Abstract
OBJECTIVE
Compelling biological pathways suggest that selenium (Se) may lower onset of type 2 diabetes mellitus (T2DM), but very few studies have evaluated this relationship, with mixed results. We examined the association between toenail Se and incidence of T2DM.
RESEARCH DESIGN AND METHODS
We performed prospective analyses in two separate U.S. cohorts, including 3,630 women and 3,535 men, who were free of prevalent T2DM and heart disease at baseline in 1982–1983 and 1986–1987, respectively. Toenail Se concentration was quantified using neutron activation analysis, and diabetes cases were identified by biennial questionnaires and confirmed by a detailed supplementary questionnaire. Hazard ratios of incident T2DM according to Se levels were calculated using Cox proportional hazards.
RESULTS
During 142,550 person-years of follow-up through 2008, 780 cases of incident T2DM occurred. After multivariable adjustment, the risk of T2DM was lower across increasing quintiles of Se, with pooled relative risks across the two cohorts of 1.0 (reference), 0.91 (95% CI 0.73–1.14), 0.78 (0.62–0.99), 0.72 (0.57–0.91), and 0.76 (0.60–0.97), respectively (P for trend = 0.01). Results were similar excluding the few individuals (4%) who used Se supplements. In semiparametric analyses, the inverse relationship between Se levels and T2DM risk appeared to be linear.
CONCLUSIONS
At dietary levels of intake, individuals with higher toenail Se levels are at lower risk for T2DM. Further research is required to determine whether varying results in this study versus prior trials relate to differences in dose, source, statistical power, residual confounding factors, or underlying population risk.
Accumulating evidence indicates that excess oxidative stress is a risk factor for insulin resistance, β-cell dysfunction, impaired glucose tolerance, and type 2 diabetes mellitus (T2DM) (1–3). Selenium (Se), an essential trace nutrient, is a critical component of numerous selenoproteins involved in antioxidant defense systems, such as glutathione peroxidase, which actively protect against damage from free radicals and reactive oxygen species (4,5). Increased free radical levels impair glucose-stimulated insulin secretion, decrease gene expression of key β-cell genes, and induce cell death (2,6–8).
Investigations into the effects of habitual Se consumption on chronic disease in humans have been limited by challenges in accurately assessing Se intake from dietary questionnaires, due to errors in recall, geographic variation in Se exposures, and wide variations in Se content of otherwise similar foods. In this setting, measurements of toenail Se concentrations provide a valid and objective biomarker of long-term (∼1 year) Se consumption (9). However, to our knowledge, no prior investigations have followed large numbers of individuals with both biomarker measures and sufficiently long durations of follow-up to assess development of T2DM.
Although compelling biological evidence suggests that Se might reduce the onset of T2DM, results of prior cross-sectional studies have been conflicting. One prior study observed an inverse association between toenail Se and prevalent T2DM (10), whereas two other studies using data from the U.S. National Health and Nutrition Examination Survey showed nonlinear positive associations between serum Se and prevalence of T2DM (11,12). Such cross-sectional studies are limited by an inability to assess temporal relationships, in that diabetes status could alter Se levels. In two randomized clinical trials (13,14), Se supplementation did not reduce the incidence of T2DM. However, these studies evaluated the effects of relatively high Se dosages in specific high–cancer risk populations rather than the effects of dietary doses derived from foods in more general populations. To our knowledge, no study has prospectively evaluated the relationship between habitual dietary Se consumption, as assessed through a valid Se biomarker, and the incidence of T2DM. Therefore, we prospectively evaluated whether Se consumption, as assessed by an objectively measured toenail-Se biomarker, was associated with lower incidence of T2DM in women and men in two separate U.S. cohort studies: the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS).
