Abstract
OBJECTIVE
The effect of fructose on cardiometabolic risk in humans is controversial. We conducted a systematic review and meta-analysis of controlled feeding trials to clarify the effect of fructose on glycemic control in individuals with diabetes.
RESEARCH DESIGN AND METHODS
We searched MEDLINE, EMBASE, and the Cochrane Library (through 22 March 2012) for relevant trials lasting ≥7 days. Data were aggregated by the generic inverse variance method (random-effects models) and expressed as mean difference (MD) for fasting glucose and insulin and standardized MD (SMD) with 95% CI for glycated hemoglobin (HbA1c) and glycated albumin. Heterogeneity was assessed by the Cochran Q statistic and quantified by the I2 statistic. Trial quality was assessed by the Heyland methodological quality score (MQS).
RESULTS
Eighteen trials (n = 209) met the eligibility criteria. Isocaloric exchange of fructose for carbohydrate reduced glycated blood proteins (SMD −0.25 [95% CI −0.46 to −0.04]; P = 0.02) with significant intertrial heterogeneity (I2 = 63%; P = 0.001). This reduction is equivalent to a ∼0.53% reduction in HbA1c. Fructose consumption did not significantly affect fasting glucose or insulin. A priori subgroup analyses showed no evidence of effect modification on any end point.
CONCLUSIONS
Isocaloric exchange of fructose for other carbohydrate improves long-term glycemic control, as assessed by glycated blood proteins, without affecting insulin in people with diabetes. Generalizability may be limited because most of the trials were <12 weeks and had relatively low MQS (<8). To confirm these findings, larger and longer fructose feeding trials assessing both possible glycemic benefit and adverse metabolic effects are required.
The number of people with type 2 diabetes is likely to double during the next 20 years (1), leading to an increased burden of cardiovascular disease (2), renal failure (2), blindness (2), and risk of colon, breast, and other cancers (3). Diabetes profoundly alters macronutrient metabolism; the roles of diet and especially carbohydrate type and quality are therefore of considerable interest. Since 1970, the total availability of sugars has increased by ∼20% (4), and high-fructose corn syrup now represents nearly 50% of caloric sweetener use in the United States (4,5). Increased total fructose consumption (from both sucrose and high-fructose corn syrup) has been implicated in the development of the obesity epidemic in the United States (6) and has been singled out in diabetes guidelines because of concerns about its effects on lipids.
Diabetes associations (2,7,8) have taken a harm-reduction approach to fructose recommendations, setting an upper threshold for intake that is based on putative adverse effects on serum lipids. The American Diabetes Association guidelines, however, acknowledge that fructose produces a lower glycemic response in people with diabetes when it replaces sucrose and starch in the diet (7). Fructose has also been shown to improve glycemia without adversely affecting lipids when exchanged for other carbohydrate in controlled feeding trials in people with type 2 diabetes (9–15). In the absence of clear guidance on the role of fructose in glycemic control, we conducted a systematic review and meta-analysis of controlled feeding trials to assess the effects of isocaloric, oral fructose exchange for carbohydrates on fasting glucose, fasting insulin, and glycated blood proteins (glycated hemoglobin [HbA1c], glycated albumin, and fructosamine) in individuals with diabetes.
RESEARCH DESIGN AND METHODS
We followed the Cochrane Handbook for Systematic Reviews of Interventions for the planning and conduct of this meta-analysis (16). The reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17).
Study selection
We searched MEDLINE, EMBASE, and Cochrane databases through 22 March 2012 with the following search terms: fructose AND (glucose OR glycemic OR glycemic OR glycaemia OR glycemia OR insulin OR OGTT OR HOMA-IR OR HbA1c OR fructosamine). Manual searches supplemented the electronic search strategy. We included controlled feeding trials that investigated the effect of oral fructose in isocaloric exchange for other sources of carbohydrate on markers of glycemic control in individuals with diabetes. Trials that had <7 days of follow-up, administered fructose intravenously, lacked an adequate carbohydrate comparator, or did not provide suitable end point data were excluded. No restriction was placed on language.
Data extraction
Reports that met the inclusion criteria were each independently reviewed and extracted by at least two investigators with a standardized form. Relevant information about study design, randomization, blinding, level of feeding control, sample size, subject characteristics, fructose format, dose, reference carbohydrate, duration of follow-up, and macronutrient profile of the background diet were obtained. We extracted mean ± SD posttreatment values for fasting glucose, fasting insulin, and percentage glycated blood proteins (HbA1c, glycated albumin, and fructosamine, with HbA1c preferred). Trials that did not report SDs had these values imputed from SD, 95% CI, P values, t or F statistics according to standard formulas (16). When these statistics were unavailable, an imputed pooled SD from the other trials included in the meta-analysis was applied (16). Imputations were necessary for 11 of 13 glycated blood protein trials, 8 of 16 fasting glucose trials, and 5 of 7 fasting insulin trials. The quality of each study was assessed with the Heyland methodological quality score (MQS) (18). Trials were considered to be of high quality if they obtained an MQS ≥8. Heyland score disagreements were reconciled by consensus. Authors were contacted to request additional information, where necessary.
