further adjusted for other comorbidities, including hypertension, high cholesterol, and cardiovascular disease.
In addition, since lung cancer is so strongly related to tobacco smoking, we also performed our analyses stratified by smoking status. Interactions between diabetes and smoking, and diabetes and hormone therapy use, were tested by entering cross-product terms into the multiplicative models. The proportionality assumption was satisfied for all exposure variables of interest and potential confounding variables based on graphs of scaled Schoenfeld residuals (27). All statistical analyses were conducted using SAS (version 9.2; SAS Institute, Cary, NC).
RESULTS
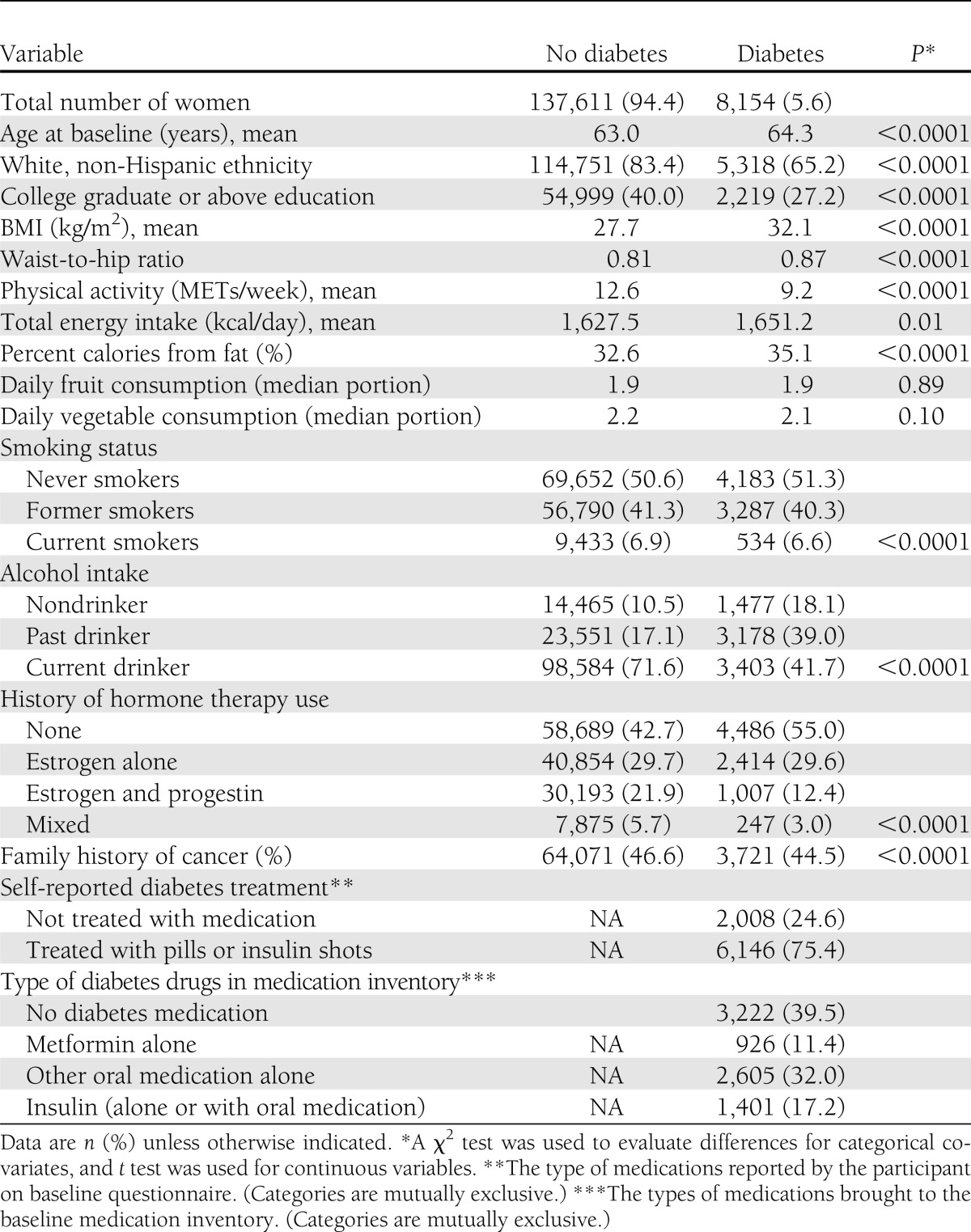
Baseline characteristics by diabetes status at enrollment are shown in Table 1. Compared with women without diabetes, women with diabetes were significantly more likely to be older and have higher BMI, waist-to-hip ratio, physical inactivity, total daily energy intake, and percent calories from fat and were significantly less likely to be white (non-Hispanic), have graduated from college, currently drink, ever have smoked, report history of estrogen plus progestin hormone therapy use, and report a