RESEARCH DESIGN AND METHODS
The population and design of the NHS and HPFS have previously been described (15,16). Briefly, NHS is a prospective cohort study among 121,700 U.S. female registered nurses aged 30–55 years at enrollment in 1976, and HPFS is a prospective cohort study among 51,529 U.S. male health professionals aged 40–75 years at enrollment in 1986. Members of both cohorts have been sent questionnaires biennially about their medical history, risk factors, lifestyle, and disease incidence. The current study included 3,535 HPFS and 3,630 NHS participants with available toenail Se data from prior or ongoing nested case-control studies of cancer or cardiovascular disease (CVD) who had available valid covariate information and were free of prevalent T2DM or heart disease (stroke, angina pectoris, and myocardial infarction) at baseline, defined as the date of toenail sampling. The Harvard School of Public Health and Brigham and Women’s Hospital Human Subjects Committee Review Board approved the study protocol.
Assessment of toenail Se concentrations
We assessed toenail Se as an objective biomarker of long-term Se consumption. Other potential measures, such as serum or plasma Se, provide a measure of short-term exposure (9,17,18). Hair and fingernail Se levels provide relatively long-term Se status, but these are more likely than toenails to be influenced by superficial contamination such as use of Se-medicated shampoos (9,17). In both cohorts, participants were asked to provide toenail clippings from all 10 toes and return them to the investigators. Follow-up mail requests were sent to initial nonrespondents. Overall, 68% of HPFS participants and 52% of NHS participants returned toenail samples in 1986–1987 and 1982–1983, respectively. No differences in the baseline characteristics were observed between participants supplying and not supplying toenail samples in both cohorts (data not shown).
Toenail Se concentrations were measured using neutron activation analysis at the University of Missouri Research Reactor by personnel unaware of the participants’ clinical information. Details of analytical methods and information regarding validation of these measures have previously been reported (9,17–20). Samples of nail clippings from all toes were combined, providing a time-integrated measure of exposure over approximately the prior year owing to the elimination half-life of toenail Se, the growth rate of toenails, and the differential length of time (distance) from cuticle synthesis to toenail clipping comparing the smallest to largest toes. Sample mass was adequate for neutron-activation analysis in all participants. Se determinations were performed in 98 analytical batches between 2009 and 2011. Potential laboratory drift was controlled by both standard comparison procedures for neutron activation analysis and repeated analysis of representative sample subsets. Intra-assay coefficients of variation were 2.4% for Se. We excluded 164 (2%) individuals with toenail Se concentrations >1.5 μg/g, which could reflect exogenous contamination or considerable excess ingestion of Se supplements (9,19,20).
Toenail Se concentrations are valid biomarkers of Se consumption, responding to long-term changes in dietary consumption and correlating with whole blood and serum Se levels (9,17). A single measurement also correlates with future exposure (9,17,21), based on reasonable within reliability of toenail Se levels over time (r = 0.48) for levels in clippings obtained 6 years apart (21).
Ascertainment of T2DM
Potential T2DM cases were identified by self-report on biennial questionnaires, and all self-reported cases were confirmed by a detailed validated supplementary questionnaire. Cases were diagnosed according to the National Diabetes Data Group criteria (22) by having at least one of the following: 1) an elevated plasma glucose concentration (fasting ≥7.8 mmol/L [or 140.4 mg/dL], random ≥11.1 mmol/L [or 199.8 mg/dL], or ≥11.1 mmol/L 2 h after an oral glucose load) plus at least one symptom related to diabetes (excessive thirst, polyuria, weight loss, or hunger), 2) no symptoms but elevated glucose concentrations as defined above on at least two occasions, or 3) treatment with insulin or oral hypoglycemic medication. The lower cutoff point for fasting plasma glucose concentrations (7.0 mmol/L) was used for cases identified after 1998 according to the American Diabetes Association criteria (23). The validity of the supplementary questionnaire for diagnosing T2DM was confirmed against medical record review in subsamples from both the NHS (98% confirmed) and the HPFS (97% confirmed) (24,25). For the present analysis, case diagnosis information of participants was included from the date of toenail sampling through the latest date of adjudicated follow-up in 2008, representing 26 years of follow-up in NHS (1982–1983 through 2008) and 22 years of follow-up in HPFS (1986–1987 through 2008).
