Abstract
OBJECTIVE
To determine if autologous nonmyeloablative hematopoietic stem cell transplantation (AHSCT) was beneficial for type 1 diabetic adolescents with diabetic ketoacidosis (DKA) at diagnosis.
RESEARCH DESIGN AND METHODS
We enrolled 28 patients with type 1 diabetes, aged 14–30 years, in a prospective AHSCT phase II clinical trial. HSCs were harvested from the peripheral blood after pretreatment consisting of a combination of cyclophosphamide and antithymocyte globulin. Changes in the exogenous insulin requirement were observed and serum levels of HbA1c, C-peptide, and anti-glutamic acid decarboxylase antibody were measured before and after the AHSCT.
RESULTS
After transplantation, complete remission (CR), defined as insulin independence, was observed in 15 of 28 patients (53.6%) over a mean period of 19.3 months during a follow-up ranging from 4 to 42 months. The non-DKA patients achieved a greater CR rate than the DKA patients (70.6% in non-DKA vs. 27.3% in DKA, P = 0.051). In the non-DKA group, the levels of fasting C-peptide, peak value during oral glucose tolerance test (Cmax), and area under C-peptide release curve during oral glucose tolerance test were enhanced significantly 1 month after transplantation and remained high during the 24-month follow-up (all P < 0.05). In the DKA group, significant elevation of fasting C-peptide levels and Cmax levels was observed only at 18 and 6 months, respectively. There was no mortality.
CONCLUSIONS
We have performed AHSCT in 28 patients with type 1 diabetes. The data show AHSCT to be an effective long-term treatment for insulin dependence that achieved a greater efficacy in patients without DKA at diagnosis.
Clinical trials show that autologous nonmyeloablative hematopoietic stem cell transplantation (AHSCT) is an exciting and promising therapy for wide-spectrum diseases, such as autoimmune disorders (1–3) and cardiac and vascular disease (4–6). For autoimmune diseases, the AHSCT treatment has shown a potent disease-remitting effect when the transplantation was performed during the inflammatory stage of these autoimmune diseases. The potential use of AHSCT to alter the disease course of type 1 diabetes was first proposed in animal studies in 1985 using allogenic bone marrow (7). After a long examination and clinical research, the immunological applications of stem cells (SCs) in type 1 diabetes have been expected to ideally preserve the remaining β-cells, restore β-cell function, and protect the replaced insulin-producing cells from autoimmunity (8).
In 2007, Voltarelli et al. (9) reported their findings on a small group of patients receiving AHSCT within 6 weeks of their diagnosis with type 1 diabetes. The patients who underwent AHSCT had increased islet β-cell secretory function, as evidenced by an increment of C-peptide levels, with good glycemic control achieved by very low doses or frank discontinuation of insulin therapy. However, in a small number of patients presenting with previous diabetic ketoacidosis (DKA), there has been no response to AHSCT. It is unclear if patients with a history of DKA should be excluded from AHSCT. Our study tries to not only confirm the original publication by Voltarelli et al. (9) but also extend those observations by documenting whether a presentation with DKA is predictive of a lower response rate.
RESEARCH DESIGN AND METHODS
Between November 2007 and November 2010, 28 patients with type 1 diabetes were prospectively enrolled at the clinical centers of Shanghai Ruijin Hospital and Nanjing Drum Tower Hospital. The group consisted of 14 males and 14 females with a mean age of 17.6 ± 3.7 years (range 14–27 years). The mean duration of time from symptoms of hyperglycemia to transplantation (beginning of SC mobilization) ranged from 4 to 26 weeks (mean 12 ± 4.7 weeks). The study was approved by the board of medical ethics of Ruijin Hospital, and written informed consent was obtained from all patients and/or their parents prior to enrollment. The inclusion criteria were a diagnosis of type 1 diabetes by clinical/metabolic parameters with positive anti-glutamic acid decarboxylase antibody (anti-GAD) of <6 months. Patients with any of the following diagnoses were excluded: cardiorespiratory insufficiency; renal or liver failure; presence of acute or chronic infections; positive serology for HIV or hepatitis B or C; and an underlying hematologic, nephrologic, cardiac, psychiatric, or hepatic disease.