family history of cancer (all P values <0.05). Among 8,154 (5.6%) women with diabetes, 24.6% reported no pharmacologic treatment for diabetes and 75.4% reported diabetes treated with pills or insulin shots (47.8% reporting a history of treatment with oral medications only and 27.6% reporting a history of treatment with insulin). According to type of drugs in the current medication inventory at baseline, 39.5% were not treated, 11.4% were treated with metformin alone, 32.0% were treated with other oral medications, and 17.1% were treated with insulin alone or in combination with other drugs (Table 1).
Table 1.
Baseline characteristics of participants by diabetes status among 145,765 women at WHI enrollment
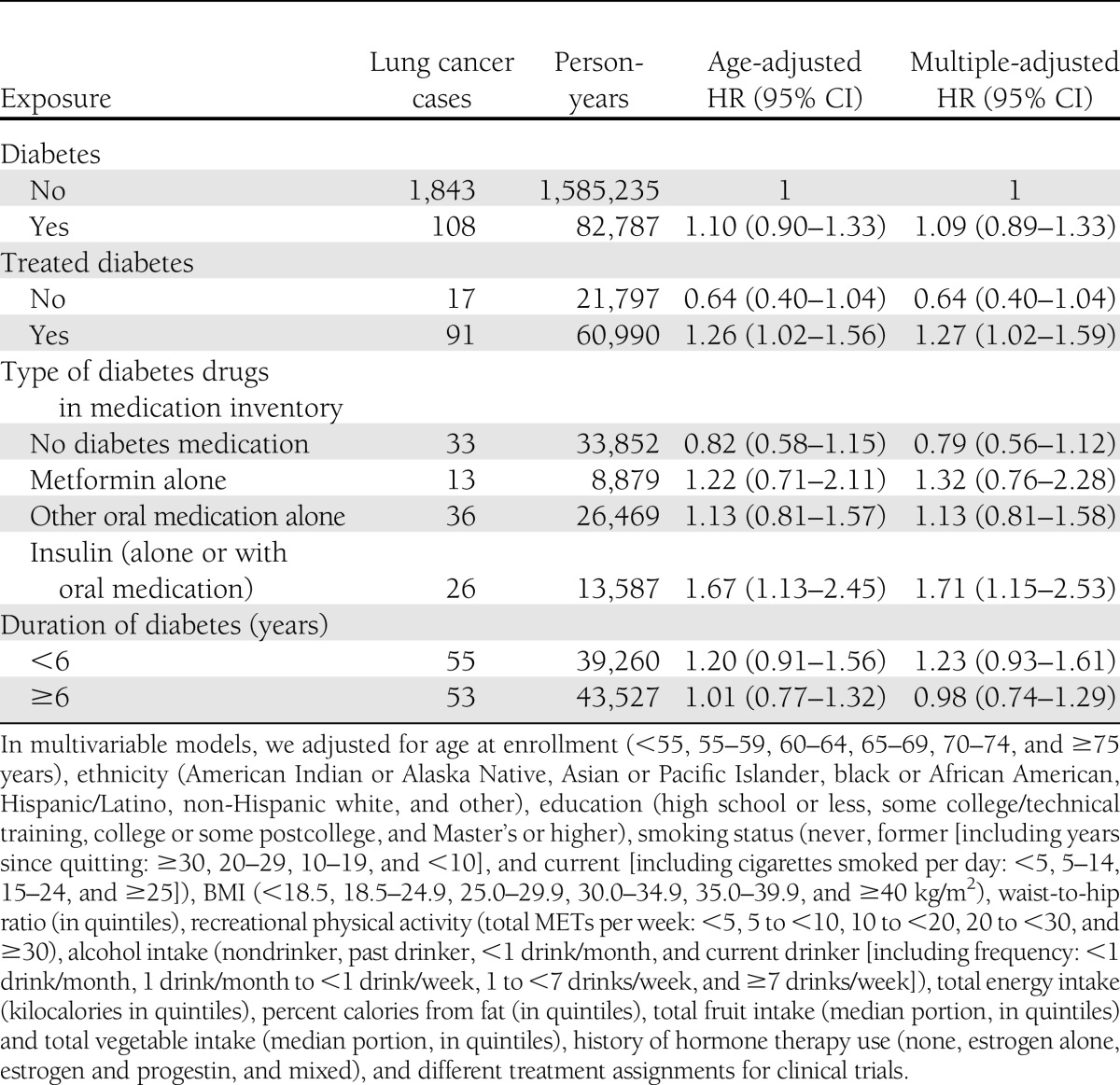
Self-reported diabetes diagnosed at enrollment was not significantly associated with risk of lung cancer (HR 1.09 [95% CI 0.89–1.33]) after adjustment for potential confounders (Table 2). However, women with self-reported treated diabetes had a 27% (95% CI 2–59) excess risk of lung cancer. When diabetes at enrollment was further divided by type of treatments according to the medication inventory, women with diabetes requiring insulin treatment had a significantly higher risk of lung cancer (HR 1.71 [95% CI 1.15–2.53]) compared with women without diabetes, as did those who self-reported use of insulin (1.45 [1.04–2.04]). However, there was no significant association between duration of diabetes and lung cancer risk (Table 2). Elevated risk was noted when considering treated diabetes at baseline and diagnosed during follow-up, but it did not reach statistical significance (1.12 [0.95–1.31] for treated diabetes). We also examined the impact of BMI and waist-to-hip ratio on the strength of the association between diabetes and lung cancer, comparing analyses adjusted and unadjusted for these factors, and observed that the strength of the association was nearly identical before and after adjustment for BMI and waist-to-hip ratio. Since other comorbidities, including hypertension, high cholesterol, and cardiovascular disease, could be mediators, we did not adjust for these factors in our primary models. However, we did a sensitivity analysis by further adjusting for these factors and found that the results were attenuated slightly, but the risk of lung cancer associated with diabetes requiring insulin treatment remained significant (1.61 [1.08–2.39]).
Table 2.