Assessment of covariates
Detailed information on demographics, risk factors, habitual diet, and other lifestyle habits was collected via validated self-administered questionnaires. We used the reports closest in time to the collection of toenail samples from each participant. Usual dietary habits were assessed with validated semiquantitative food frequency questionnaires that inquired about the usual intake of foods and beverages and the use of dietary supplements, including multivitamins, Se supplements, and other dietary supplements over the past 12 months. The reproducibility and validity of the Food Frequency Questionnaire have previously been reported (26,27). Smoking status was assessed as never, former, and current (1–14, 15–34, and ≥35 cigarettes per day). Self-reported height was obtained from the baseline questionnaire. Information on weight was obtained at baseline, and self-reported weight was updated every 2 years; self-reported weight was validated against technician-measured weight (r = 0.96) among both men and women in these cohorts (28). In a validation study, self-reported weight was, on average, 1.0 kg lower than technician-measured weight (28).
BMI was calculated as the ratio of weight in kilograms to the square of height in meters. Physical activity was assessed as MET tasks per week, using the 1986 HPFS questionnaire. In the NHS, physical activity levels were evaluated qualitatively (rather than as METs) in 1980 and 1982. To minimize misclassification yet still capture physical activity at or before the time of toenail sampling, we calculated the average physical activity level in the NHS based on the average of information from the 1980 and 1982 questionnaires (29). We used sex-specific quintiles of physical activity for the multivariate analyses models.
Statistical analyses
The Cox proportional hazards model was used to estimate the hazard ratio (hereafter referred to as relative risk [RR]) of incident T2DM according to Se levels. Person-months of follow-up accrued from the date of toenail sample return until the diagnosis of T2DM, death, or latest date of adjudicated follow-up in 2008—whichever occurred first. Participants were classified into sex-specific quintiles of toenail Se. In preliminary analyses, the relationships between toenail Se and incident T2DM did not differ according to sample selection