Study design
The primary study measurement was the change in the effective exogenous insulin requirement (daily dose and duration of usage). The daily insulin dose and duration of treatment were monitored for all patients throughout the entire study period. According to the response of the patients to AHSCT, a 100% reduction in the daily insulin dose (insulin free) was defined as a complete remission (CR). Secondary measurements included the serum levels of HbA1c, fasting C-peptide, peak value of C-peptide during a 75-g oral glucose tolerance test (Cmax), and area under C-peptide release curve during an oral glucose tolerance test (AUCC) and GAD levels prior to transplantation and at 1, 3, 6, 12, 18, and 24 months posttransplantation.
SC mobilization regimen.
Peripheral HSCs were mobilized with cyclophosphamide (Hualian Co., Shanghai, China) and granulocyte colony-stimulating factor (Qilu Pharm, Shandong, China). Cyclophosphamide (2 g/m2) was infused in a single dose in 250 mL saline solution during 1 h. Granulocyte colony-stimulating factor (10 μg/kg/day) was injected subcutaneously, and the injections started 1 day after the cyclophosphamide infusion and were continued until the leukapheresis was completed. Leukapheresis using a continuous-flow blood cell separator was initiated when the rebounding CD34+ cells reached 10 cells/μL. Peripheral blood SCs were frozen in 10% dimethyl sulfoxide in a rate-controlled freezer and stored in the vapor phase of liquid nitrogen.
Patient conditioning (immune ablative) regimen.
Patient conditioning was performed using cyclophosphamide and rabbit anti-thymocyte globulin (rATG) (Thymoglobuline; Genzyme, Cambridge, MA). Cyclophosphamide was given intravenously in divided doses of 50 mg/kg/day during 1 h on days 5, 4, 3, and 2 before the SC infusion. Rabbit anti-thymocyte globulin was also initiated 5 days prior to the transplantation with a single dose of 0.5 mg/kg, which was subsequently followed with daily doses of 1 mg/kg/day on days 4, 3, 2, and 1 before the SC infusion.
SC infusion.
Frozen SCs were gently thawed and brought to 37°C in a regulated water bath. Cell infusion was performed on day 0, and granulocyte colony-stimulating factor (5 μg/kg/day) was administered subcutaneously from day 5 after the SC infusion until the neutrophil count was >1,000/μL.
Laboratory assessment of diabetes status.
The serum C-peptide levels were measured by a radioimmunoassay using a commercially available kit (Roche Diagnostics, Penzberg, Germany). The lower limit of detection was 0.1 ng/mL, and undetected values were reported as 0.1 ng/mL. The serum levels of anti-GADs were also measured by a radioimmunoassay using a commercial kit (RSR Limited, Cardiff, U.K.), and the results were considered positive if the values were >7.5 units/mL. HbA1c was measured by high-pressure liquid chromatography.
Statistical analysis
Statistical analysis was performed with SPSS version 13.0 (SPSS Inc., Chicago, IL). The baseline data were reported individually in a tabular form. ANOVA analyses were performed between the two study groups and among the different follow-up times after transplantation (1, 3, 6, 12, 18, and 24 months) with a model that included the baseline values of the duration of diabetes, age, and sex as covariates. All hypothesis testing was two tailed. The statistical significance was set a priori at P < 0.05.