Statistical analyses
Data were analyzed with Review Manager (RevMan) software version 5.0.25 (Nordic Cochrane Centre, Copenhagen, Denmark). Stratified aggregate analyses were conducted for undifferentiated diabetes, type 1 diabetes, and type 2 diabetes with the generic inverse variance method with random-effects models. Change from baseline differences between fructose and carbohydrate comparator for fasting glucose, fasting insulin, and percentage glycated protein were extracted as the primary end points. When these data were unavailable, end-of-treatment differences were used. Paired analyses were applied to all crossover trials (19). A weighted average was applied within studies to combine multiple comparator arms. When two separate control phases were present within the same crossover study, both phases were averaged and compared with the fructose intervention. Data were expressed as mean difference (MD) for fasting glucose and insulin, and standardized MD (SMD) for glycated blood proteins, all with 95% CI. Although baseline subject characteristics were reported in terms of HbA1c, certain studies reported end values in terms of glycated albumin, necessitating the use of SMDs in our analysis. Between-trial heterogeneity was tested by the Cochran Q statistic with a significance level set at P < 0.10. Heterogeneity was quantified by the I2 statistic, where I2 ≥ 50% was considered evidence of substantial heterogeneity (16). Sources of heterogeneity were investigated by a priori subgroup analyses assessing the effects of carbohydrate comparator, fructose form, dose, baseline values, trial quality, trial design, length of follow-up, and randomization. Sensitivity analyses were performed to determine if any single study exerted an undue influence on the overall result. To address this point, we systematically removed each individual study from the meta-analysis and recalculated the pooled effect size from the remaining studies. Meta-regression was performed to assess the significance of subgroup effects with Stata software version 11.2 (StataCorp, College Station, TX). Publication bias was investigated by inspection of funnel plots and quantitatively assessed with Begg and Egger tests.
RESULTS
Search results
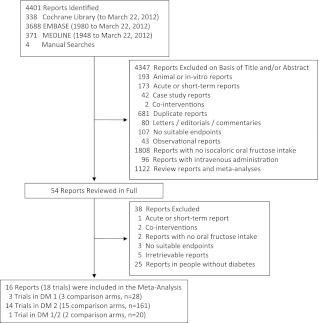
A total of 4,401 eligible reports were identified with the search; of these, 4,347 were determined to be irrelevant on review of the titles and abstracts. The remaining 54 reports were retrieved and reviewed in full, and a further 38 were excluded. A total of 16 reports (18 trials) were selected for pooled analyses (Fig. 1). The characteristics of the 18 included trials are shown in Table 1.
Figure 1.
Flowchart of literature search for the effect of fructose on glycemic end points (fasting glucose, fasting insulin, and glycated blood proteins [HbA1c and glycated albumin]). Electronic searches of Cochrane Library, EMBASE and MEDLINE databases were supplemented by manual searches of the references of included trials. DM 1, type 1 diabetes; DM 2, type 2 diabetes; DM 1/2, both type 1 and type 2 diabetes.
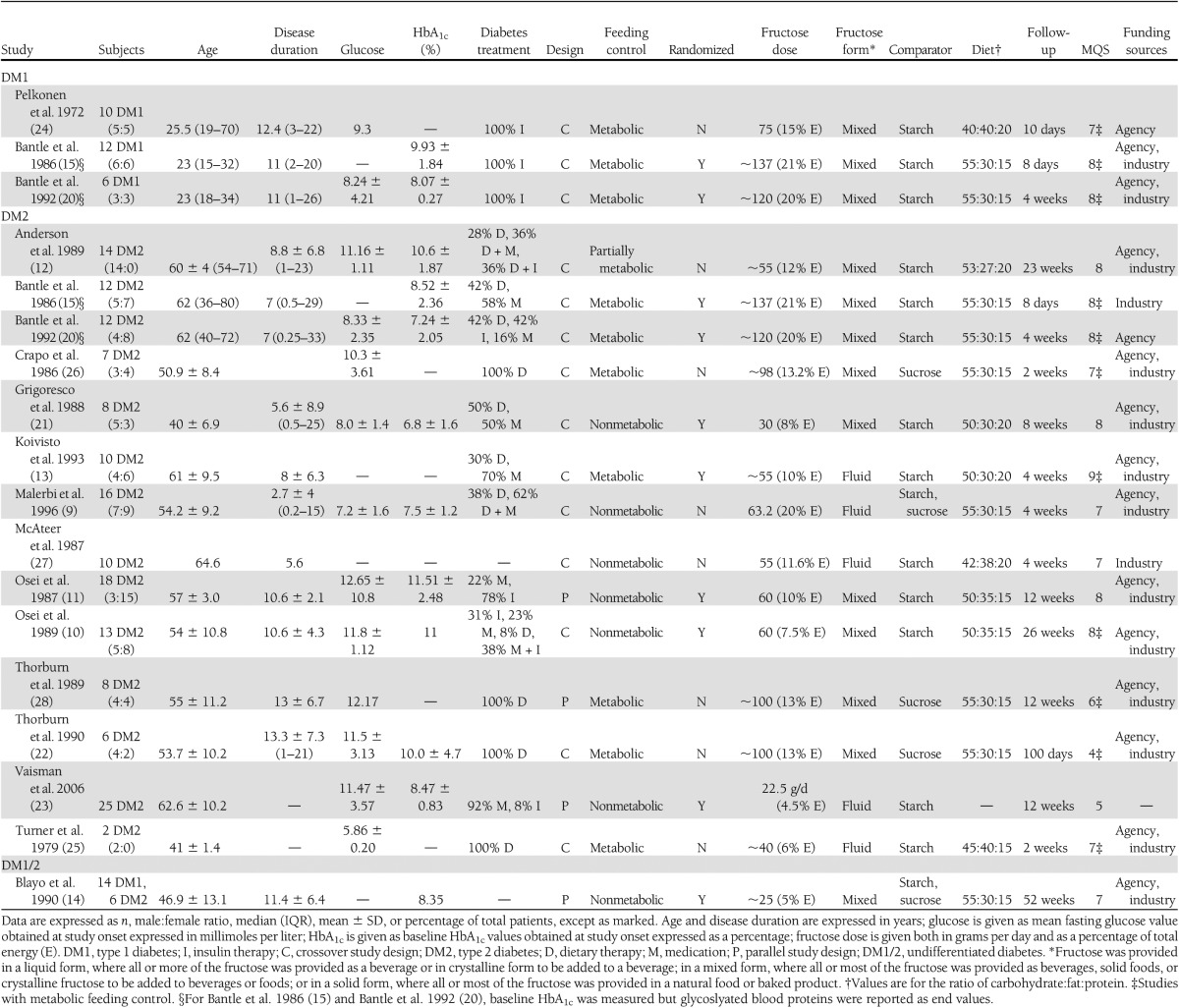
Table 1.