HRs (95% CI) for lung cancer incidence associated with diabetes status and treatment of diabetes at baseline
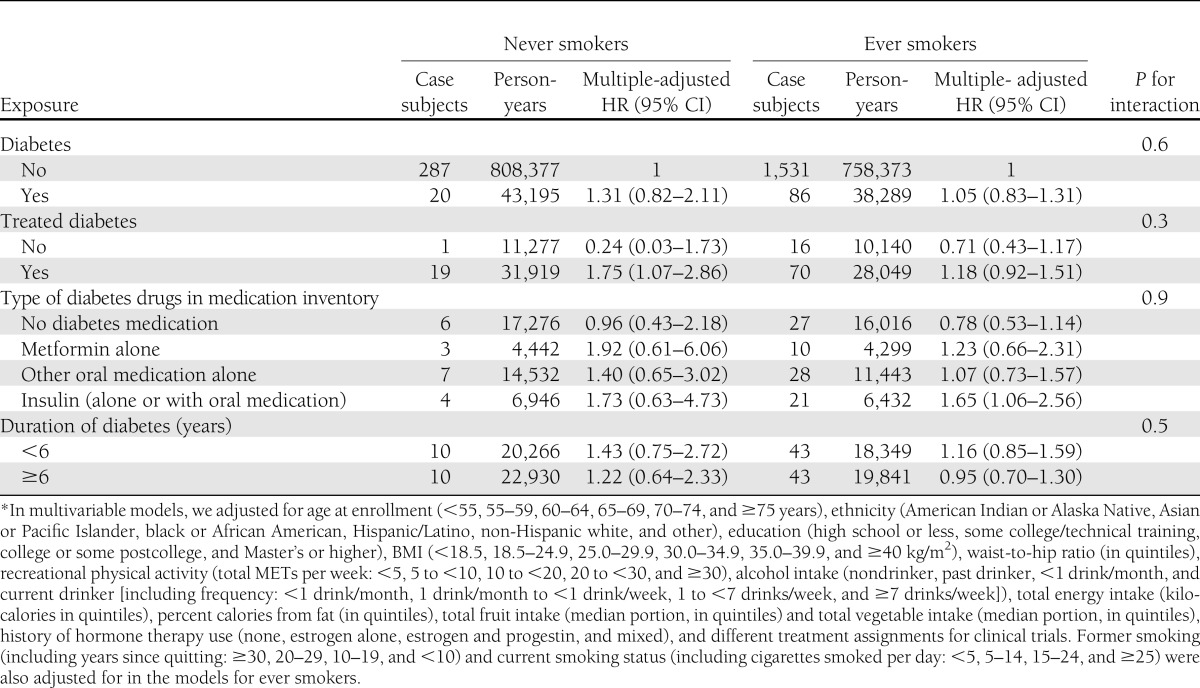
We further performed analyses stratified by smoking status (Table 3). The overall findings among never smokers were generally similar to those of the entire population, except that there was a statistically significant association of self-reported treatment with oral medications and lung cancer. However, the risk estimates had wide CIs, due to small sample sizes. Among ever smokers, only women who had insulin in their medication inventory had a significantly elevated risk of lung cancer. In addition, we did not observe that the association between treated diabetes and lung cancer was significantly modified by smoking (Table 3) or hormone therapy use (P for interaction = 0.8, data not shown).
Table 3.
HRs (95% CI) for lung cancer incidence associated with diabetes status and treatment of diabetes at baseline stratified by smoking status*
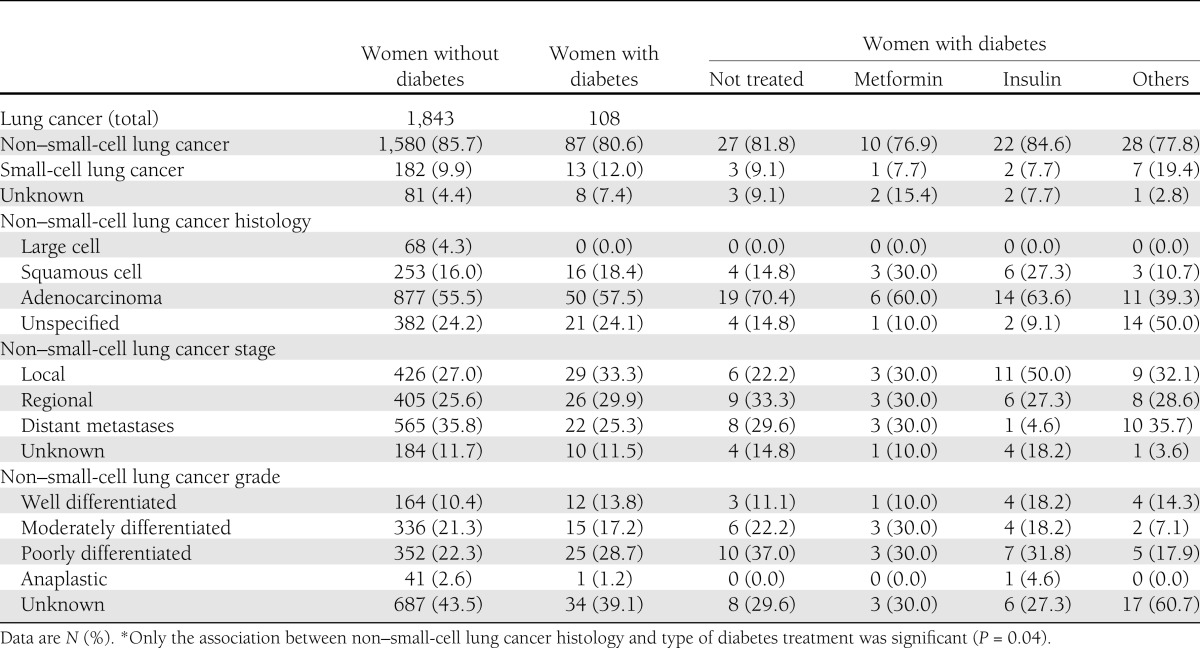
Compared with lung cancers in women using metformin, lung cancers in insulin users were somewhat more likely to be non–small-cell lung cancer, to be localized, and to be well differentiated. However, none of these differences were significant, due to small sample size (Table 4). We also repeated all analyses for non–small-cell lung cancer only and found results similar to those for overall lung cancer (data not shown).
Table 4.
Lung cancer characteristics by diabetes and type of treatment*
CONCLUSIONS
In this large prospective study in postmenopausal women, women with treated diabetes, especially those requiring insulin, had significantly higher risk of lung cancer. The risk of lung cancer did not differ significantly by duration of diabetes.
Previous epidemiological evidence on the association of diabetes with lung cancer is limited and conflicting (1). Compared with that in persons without diabetes, the relative risk of lung cancer in persons with diabetes varied from a significant positive association (9,28,29), or no clear association (10–13,15,16), to a significant negative association (14,17). However, in the two studies that reported a