RESULTS
Pretransplantation characteristics of the patients with type 1 diabetes undergoing AHSCT
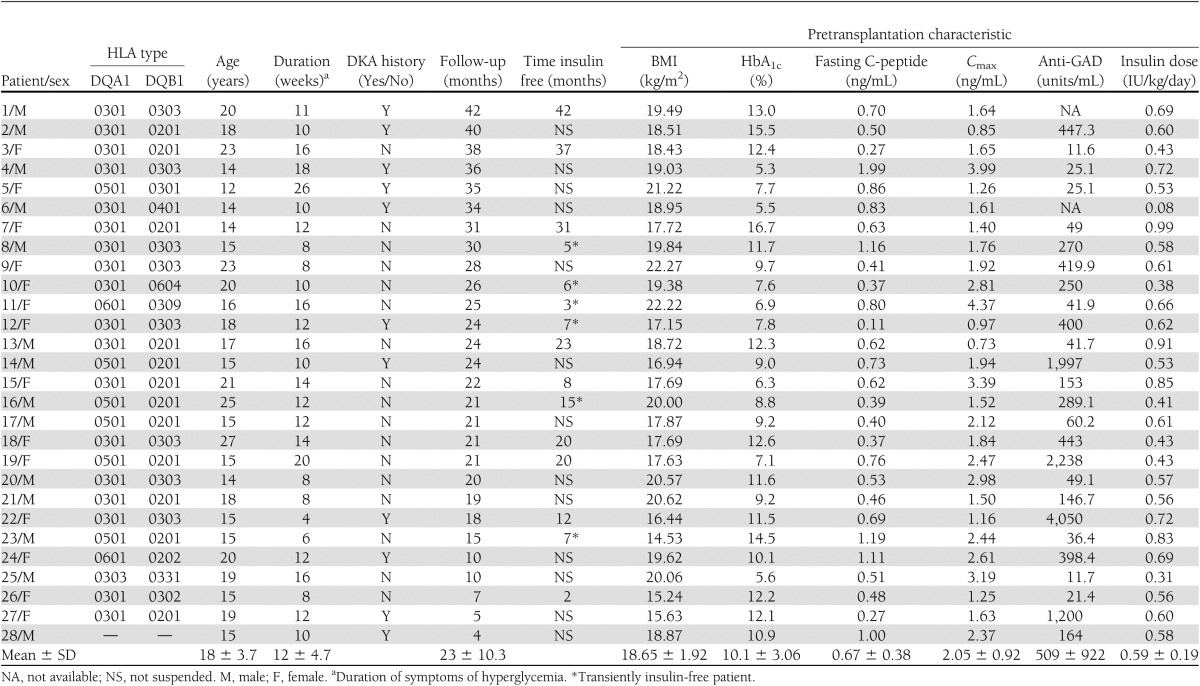
All of the 28 patients included in this study met the 2007 American Diabetes Association diagnostic criteria for type 1 diabetes of a positive islet cell antibody (GAD), a low C-peptide level, and the requirement of insulin injection for treatment. A total of 11 patients presented DKA at diagnosis (DKA group), and 17 patients were diagnosed with hyperglycemia accidently or as a result of obvious symptoms of polyuria, polydipsia, or weight loss (non-DKA group). Insulin therapies were immediately started once diabetes was diagnosed. The mean duration of time from symptoms of hyperglycemia to the transplantation (beginning of SC mobilization) ranged from 4 to 26 weeks (mean 12 ± 4.7 weeks). Pretransplantation, the mean BMI was 18.95 ± 1.92 kg/m2 and the mean HbA1c level was 10.1 ± 3.06% (Table 1).
Table 1.
Pretransplantation characteristics of type 1 diabetic patients undergoing AHSCT
AHSCT procedure and side effects
The mean duration of the hospital stay for transplantation (from SC mobilization to infusion) was 20.2 days (range 18–36 days) with an additional 10–14 days of stay in a laminar flow unit until the neutrophil cells reached a level greater than 1.0 × 106/L.
The white blood cell levels declined immediately after the SC infusion and reached the minimum on day 5 or day 6 after transplantation. Once the administration of granulocyte colony-stimulating factor (5 μg/kg/day) began from day 5 after the SC infusion, the number of white blood cells elevated and kept a level greater than 5 × 109/L since day 9. The change of platelet was similar with that of white blood cell, presenting a U-style curve. The lowest point was at day 6 or day 7 after transplantation. However, the curve of erythrocyte count was flat, and the cell count fluctuated within the lower limit of the normal range. There were five patients (17.9%) who received a blood transfusion and five patients (17.9%) who received a platelet transfusion after transplantation.