Characteristics of experimental trials included in the meta-analysis
Glycated blood proteins
A total of 13 glycated blood protein comparisons were made among 172 subjects with type 1 diabetes (2 trials, n = 18) (15,20), type 2 diabetes (10 trials, n = 134) (9–13,15,20–23), or undifferentiated diabetes (1 trial, n = 20) (14). Patients had a median age of 54.2 years (interquartile range [IQR] 46.9–61 years) and a diabetes duration of 9.7 years (7–11 years). Their median baseline HbA1c values were 8.5% (IQR 7.9–10.1%). Ten trials were randomized (77%). Ten trials used crossover designs (77%), and three used parallel designs (23%). Starch (77%) and sucrose (7.7%) were used as carbohydrate comparators, and notably Malerbi et al. (9) and Blayo et al. (14) used both starch and sucrose comparisons (15.3%). Fructose was administered in mixed (77%) and fluid (23%) formats at a median dose of 60.0 g/day (IQR 55–120 g/day), with 6 trials (46%) exceeding the Canadian Diabetes Association (CDA) threshold of 60 g/day (2) and 10 trials (77%) exceeding the European Association for the Study of Diabetes (EASD) threshold of 30 g/day (8). Six trials (46.2%) were metabolically controlled, providing all foods consumed, six trials (46.2%) were not metabolically controlled, and one trial (7.6%) was partially metabolically controlled, providing some of all foods consumed. Background diets were 50–55% carbohydrate, 20–35% fat, and 15–30% protein. The median follow-up was 8 weeks (IQR 4–14.3 weeks). Nine trials were of high quality (MQS ≥ 8), with a median MQS of 8 (IQR 7–8). No eligible study measured fructosamine. No hypercaloric feeding trials met the inclusion criteria.
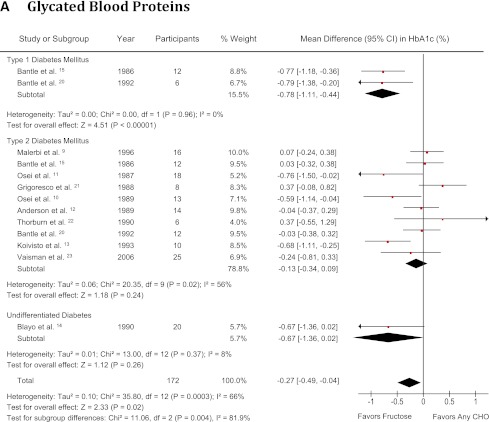
Figure 2A shows the effect of isocaloric fructose exchange for other carbohydrates on glycated blood proteins. There was a significant reduction in the percentage of glycated blood proteins (SMD −0.27 [95% CI −0.49 to −0.04]; P = 0.02), with significant evidence of interstudy heterogeneity (I2 = 66% [95% CI 40–81]; P < 0.001) in people with type 1 and type 2 diabetes combined. A significant reducing effect on glycated blood proteins was also seen in subjects with type 1 diabetes (SMD −0.78 [95% CI −1.11 to −0.44]; P < 0.001), with no evidence of interstudy heterogeneity (I2 = 0%, [95% CI not estimable]; P = 0.96). No effect was seen in the type 2 diabetes stratum, (SMD −0.13 [95% CI −0.34 to 0.09]; P = 0.24), with evidence of significant interstudy heterogeneity (I2 = 56% [95% CI 10–78%]; P = 0.02). Systematic removal of individual studies did not alter the results. Meta-regression revealed no statistically significant subgroup effects (Supplementary Fig. 1).
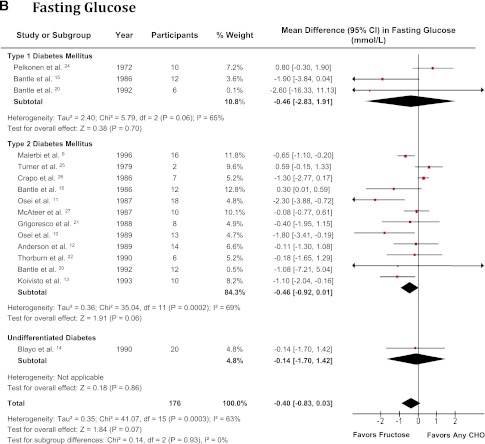
Figure 2.