significant negative association (14,17), the study by Armstrong et al. (14) measured standardized mortality ratio for lung mortality with the general population as reference. The other study (17) was conducted among U.S. veterans. Both studies were unable to adjust for important confounders, such as BMI and smoking habits. Among studies with no clear association (10–13,15,16), two were case-control studies (15,16); and three studies had less than 10 cases among women (11–13). Diabetes was self-reported in all 11 identified studies but one, in which the diagnosis of diabetes was based on hospital discharge records (17). Saydah et al. (11) examined abnormal glucose tolerance and the risk of cancer death and found a nonsignificant increase in risk of lung cancer mortality for participants with impaired glucose tolerance (HR 1.57 [95% CI 0.70–3.54]) compared with participants who had normal glucose tolerance. To our knowledge, this is the first prospective study to examine whether the risk of lung cancer incidence is associated with diabetes treatments or duration.
Our data show that women who were treated for diabetes, especially those requiring insulin, appeared to have higher risk of lung cancer. Patients with diabetes begin to require insulin therapy when endogenous insulin production declines, and insulin is more commonly prescribed in those with one or more comorbid conditions that preclude the use of oral medications (1). Thus, treated diabetes or diabetes requiring insulin treatment may serve as a marker of more severe diabetes, which may have greater risk of cancer. However, our study did not observe a stronger association with longer duration of diabetes (another marker of diabetes severity). This suggests that lung cancer risk may be more strongly influenced by specific treatments. There are a variety of different types and analogs of insulin that may have different pharmacokinetic and pharmocodynamic profiles (30). In addition, studies have reported that patients who have type 2 diabetes and are exposed to both sulfonylureas and exogenous insulin have a significantly increased risk of cancer-related mortality compared with patients exposed to metformin (31,32).