Most patients experienced febrile neutropenia, nausea, vomiting, alopecia, and bone marrow suppression as a result of the drugs used for the mobilization and conditioning. No severe acute drug toxicity, infection, or organ damage occurred. Most side effects disappeared at 2–4 weeks after the AHSCT, except that recovery from the neutropenia was the slowest. Patient 13 and patient 23 were diagnosed with autoimmune hypothyroidism before transplantation and were treated successfully with levothyroxine during the long-term follow-up period. Patient 6 was diagnosed with Graves disease and was treated with tapazole before transplantation. The thyroid function was restored 3 months after transplantation, and the antithyroid drug therapy was stopped at 6 months after transplantation. Patient 25, who had autoimmune hyperthyroidism before transplantation, developed hypothyroidism after the transplantation and was still being treated with levothyroxine as this manuscript was being drafted. Measurements of gonadal function (levels of follicle-stimulating hormone and luteinizing hormone in both sexes, testosterone in men, and estradiol in women) were in the normal range for all patients. Patient 19 became pregnant 1 year after transplantation (by natural means) and delivered a baby girl. There was no mortality.
Responses after treatment with AHSCT in type 1 diabetic patients
Of the 28 patients studied over a follow-up period ranging from 4 to 42 months (median 23 months), 15 of 28 patients (53.6%) had a CR after treatment that lasted from 3 to 42 months (median 24 months). The non-DKA group achieved a greater CR rate than the DKA group (70.6% [12 of 17] in the non-DKA group vs. 27.3% [3 of 11] in the DKA group, P = 0.051). Of the patients, 8 (28.6%) maintained a CR for a mean duration of 23.8 ± 12.7 months (range 3–42 months), and 7 patients relapsed and resumed insulin use. Of the seven patients with a demonstrated relapse, four relapses were associated with an upper respiratory infection within 6 months after treatment and one patient failed to adapt fully to the recommended dietary and lifestyle changes. Another two relapses had indeterminate causes.
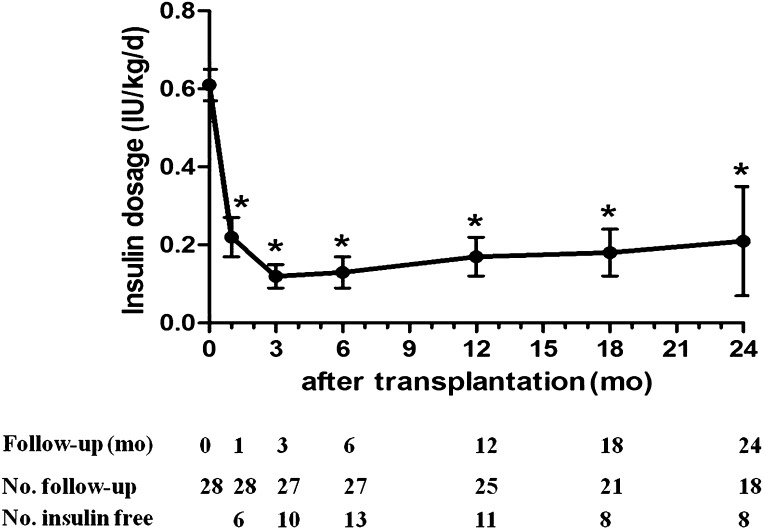
The average daily insulin dose requirements were significantly decreased beginning at 1 month after transplantation, reached their lowest levels at 3 months, and remained at a stable level for at least 24 months (compared with the level prior to transplantation, all P < 0.01) (Fig. 1). At 3 months after transplantation, the insulin dose was <0.15 IU/kg/day and remained at that level for 2 years (mean 0.15 ± 0.07 IU/kg/day) in the non-DKA group. However, in the DKA group, the insulin dosage was lowest at 6 months after transplantation (0.17 ± 0.09 IU/kg/day) and increased to a mean level of 0.37 ± 0.08 IU/kg/day at 24 months. A significant difference in insulin dose reduction was observed at 18 and 24 months between the two groups (P = 0.013 and 0.019, respectively) (Fig. 2).
Figure 1.
Insulin dose (IU/kg/day) in type 1 diabetic patients after treatment of AHSCT. *P < 0.001 compared with month 0 (pretransplantation). d, day; mo, month.