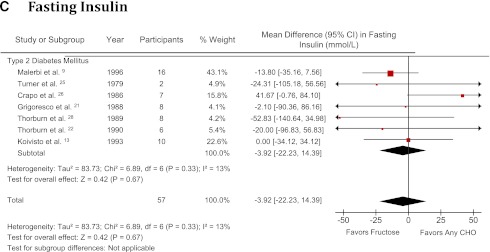
Forest plot of controlled feeding trials investigating the effect of isocaloric exchange of fructose for other carbohydrate on (A) glycated blood proteins (HbA1c and glycated albumin), (B) fasting glucose, and (C) fasting insulin. Data are SMD for glycated blood proteins and MD for fasting glucose and insulin with 95% CI (16). P values are for generic inverse variance random effects models. Interstudy heterogeneity was tested by the Cochran Q statistic (χ2) at a significance level of P < 0.1 and quantified by I2 (2,16). There were no studies investigating type 1 or undifferentiated diabetes for fasting insulin. CHO, carbohydrate. (A high-quality color representation of this figure is available in the online issue.)
Fasting glucose
A total of 16 fasting glucose comparisons were made among 176 subjects with type 1 diabetes (3 trials, n = 28) (15,20,24), type 2 diabetes (13 trials, n = 128) (9–13,15,20–22,25–27), and undifferentiated diabetes (1 trial, n = 20) (14). Patients had a median age of 53.9 years (IQR 40.8–60.3 years) and a diabetes duration of 9.7 years (7–11 years). Their median baseline fasting glucose values were 9.3 mmol/L (IQR 8.1–11.3 mmol/L). Nine trials were randomized (56%). Fourteen trials used crossover designs (88%), and two used parallel designs (12%). Starch (75%) and sucrose (13%) were used as carbohydrate comparators, and notably Malerbi et al. (9) and Blayo et al. (14) used both starch and sucrose comparisons (12%). Fructose was administered in mixed (75%) and fluid (25%) formats at a median dose of 61.6 g/day (IQR 55–105 g/day), with 8 trials (50%) exceeding the CDA threshold of 60 g/day (2) and 14 trials (88%) exceeding the EASD threshold of 30 g/day (8). Nine trials (56%) were metabolically controlled, six trials (38%) were nonmetabolically controlled, and one trial (6%) was partially metabolically controlled. Background diets were 40–55% carbohydrate, 20–40% fat, and 15–30% protein. The median follow-up was 4 weeks (IQR 2–12.6 weeks). Nine trials were of high quality, with a median MQS of 8 (IQR 7–8). No hypercaloric feeding trials met the inclusion criteria.
Fig. 2B shows the effect of isocaloric fructose exchange for other carbohydrates on fasting glucose. There was a borderline reducing effect on fasting glucose (MD −0.40 mmol/L [95% CI −0.83 to 0.03]; P = 0.07) in the overall analysis, with evidence of substantial and significant interstudy heterogeneity (I2 = 63% [95% CI 38–79%]; P < 0.001). There was a trend favoring a reduction in fasting glucose in people with type 2 diabetes (MD −0.46 mmol/L [95% CI −0.92 to 0.01]; P = 0.06) but not type 1 diabetes (MD −0.46 mmol/L [95% CI −2.83 to 1.91]; P = 0.7), although both strata had evidence of substantial and significant interstudy heterogeneity (I2 = 69% [95% CI 43–83%]; P < 0.001; and I2=65% [95% CI 0–90%]; P = 0.06, respectively). A significant fasting glucose lowering effect was seen in the overall analysis after the systematic removal of either Bantle et al. (15) (MD −0.50 mmol/L [95% CI −0.94 to −0.05]; P = 0.03) or Turner et al. (25) (MD −0.51 mmol/L [95% CI −0.96 to −0.06]; P = 0.03) during our sensitivity analysis. Similarly, the removal of either study achieved significance in the type 2 diabetes subset, with an MD of −0.57 mmol/L (95% CI −1.05 to −0.10; P = 0.02) after removal of Bantle et al. (15) and an MD of −0.59 mmol/L (95% CI −1.09 to −0.09; P = 0.02) after removal of Turner et al. (25). There was no change in the interstudy heterogeneity during sensitivity analyses. Meta-regression revealed no statistically significant subgroup effects (Supplementary Fig. 2).