status (control subjects vs. future case subjects in nested prospective case-control studies of cancer or CVD), smoking status (P = 0.69), or sex (P = 0.85). To account for geographic variation in Se exposures, U.S. states were grouped into five regions based on other analyses to increase interpretability and minimize confounding by geographic location (30). To minimize other potential confounding factors, we evaluated three covariate models: model 1, adjusted for age, sex, and future case status; model 2, further adjusted for other environmental and lifestyle factors, including geographic region, smoking, alcohol intake, physical activity, BMI, and use of Se or multivitamin supplements; and model 3, further adjusted for other dietary risk factors for T2DM including consumption of total energy, trans fat, whole grains, coffee, and the ratio of polyunsaturated to saturated fat. Potential effect modification was assessed by sex, smoking, time, and sample selection status (control subjects vs. future case subjects in nested prospective case-control studies of cancer or CVD). We included multiplicative terms in the Cox regression models, with adjustment for other potential confounders. The median value of each Se-level category was evaluated as a continuous variable to test for linear trends. Nonlinear relationships were examined semiparametrically using restricted cubic splines, excluding values outside the 5th and 95th percentiles. All P values were two tailed, α = 0.05. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
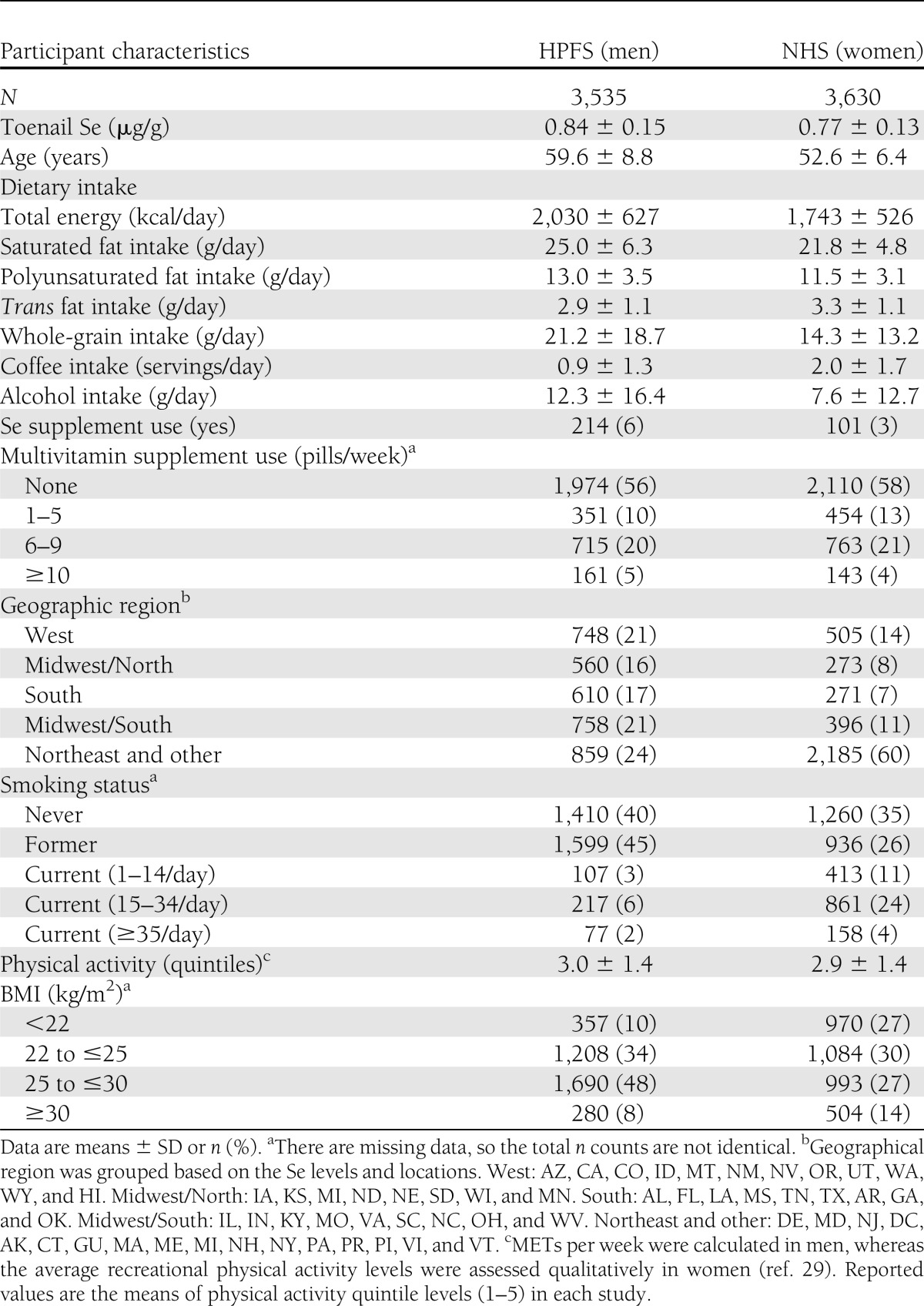
Baseline characteristics of the cohorts, including 3,535 men and 3,630 women, are presented in Table 1. The mean concentration of toenail Se was 0.84 μg/g in men and 0.77 μg/g in women. Use of Se supplements was infrequent, including only 6% of men and 3% of women. General multivitamin supplement use was more common, taken by 35% of men and 38% of women.
Table 1.
Baseline characteristics of the NHS and the HPFS populations
Individuals with higher Se levels consumed more whole grains and less saturated fat, coffee, and alcohol (Supplementary Table). They were also less likely to be current smokers, and geographic variation in Se levels was also seen, as previously reported (30). We therefore adjusted for these and other potential confounding factors in multivariable models.