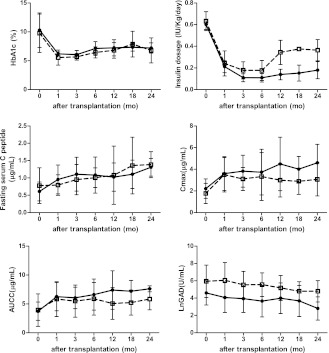
Figure 2.
Time course of HbA1c, insulin dose, fasting C-peptide, Cmax, AUCC, and LnGAD level (because of the uneven data distribution) in the non-DKA group and DKA group, respectively. Solid line, non-DKA group; dotted line, DKA group. mo, month.
In the non-DKA group, a stable reduction of HbA1c levels was observed for the entire 24 months of follow-up (compared with the level prior to transplantation, all P < 0.01). The fasting C-peptide levels, Cmax, and AUCC were enhanced significantly 1 month after transplantation and remained elevated during the 24 months of follow-up (all P < 0.05). In the DKA group, despite decreased HbA1c levels, significant elevations of the fasting C-peptide level were observed only at the 18-month follow-up time point (compared with the level prior to transplantation, P = 0.049), and the Cmax level was significantly increased only at 6 months (P = 0.031) (Fig. 2).
An evaluation of the GAD levels showed a downward trend in both groups after the transplantation (Fig. 2). A lower GAD level (analysis of LnGAD, log form of GAD) in the non-DKA group was observed after transplantation during the 24-month follow-up period. Furthermore, despite a large variance, an obvious difference in the LnGAD between the two study groups appeared at 6 months after transplantation (non-DKA 3.65 ± 1.83 vs. DKA 5.55 ± 1.35 units/mL, P = 0.059). Correlation analyses showed that the LnGAD had a significantly negative relationship with the fasting C-peptide level (r = −0.316, P = 0.001).
Prognostic factors associated with CR between the non-DKA and DKA groups
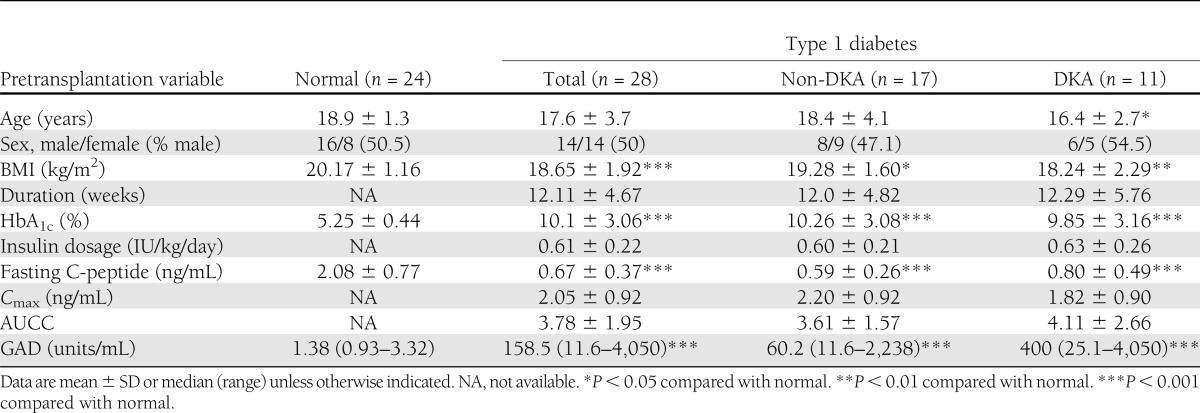
Prognostic factors that were associated with a CR were analyzed by a comparison of the baseline values between the two study groups, which included demographic indicators, metabolic variables, and cytokine and chemokine levels. There were no significant differences in age, sex, BMI, duration of diabetes, and baseline HbA1c level between the non-DKA and DKA groups. In addition, the indices of β-cell secretory function, as assessed by fasting C-peptide level, Cmax, and AUCC, were unchanged. There was a trend for a decreased GAD level in the non-DKA group compared with the DKA group (60.2 [11.6–2,238] vs. 400 [25.1–4,050] units/mL, P = 0.071) (Table 2).