Fasting insulin
A total of 7 comparisons were made in 57 subjects with type 2 diabetes (7 trials, n = 57) (9,13,21,22,25,26,28). Patients had a median age of 53.7 years (IQR 46.0–54.6 years) and a diabetes duration of 8 years (5.6–13 years). Four trials (57%) used diet-only interventions, and three trials (43%) used a combination of diet and medications for their insulin treatment before study onset. Two trials were randomized (29%). Six trials used crossover designs (86%), and one used a parallel design (14%). Starch (43%) and sucrose (43%) were used as carbohydrate comparators, and notably Malerbi et al. (9) used both starch and sucrose comparisons (14%). Fructose was administered in mixed (57%) and fluid (43%) formats at a median dose of 63.2 g/day (IQR 47.5–99 g/day), with four trials (57%) exceeding the CDA threshold of 60 g/day (2) and six trials (86%) exceeding the EASD threshold of 30 g/day (8). Five trials (71%) were metabolically controlled, and two were nonmetabolically controlled (29%). Background diets were 45–55% carbohydrate, 20–40% fat, and 15–30% protein. The median follow-up was 4 weeks (IQR 3–10 weeks). Two trials were of high quality, with a median MQS of 7 (IQR 5.5–7.5). No hypercaloric feeding trial met the inclusion criteria.
Fig. 2C shows the effect of isocaloric fructose exchange for other carbohydrates on fasting insulin. There was no effect on fasting insulin (MD −3.92 pmol/L [95% CI −22.23 to 14.39 pmol/L]), with no evidence of interstudy heterogeneity (I2 = 13% [95% CI 0–75]; P = 0.33) in the type 2 diabetes stratum. Sensitivity analyses did not alter the effect estimate or degree of heterogeneity for fasting insulin, and meta-regression revealed no statistically significant subgroup effects (Supplementary Fig. 3). None of the subjects were treated with insulin.
Publication bias
Supplementary Figs. 4 and 5 show the funnel plots and Egger regression plots, respectively, for investigating publication bias. There was evidence of funnel plot asymmetry for fasting glucose (P < 0.05 by Egger test; P = 0.12 by Begg test), consistent with small-study effects. There was no evidence of publication bias for fasting insulin (P = 0.91 by Egger test; P = 1.00 by Begg test) or glycated blood protein analyses (P = 0.20 by Egger test; P = 0.43 by Begg test).
CONCLUSIONS
In the current aggregate analyses of 18 controlled feeding trials with 209 subjects with type 1 and 2 diabetes, isocaloric fructose exchange for other carbohydrate decreased glycated blood proteins (aggregated glycated albumin and HbA1c) but not fasting glucose or insulin. The observed SMD reduction in glycated blood proteins may be considered clinically significant, because it was equivalent to an absolute reduction of ∼0.53%. This reduction exceeds the clinically meaningful threshold of ≥0.3% proposed by the U.S. Food and Drug Administration for the development of new drugs for diabetes (29) and lies at the lower limit of efficacy expected for oral hypoglycemic agents (30). The lack of change in fasting glucose and insulin suggests that fructose consumption does not promote hepatic and systemic insulin resistance. Future meta-analyses of direct measures of insulin sensitivity would be of value.
Our observed reduction in glycated blood proteins was consistent with the findings of an earlier meta-analysis by Livesey and Taylor (31), who found an improvement in HbA1c (31). It is important to note, however, that their analysis, in contrast to the current meta-analysis, did not focus exclusively on diabetes. Unlike Livesey and Taylor (31), we found the reduction in glycated blood proteins to be significant only in people with type 1 diabetes in stratified analyses. This discrepancy is likely due in part to the use of glycated albumin exclusively in the type 1 diabetes studies (15,20). The null finding in individuals with type 2 diabetes might be explained by the choice of glycated protein, because those trials used both HbA1c (9–13,21–23) and glycated albumin (15,20). Because the half-life of glycated albumin (∼20 days) (32) is shorter than that of HbA1c (∼35 days) (38), it is possible that the shorter type 2 diabetes studies may not have been of sufficient duration to detect true HbA1c changes, with the effect size too small. An improvement in glycemic control in individuals with type 2 diabetes is supported by an improvement in fasting glucose after removal of either Bantle et al. (15) or Turner et al. (25) during sensitivity analyses. It is noteworthy that the small, unusually precise study of Turner et al. (25) was a seemingly disproportionate contributor to the pooled effect, including only two participants and carrying a weight of 9%. Individual patient data revealed that one patient showed a dramatic increase in fasting glucose after fructose consumption, whereas the second patient’s fasting glucose remained constant.
Subgroup analyses revealed no significant effect modification for glycated blood proteins, fasting glucose, or insulin. Although Livesey and Taylor (31) in their earlier meta-analysis found that the improvement in HbA1c was dependent on the degree of dysglycemia, fructose dose, and follow-up, we did not find that these conditions altered any of the outcomes, nor in a separate analysis did we see any effect of fructose dose, follow-up, or comparator on triglycerides in type 2 diabetes with the same subgroup criteria (33). There was, however, evidence of significant interstudy heterogeneity across most subgroup categories. These may be related to real biological differences between study populations or to methodological differences between trials that were not assessed in our a priori subgroup analyses.
A number of potential mechanisms have been proposed to explain the improvements in glycemia seen with the consumption of fructose. One possibility is that the addition of fructose to the diet may help control postprandial glycemic excursions. Replacement of a high–glycemic index (GI) carbohydrate such as starch (for example, white bread, GI = 100) with a low-GI carbohydrate source such as fructose (GI = 16) (34) may decrease the GI of the diet sufficiently to result in improvement in glycemic control (35). Alternatively, an emerging body of evidence has shown that low doses of fructose (≤10 g/meal) may improve glycemic control through upregulation of the glucokinase enzyme (36), exerting a “catalytic” effect. The resulting fructose-1-P is able to displace fructose-6-P from its binding site on the glucokinase regulatory protein, allowing increased translocation of glucokinase from the nucleus to the cytosol, where it is active. Single catalytic doses of fructose infused have shown a ∼30% reduction in postprandial hepatic glucose output under hyperglycemic conditions in people with type 2 diabetes (36) and a roughly threefold increase in glycogen synthesis under euglycemic hyperinsulinemic conditions in people without diabetes (37). Both these mechanisms may be operating.