Somewhat surprisingly, we observed that women with diabetes who did not use any medication treatment had a nonsignificantly lower lung cancer risk. It is possible that women who did not use medications for diabetes had lower lung cancer risk related to unmeasured lifestyle changes or that they visited doctors less often and were thus less likely to have lung cancer detected (surveillance bias). In addition, our study observed that including incident diabetes status as an exposure resulted in a weaker association than results using baseline diabetes status alone. Since newly diagnosed patients with diabetes are more likely to be treated with lifestyle or oral medications than with insulin, addition of incident diabetes cases would have been likely to weaken the association with lung cancer.
Despite inconsistent epidemiological evidence on the association of diabetes and lung cancer, it is biologically plausible that diabetes could increase the risk of lung cancer (33). Studies have shown that elevated insulin potentiates the activity of IGF-I either via direct upregulation or indirectly through the downregulation of IGF-binding protein 1 (34), which can lead to higher risk of lung cancer (35). A hospital-based case-control study also detected a dose-dependent association between plasma IGF-I levels and lung cancer risk (36). In addition, it has been proposed that hyperglycemia activates the polyol pathway, increasing the production of sorbitol, which in turn results in cellular stress and a decrease in the intracellular antioxidant defenses (3). Studies also show that inadequate glucose control is simultaneously associated with inflammation and decreased lung function in diabetic patients (4,5). The combination of these mechanisms may lead to an increase in cell damage and risk for lung cancer (6–8).
Strengths of our study include the prospective cohort design and the large, diverse population well characterized for tobacco use and other potential confounders, including data on waist-to-hip ratio, a better measure of lung cancer risk than obesity (26). Limitations include lack of information on diabetes severity such as HbA1C levels and information on diabetes therapy only at baseline precluding adjustments over time for change in diabetes management. Information on radon, asbestos, or occupational exposures was not available. In addition, patients may change their treatment plans during the course of diabetes. Classifying treatment based on the WHI current medication inventory collected at baseline would not capture these treatment changes. However, if this exposure misclassification is nondifferential, it would bias our estimates of effect toward the null. We do not have any reason to suspect that patients who are destined to develop lung cancer were more likely to start insulin; thus, any misclassification is likely to have resulted in underestimating the lung cancer risk associated with insulin treatment. Finally, study of associations of diabetes and lung cancer by cell type or stage was limited by subgroup sample size. Diabetes diagnoses were by ongoing self-report and review of diabetes medication use rather than by medical record review. However, this approach has been evaluated (24) and found to have high concordance with a gold standard based on fasting glucose level and medical records.
In conclusion, postmenopausal women with treated diabetes, especially those with diabetes requiring insulin treatment, have a significantly increased risk of lung cancer. More large prospective studies are needed to examine whether the increased risk of lung cancer among women with treated diabetes is driven by specific types of oral diabetes drugs, exogenous insulin treatment, high endogenous insulin levels, poor glycemic control, or longer diabetes severity.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
No potential conflicts of interest relevant to this article were reported.
J.L. contributed to the study concept and design, acquisition of data, data management, analysis and interpretation of data, and drafting of the manuscript. R.C. and K.L.M. contributed to the study concept and design, interpretation of data, and critical review and revision of the manuscript. J.W.-W., N.F.S., and L.T. contributed to interpretation of data and critical review and revision of the manuscript. J.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2108/-/DC1.
A short list of the Women's Health Initiative investigators can be found in the Supplementary Data online.