Table 2.
Pretransplantation variables of the patients, grouping by history of DKA at diagnosis
CONCLUSIONS
The most common indications for AHSCT were systemic lupus erythematous, multiple sclerosis, systemic sclerosis, and Crohn disease. Researchers at Northwestern University have performed >200 AHSCTs for immune-mediated diseases (10,11). The studies show that SCs harvested from blood (HSCs) or marrow, under appropriate conditions in select patients, provide disease-ameliorating effects. For type 1 diabetes, the first promising data were from Couri et al. (12) in Brazil and Snarski et al. (13) in Poland. In the Couri et al. (12) study, after a mean follow-up of 29.8 months post AHSCT, 20 of 23 patients became insulin free; 12 patients maintained this status for a mean of 31 months (range 14–52 months), while 8 patients relapsed and resumed insulin use at a low dose (0.1–0.3 IU/kg). In the Snarski et al. (13) study, all of the eight patients became independent of exogenous insulin after the transplantation; one patient resumed using low-dose insulin 7 months after the transplantation, and six patients were given acarbose for better glycemic control after transplantation. In our study, of all 28 patients, 15 remained exogenous insulin free for a mean 24 months, and 7 patients relapsed and resumed insulin use. Both continuously and transiently insulin-free groups presented an increase in C-peptide levels for up to 2 years of follow-up. Thus far, three trials using similar protocol have obtained good results. The significantly high rate of insulin-free patients and increased serum levels of C-peptide in the patients with AHSCT treatment show well-improved endogenous β-cell function, which cannot be achieved in patients who accept traditional insulin therapy.
Instead of cell replacement therapy to restore functional islet β-cells, AHSCT is a good combination therapy of an intensive immunosuppressive strategy and an application of HSCs. Anti–T-cell globulin, which is used for bone marrow conditioning, is a potent immunosuppressive drug. It depletes almost the entire T-cell population in treated patients and is primarily used during the induction period after solid organ transplantation or within acute rejection settings in transplant patients. A 2004 study from Prague, Czech Republic, suggests that short-term anti–T-cell globulin treatment in type 1 diabetic patients had remarkable effects for preserving C-peptide levels (14). In addition, HSCs are among the most immunogenic SCs and can differentiate into the myeloid lineages and lymphoid lineages. Murine HSCs display the ability to induce immune tolerance, which may delete effector cells by the Fas/Fas ligand interaction, via the tumor necrosis factor-α pathways, or by neutralizing precursors of cytotoxic T cells (15–17). Furthermore, it has been revealed that diabetes in streptozocin-induced type 1 diabetic mice can be reversed by syngeneic bone marrow transplantation, probably via regeneration of CD4+CD25+FoxP3+ regulatory T cells (18).
When considering AHSCT as a potential treatment for type 1 diabetes, it is essential that the risk-to-benefit ratio be in favor of the AHSCT treatment. It is evident that this may not be the case for someone whose islet β-cells have been too damaged. However, in those patients with sufficient β-cells available for salvage, the achievement of a stable glucose control with low doses or a complete discontinuation of insulin therapy that is offered by AHSCT appears worthwhile. All of our patients considered the transplantation to be worthwhile and beneficial, though some treated patients experienced side effects, including febrile neutropenia, nausea, vomiting, alopecia, and bone marrow suppression transiently. The 1-year results from the randomized, placebo-controlled trial of teplizumab for the treatment of type 1 diabetes (Protégé study) show that no patients in the placebo group (regular insulin use plus intravenous use of placebo) and 5% (19 of 415) of the patients in the teplizumab group were not taking insulin after 1 year (19), while 44% (11 of 25) of patients with the AHSCT treatment stopped requiring insulin injections in our study. Long-term complications related to immunosuppression therapy, such as tumor formation, endocrine disorders, and potential infertility, are disadvantages of using AHSCT. It will take further follow-ups to determine the occurrence of relapses and whether there is a net benefit. Even so, the current results are certainly encouraging. AHSCT is much more effective in restoring normoglycemia than any other antigen-specific vaccines or single utility immunosuppressive drugs (20).