Although it appears that isocaloric fructose feeding benefits glycemia, a dose threshold for harm must also be considered because fructose, more than other sources of carbohydrate, may increase serum triglycerides. We previously showed in a meta-analysis of controlled feeding trials that fructose at doses >60 g/day (in excess of CDA recommendations) or >10% energy in isocaloric exchange for other carbohydrate increases serum triglyceride levels in type 2 diabetes (33). Livesey and Taylor (31) in their meta-analysis also showed a consistent triglyceride-raising effect of fructose at high doses (>100 g/day) across different subject types. We therefore must consider the possible adverse effects of substituting fructose for other carbohydrates at high doses. There are currently no meta-analyses investigating the effect of fructose on LDL.
A number of limitations complicate the interpretation of these aggregate analyses. First, most of these trials were relatively short, with only four trials ≥12 weeks. It is therefore possible that these shorter trials may have underestimated the HbA1c reduction, given the evidence that HbA1c reduces at ∼0.1%/day at a steady state, with a half-life of 5 weeks (38). Second, several studies included participants who were receiving insulin or oral hypoglycemic agents, treatments that in themselves would be expected to influence glycemia. Third, given the small number of trials included in each stratum, meta-regression may have been underpowered to detect true differences. Fourth, a significant amount of unexplainable heterogeneity was detected in both primary and subgroup analyses, although our random-effects model did account for this heterogeneity. Fifth, study quality was poor (MQS <8) in 50% of the included trials. These deficiencies were especially of concern in the context of the small sample sizes, with most of the trials having 15 or fewer participants. There was, however, no effect of MQS (<8 vs. ≥8) in subgroup analyses. Finally, because only published trials were included, publication bias remains a possibility for all outcomes, although we noted statistical evidence of publication bias only for fasting glucose.
In conclusion, aggregate analyses of short-term controlled feeding trials showed that isocaloric fructose replacement of other carbohydrates resulted in clinically significant improvements in glycemic control, equivalent to a ∼0.53% reduction in HbA1c, without significantly affecting insulin in diabetic individuals. This benefit was seen across a full dose range (20–160 g/day), including at doses below the CDA threshold of 60 g/day, a level of exposure that is unlikely to have an adverse effect on other aspects of metabolic control. The harm-reduction approach to fructose taken by diabetes associations (2,7,8), which is based on possible adverse serum lipid effects, may need to be reconciled with a possible glycemic benefit. These conclusions, however, are limited by the short follow-up, small sample size, and poor quality of most trials included in our meta-analysis, as well as the large degree of unexplained significant heterogeneity. Larger, longer, and higher-quality trials of controlled fructose feeding that also weigh any possible glycemic benefit against adverse metabolic effects are required for definitive confirmation of these findings.
Acknowledgments
This work was funded by a Canadian Institutes of Health Research (CIHR) Knowledge Synthesis to J.L.S., R.J.d.S., A.M., A.J.C., M.D., A.L.J., L.A.L., T.M.S.W., J.B., C.W.C.K., and D.J.A.J. and an unrestricted research grant from the Calorie Control Council to J.L.S., R.J.D., J.B., C.W.C.K., and D.J.A.J. J.L.S. was supported in the initial stages of this work by a Province of Ontario Postdoctoral Fellowship, the Edie Steinberg Scholarship Fund, and the Edward Christie Stevens Fellowship in Medicine. R.J.d.S. was funded by a CIHR Postdoctoral Fellowship Award and A.M. by a CIHR Canada Graduate Scholarship Master's award. D.J.A.J. was funded by the Government of Canada through the Canada Research Chair Endowment. None of the sponsors had a role in any aspect of the current study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.L.S. has received consultant fees, travel funding, honoraria, or research support from the CIHR, Canadian Diabetes Association (CDA), Calorie Control Council, The Coca-Cola Company, Archer Daniels Midland, International Life Sciences Institute (ILSI) North America and Brazil, Abbott Laboratories, and Pulse Canada. R.J.d.S. has received research support from the CIHR and The Coca-Cola Company. L.C. is a casual Clinical Research Coordinator at Glycemic Index Laboratories, Toronto, Canada, and has received research support from the CIHR. V.H., A.M., and A.J.C. have received research support from the CIHR. T.M.S.W. is a part owner and the President of Glycemic Index Laboratories, Toronto, Canada, and has authored several popular diet books on the glycemic index for which he has received royalties from Phillipa Sandall Publishing Services and CABI Publishers. He has received consultant fees, honoraria, travel funding, or research support from or served on the scientific advisory board for the CIHR; CDA; Dairy Farmers of Canada; McCain Foods; Temasek Polytechnic; Northwestern