References
- 1.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–249 [DOI] [PubMed] [Google Scholar]
- 3.Forgiarini LA, Jr, Kretzmann NA, Porawski M, Dias AS, Marroni NA. Experimental diabetes mellitus: oxidative stress and changes in lung structure. J Bras Pneumol 2009;35:788–791 [DOI] [PubMed] [Google Scholar]
- 4.Dennis RJ, Maldonado D, Rojas MX, et al. Inadequate glucose control in type 2 diabetes is associated with impaired lung function and systemic inflammation: a cross-sectional study. BMC Pulm Med 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori H, Okubo M, Okamura M, et al. Abnormalities of pulmonary function in patients with non-insulin-dependent diabetes mellitus. Intern Med 1992;31:189–193 [DOI] [PubMed] [Google Scholar]
- 6.Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr Opin Pulm Med 2009;15:303–307 [DOI] [PubMed] [Google Scholar]
- 7.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev 2008;11:1–15 [DOI] [PubMed] [Google Scholar]
- 8.Nomura A, Stemmermann GN, Chyou PH, Marcus EB, Buist AS. Prospective study of pulmonary function and lung cancer. Am Rev Respir Dis 1991;144:307–311 [DOI] [PubMed] [Google Scholar]
- 9.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 2004;159:1160–1167 [DOI] [PubMed] [Google Scholar]
- 11.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol 2003;157:1092–1100 [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 2006;166:1871–1877 [DOI] [PubMed] [Google Scholar]
- 13.Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol Biomarkers Prev 1995;4:807–811 [PubMed] [Google Scholar]
- 14.Armstrong B, Lea AJ, Adelstein AM, Donovan JW, White GC, Ruttle S. Cancer mortality and saccharin consumption in diabetics. Br J Prev Soc Med 1976;30:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Mara BA, Byers T, Schoenfeld E. Diabetes mellitus and cancer risk: a multisite case-control study. J Chronic Dis 1985;38:435–441 [DOI] [PubMed] [Google Scholar]
- 16.Rousseau MC, Parent ME, Pollak MN, Siemiatycki J. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer 2006;118:2105–2109 [DOI] [PubMed] [Google Scholar]
- 17.Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer 2011;128:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 19.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 2003;13(Suppl.):S18–S77 [DOI] [PubMed] [Google Scholar]
- 20.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(Suppl.):S98–S106 [DOI] [PubMed] [Google Scholar]
- 21.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13(Suppl.):S107–S121 [DOI] [PubMed] [Google Scholar]
- 22.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women’s Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(Suppl.):S87–S97 [DOI] [PubMed] [Google Scholar]
- 23.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(Suppl.):S78–S86 [DOI] [PubMed] [Google Scholar]
- 24.Margolis KL, Lihong Qi, Brzyski R, et al. Women Health Initiative Investigators Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson JM, DeFor TA, Crain AL, et al. Validity of diabetes self-reports in the Women's Health Initiative. J Clin Epi. In press [DOI] [PMC free article] [PubMed]
- 26.Kabat GC, Kim M, Hunt JR, Chlebowski RT, Rohan TE. Body mass index and waist circumference in relation to lung cancer risk in the Women’s Health Initiative. Am J Epidemiol 2008;168:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995;14:1707–1723 [DOI] [PubMed] [Google Scholar]
- 28.Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev 2007;16:83–89 [DOI] [PubMed] [Google Scholar]
- 29.Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia 2012:55;948–958 [DOI] [PubMed] [Google Scholar]
- 30.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 31.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254–258 [DOI] [PubMed] [Google Scholar]
- 33.Petridou ET, Sergentanis TN, Antonopoulos CN, et al. Insulin resistance: an independent risk factor for lung cancer? Metabolism 2011;60:1100–1106 [DOI] [PubMed] [Google Scholar]
- 34.Petridou ET, Sergentanis TN, Antonopoulos CN, et al. Insulin resistance: an independent risk factor for lung cancer? Metabolism 2011:60;1100–1106 [DOI] [PubMed]
- 35.Kim WY, Jin Q, Oh SH, et al. Elevated epithelial insulin-like growth factor expression is a risk factor for lung cancer development. Cancer Res 2009;69:7439–7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 1999;91:151–156 [DOI] [PubMed] [Google Scholar]