The costs attributed to type 1 diabetes are disproportionately higher than the number of type 1 patients compared with type 2 patients according to a study by Tao et al. (21). And the cost of AHSCT is approximately equivalent to 10-year spending on standard care for patients with type 1 diabetes and negative diabetes complications (G.N. et al., unpublished data). Nevertheless, it is a one-time expenditure and is valuable. The economic benefit comes from avoiding the additional costs and higher disability rate from later complications. Furthermore, an economic value will be generated from productivity regained, changes in personal situations, and enhanced well-being and quality of life of patients with type 1 diabetes.
Meanwhile, we try to determine good candidates for AHSCT therapy by specifically addressing the clinical value of AHSCT in patients who previously presented with DKA. Older age at onset, sex, a mild initial metabolic derangement, and an absence of frank DKA are known factors predicting diabetic remission (22,23). We compared the CR rates between groups stratified by baseline values and found that a prior occurrence of DKA affects the outcome of AHSCT treatment. The non-DKA patients required lower doses of insulin to achieve normoglycemia during the 24-month follow-up, while the DKA patient group showed a slightly delayed response to transplantation and began trending toward a higher insulin dose requirement after 1 year. We observed that the ongoing destruction of the remaining β-cells slowed down and was even arrested in the non-DKA group. The levels of fasting C-peptide, Cmax, and AUCC in the patients without prior DKA were increased significantly 1 month after treatment and remained at a high level during the following 24 months. In a Finland study, low serum C-peptide concentrations, a high requirement of exogenous insulin, a low prevalence of remission, and high glycated hemoglobin concentrations were observed during the follow-up period in the group of probands having DKA at diagnosis of type 1 diabetes (24). Thus, the occurrence of DKA at diagnosis was related to a decreased capacity for β-cell recovery after the clinical manifestation of type 1 diabetes in children and adolescents.
In this phase II study involving 28 patients, we have demonstrated a benefit of AHSCT treatment in type 1 diabetes. Within our study population, 15 patients achieved insulin independence. Despite the relapse of seven patients, eight patients have remained insulin independent on average for nearly 2 years, with the longest patients currently at 42 months. There were significant differences in the long-term remission after AHSCT, with the CR rate nearly three times greater in the non-DKA group. This suggests that the patients with DKA at diagnosis may not be suitable for treatment with AHSCT. However, whether the clinical benefits of this protocol can be provided to a majority of patients remains debatable. The costs and risks involved in this experimental procedure require that AHSCT be performed in a well-controlled environment. We are optimistic that with a greater understanding of the AHSCT treatment, this approach will offer a targeted cure to a subset of patients with type 1 diabetes.
Acknowledgments
This study was supported by grants from the National 863 Program (2011AA020107), the Chinese National Natural Science Foundation (81130016, 81100588), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01040102), and the Shanghai Natural Science and Technology Foundation (11ZR143200).
No potential conflicts of interest relevant to this article were reported.
W.G. and J.H. researched data and wrote the manuscript. L.L., W.T., and W.C. researched data. W.W., S.S., L.Y., Y.Z., and J.H. contributed to discussion. G.N. and D.Z. reviewed and edited the manuscript. G.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Gilbert Cote, Department of Endocrine Neoplasia and Hormonal Disorders, MD Anderson Cancer Center, for help with preparing the manuscript.
Footnotes
Clinical trial reg. no. NCT00807651, clinicaltrials.gov.