University; Royal Society of London; Glycemic Index Symbol program; CreaNutrition AG; McMaster University; Canadian Society for Nutritional Sciences; National Sports and Conditioning Association; Faculty of Public Health and Nutrition, Autonomous University of Nuevo Leon; and the Diabetes and Nutrition Study Group of The European Association for the Study of Diabetes. J.B. has received research support from the CIHR and The Coca-Cola Company. C.W.C.K. has received consultant fees, honoraria, travel funding, or research support from or served on the scientific advisory board for the CIHR, The Coca-Cola Company, Abbott Laboratories, Advanced Food Materials Network, Almond Board of California, American Peanut Council, American Pistachio Growers, Barilla, California Strawberry Commission, Canola Council of Canada, Danone, General Mills, Hain Celestial, International Tree Nut Council, Kellogg, Loblaw Brands Ltd, Oldways, Orafti, Paramount Farms, Pulse Canada, Saskatchewan Pulse Growers, Solae, and Unilever. D.J.A.J. has received consultant fees, honoraria, travel funding, or research support from or served on the scientific advisory board for the CIHR; the Canadian Foundation for Innovation (CFI); Ontario Research Fund (ORF); and Advanced Foods and Material Network (AFMNet); Calorie Control Council; The Coca-Cola Company; Barilla; Solae; Unilever; Hain Celestial; Loblaws Supermarkets, Inc.; Sanitarium Company; Herbalife International; Pacific Health Laboratories, Inc.; Metagenics/MetaProteomics; Bayer Consumer Care; Oldways Preservation Trust; The International Tree Nut Council Nutrition Research & Education; The Peanut Institute; Procter & Gamble Technical Centres Limited; Griffin Hospital for the development of the NuVal System; Soy Advisory Board of Dean Foods; Alpro Soy Foundation; Nutritional Fundamentals for Health; Pacific Health Laboratories; Kellogg's; Quaker Oats; The Coca-Cola Sugar Advisory Board; Pepsi Company; Agrifoods and Agriculture Canada (AAFC); Canadian Agriculture Policy Institute (CAPI); The Almond Board of California; The California Strawberry Commission; Orafti; the Canola and Flax Councils of Canada; Pulse Canada; the Saskatchewan Pulse Growers; and Abbott Laboratories. A.L.J. is a part owner, Vice-President, and Director of Research of Glycemic Index Laboratories, Toronto, Canada. She has received research support from the CDA and the CIHR. No other potential conflicts of interest relevant to this article were reported.
A.I.C. was responsible for acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis. J.L.S. was responsible for conception and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, and supervision. J.L.S. had full access to all the data in the study and had final responsibility for the decision to submit for publication. R.J.d.S. was responsible for the analysis and interpretation of data, critical revision of manuscript for important intellectual content, statistical analysis, supervision, and obtaining funding. L.C. was responsible for the acquisition of data, critical revision of the manuscript for important intellectual content, and administrative support. V.H., D.D.W., and M.E.Y. were responsible for acquisition of the data and critical revision of the manuscript for important intellectual content. A.M. and A.J.C. were responsible for acquisition of data, critical revision of the manuscript for important intellectual content, and obtaining funding. M.D., A.L.J., L.A.L., T.M.S.W., and C.W.C.K. were responsible for critical revision of the manuscript for important intellectual content and obtaining funding. J.B. was responsible for critical revision of the manuscript for important intellectual content, statistical analysis, and obtaining funding. D.J.A.J. was responsible for analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, and supervision.
Parts of this work were presented at the 14th Canadian Society of Endocrinology and Metabolism/CDA Professional Conference and Annual Meetings, Toronto, Ontario, Canada, 26–29 October 2011 (39).
Footnotes
Clinical trial reg. no. NCT01363791, clinicaltrials.gov.
References
- 1.World Health Organization. The World Health Report 1998: Life in the 21st Century: A Vision for All Geneva, World Health Organization, 1998
- 2.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32:S1–S201 [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Rimm EB, Liu Y, Willett WC. Height, predictors of C-peptide and cancer risk in men. Int J Epidemiol 2004;33:217–225 [DOI] [PubMed] [Google Scholar]
- 4.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228S–1235S [DOI] [PubMed] [Google Scholar]
- 5.Park YK, Yetley EA. Intakes and food sources of fructose in the United States. Am J Clin Nutr 1993;58(Suppl):737S–747S [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–543 [DOI] [PubMed] [Google Scholar]
- 7.Bantle JP, Wylie-Rosett J, Albright AL, et al. American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 8.Mann JI, De Leeuw I, Hermansen K, et al. Diabetes and Nutrition Study Group (DNSG) of the European Association Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis 2004;14:373–394 [DOI] [PubMed] [Google Scholar]