References
- 1.Farge D, Labopin M, Tyndall A, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica 2010;95:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gualandi F, Bruno B, Van Lint MT, et al. Autologous stem cell transplantation for severe autoimmune diseases: a 10-year experience. Ann N Y Acad Sci 2007;1110:455–464 [DOI] [PubMed] [Google Scholar]
- 3.Binks M, Passweg JR, Furst D, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis 2001;60:577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansour S, Roy DC, Lemieux B, Ouellet C, Stevens LM, Noiseux N. Stem cell therapy for the broken heart: mini-organ transplantation. Transplant Proc 2009;41:3353–3357 [DOI] [PubMed] [Google Scholar]
- 5.Kaminski A, Steinhoff G. Current status of intramyocardial bone marrow stem cell transplantation. Semin Thorac Cardiovasc Surg 2008;20:119–125 [DOI] [PubMed] [Google Scholar]
- 6.Stamm C, Nasseri B, Drews T, Hetzer R. Cardiac cell therapy: a realistic concept for elderly patients? Exp Gerontol 2008;43:679–690 [DOI] [PubMed] [Google Scholar]
- 7.Ikehara S, Ohtsuki H, Good RA, et al. Prevention of type I diabetes in nonobese diabetic mice by allogenic bone marrow transplantation. Proc Natl Acad Sci U S A 1985;82:7743–7747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorina P, Voltarelli J, Zavazava N. Immunological applications of stem cells in type 1 diabetes. Endocr Rev 2011;32:725–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568–1576 [DOI] [PubMed] [Google Scholar]
- 10.Burt RK, Slavin S, Burns WH, Marmont AM. Induction of tolerance in autoimmune diseases by hematopoietic stem cell transplantation: getting closer to a cure? Blood 2002;99:768–784 [DOI] [PubMed] [Google Scholar]
- 11.Burt RK, Testori A, Craig R, Cohen B, Suffit R, Barr W. Hematopoietic stem cell transplantation for autoimmune diseases: what have we learned [corrected in: J Autoimmun 2008;31:90]? J Autoimmun 2008;30:116–120 [DOI] [PubMed] [Google Scholar]
- 12.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–1579 [DOI] [PubMed] [Google Scholar]
- 13.Snarski E, Milczarczyk A, Torosian T, et al. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant 2011;46:562–566 [DOI] [PubMed] [Google Scholar]
- 14.Saudek F, Havrdova T, Boucek P, Karasova L, Novota P, Skibova J. Polyclonal anti-T-cell therapy for type 1 diabetes mellitus of recent onset. Rev Diabet Stud 2004;1:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gur H, Krauthgamer R, Berrebi A, et al. Tolerance induction by megadose hematopoietic progenitor cells: expansion of veto cells by short-term culture of purified human CD34(+) cells. Blood 2002;99:4174–4181 [DOI] [PubMed] [Google Scholar]
- 16.Gur H, Krauthgamer R, Bachar-Lustig E, et al. Immune regulatory activity of CD34+ progenitor cells: evidence for a deletion-based mechanism mediated by TNF-α. Blood 2005;. 105:2585–2593 [DOI] [PubMed] [Google Scholar]
- 17.Rachamim N, Gan J, Segall H, et al. Tolerance induction by “megadose” hematopoietic transplants: donor-type human CD34 stem cells induce potent specific reduction of host anti-donor cytotoxic T lymphocyte precursors in mixed lymphocyte culture. Transplantation 1998;65:1386–1393 [DOI] [PubMed] [Google Scholar]
- 18.Wen Y, Ouyang J, Yang R, et al. Reversal of new-onset type 1 diabetes in mice by syngeneic bone marrow transplantation. Biochem Biophys Res Commun 2008;374:282–287 [DOI] [PubMed] [Google Scholar]
- 19.Sherry N, Hagopian W, Ludvigsson J, et al. Protégé Trial Investigators Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011;378:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couri CE, Voltarelli JC. Stem cell-based therapies and immunomodulatory approaches in newly diagnosed type 1 diabetes. Curr Stem Cell Res Ther 2011;6:10–15 [DOI] [PubMed] [Google Scholar]
- 21.Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS ONE 2010;5:e11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelkonen R, Aro A. Factors predicting remission in type I diabetes. Ann Clin Res 1984;16:94–97 [PubMed] [Google Scholar]
- 23.Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes 2006;7:101–107 [DOI] [PubMed] [Google Scholar]
- 24.Komulainen J, Lounamaa R, Knip M, Kaprio EA, Akerblom HK, Childhood Diabetes in Finland Study Group Ketoacidosis at the diagnosis of type 1 (insulin dependent) diabetes mellitus is related to poor residual beta cell function. Arch Dis Child 1996;75:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]