- 9.Malerbi DA, Paiva ES, Duarte AL, Wajchenberg BL. Metabolic effects of dietary sucrose and fructose in type II diabetic subjects. Diabetes Care 1996;19:1249–1256 [DOI] [PubMed] [Google Scholar]
- 10.Osei K, Bossetti B. Dietary fructose as a natural sweetener in poorly controlled type 2 diabetes: a 12-month crossover study of effects on glucose, lipoprotein and apolipoprotein metabolism. Diabet Med 1989;6:506–511 [DOI] [PubMed] [Google Scholar]
- 11.Osei K, Falko J, Bossetti BM, Holland GC. Metabolic effects of fructose as a natural sweetener in the physiologic meals of ambulatory obese patients with type II diabetes. Am J Med 1987;83:249–255 [DOI] [PubMed] [Google Scholar]
- 12.Anderson JW, Story LJ, Zettwoch NC, Gustafson NJ, Jefferson BS. Metabolic effects of fructose supplementation in diabetic individuals. Diabetes Care 1989;12:337–344 [DOI] [PubMed] [Google Scholar]
- 13.Koivisto VA, Yki-Järvinen H. Fructose and insulin sensitivity in patients with type 2 diabetes. J Intern Med 1993;233:145–153 [DOI] [PubMed] [Google Scholar]
- 14.Blayo A, Fontveille AM, Rizkalla S, Bruzzo F, Slama G. Effets metaboliques de la consommation quotidienne pendant un an de saccharose ou de fructose par des diabetiques. Médecine et Nutrition 1990;26:909–913 [in French] [Google Scholar]
- 15.Bantle JP, Laine DC, Thomas JW. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA 1986;256:3241–3246 [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 510. Oxford, UK, Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. Accessed on 15 May 2012 [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001;286:944–953 [DOI] [PubMed] [Google Scholar]
- 19.Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140–149 [DOI] [PubMed] [Google Scholar]
- 20.Bantle JP, Swanson JE, Thomas W, Laine DC. Metabolic effects of dietary fructose in diabetic subjects. Diabetes Care 1992;15:1468–1476 [DOI] [PubMed] [Google Scholar]
- 21.Grigoresco C, Rizkalla SW, Halfon P, et al. Lack of detectable deleterious effects on metabolic control of daily fructose ingestion for 2 mo in NIDDM patients. Diabetes Care 1988;11:546–550 [DOI] [PubMed] [Google Scholar]
- 22.Thorburn AW, Crapo PA, Griver K, Wallace P, Henry RR. Long-term effects of dietary fructose on carbohydrate metabolism in non-insulin-dependent diabetes mellitus. Metabolism 1990;39:58–63 [DOI] [PubMed] [Google Scholar]
- 23.Vaisman N, Niv E, Izkhakov Y. Catalytic amounts of fructose may improve glucose tolerance in subjects with uncontrolled non-insulin-dependent diabetes. Clin Nutr 2006;25:617–621 [DOI] [PubMed] [Google Scholar]
- 24.Pelkonen R, Aro A, Nikkilä EA. Metabolic effects of dietary fructose in insulin dependent diabetes of adults. Acta Med Scand Suppl 1972;542:187–193 [DOI] [PubMed] [Google Scholar]
- 25.Turner JL, Bierman EL, Brunzell JD, Chait A. Effect of dietary fructose on triglyceride transport and glucoregulatory hormones in hypertriglyceridemic men. Am J Clin Nutr 1979;32:1043–1050 [DOI] [PubMed] [Google Scholar]
- 26.Crapo PA, Kolterman OG, Henry RR. Metabolic consequence of two-week fructose feeding in diabetic subjects. Diabetes Care 1986;9:111–119 [DOI] [PubMed] [Google Scholar]
- 27.McAteer EJ, O’Reilly G, Hadden DR. The effects of one month high fructose intake on plasma glucose and lipid levels in non-insulin-dependent diabetes. Diabet Med 1987;4:62–64 [DOI] [PubMed] [Google Scholar]
- 28.Thorburn AW, Crapo PA, Beltz WF, Wallace P, Witztum JL, Henry RR. Lipid metabolism in non-insulin-dependent diabetes: effects of long-term treatment with fructose-supplemented mixed meals. Am J Clin Nutr 1989;50:1015–1022 [DOI] [PubMed] [Google Scholar]
- 29.Center for Drug Evaluation and Research Guidance for Industry: Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention (DRAFT GUIDANCE). Rockville, Md, U.S. Department of Health and Human Services Food and Drug Administration, 2008, p. 1–30 [Google Scholar]
- 30.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002;287:360–372 [DOI] [PubMed] [Google Scholar]
- 31.Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 2008;88:1419–1437 [DOI] [PubMed] [Google Scholar]
- 32.Jones IR, Owens DR, Williams S, et al. Glycosylated serum albumin: an intermediate index of diabetic control. Diabetes Care 1983;6:501–503 [DOI] [PubMed] [Google Scholar]
- 33.Sievenpiper JL, Carleton AJ, Chatha S, et al. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: systematic review and meta-analysis of experimental trials in humans. Diabetes Care 2009;32:1930–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802 [DOI] [PubMed] [Google Scholar]
- 36.Hawkins M, Gabriely I, Wozniak R, Vilcu C, Shamoon H, Rossetti L. Fructose improves the ability of hyperglycemia per se to regulate glucose production in type 2 diabetes. Diabetes 2002;51:606–614 [DOI] [PubMed] [Google Scholar]
- 37.Petersen KF, Laurent D, Yu C, Cline GW, Shulman GI. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes 2001;50:1263–1268 [DOI] [PubMed] [Google Scholar]
- 38.Rech ME. Observations on the decay of glycated hemoglobin HbA1c in diabetic patients. Exp Clin Endocrinol Diabetes 1996;104:102–105 [DOI] [PubMed] [Google Scholar]
- 39.Cozma AI, Sievenpiper JL, Chiavaroli L, et al. Effect of fructose on glycemic control in diabetes: a meta-analysis of controlled feeding trials. Can J Diabetes 2011;35:420–421 [DOI] [PMC free article] [PubMed] [Google Scholar]