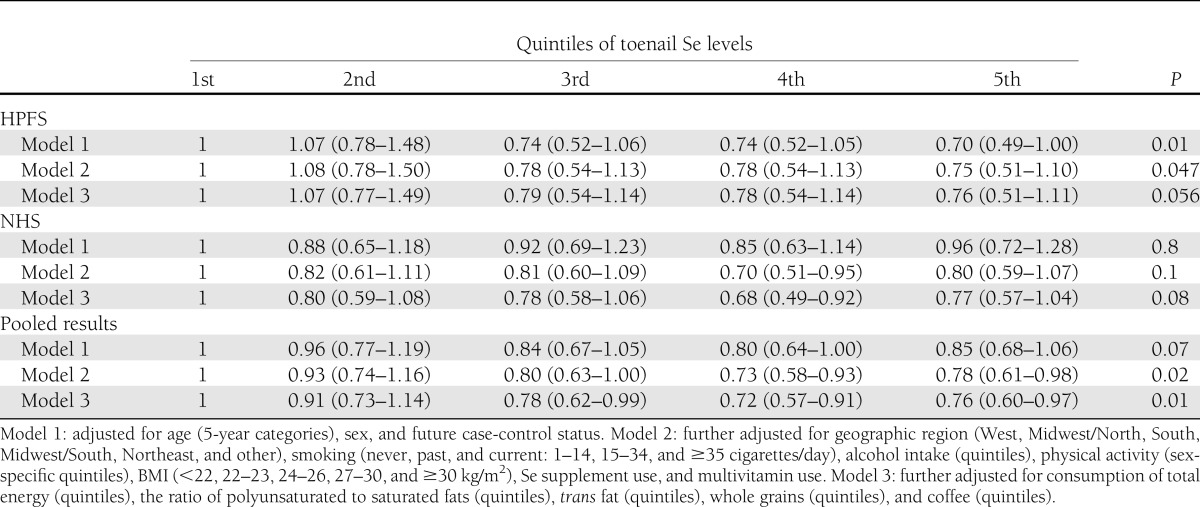
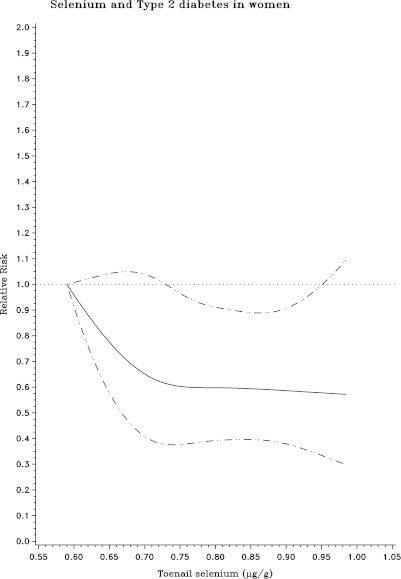
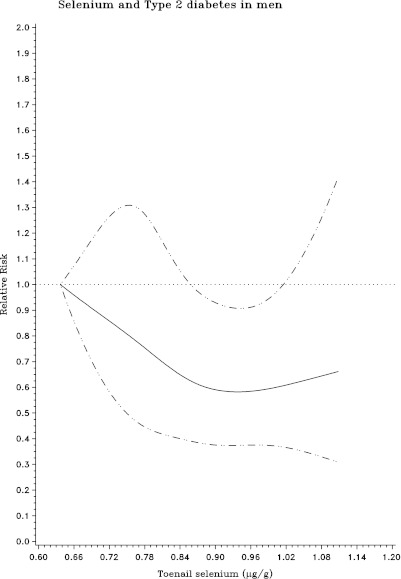
During 142,550 person-years of follow-up, we documented 780 cases of incident T2DM. Higher toenail Se levels were associated with lower risk of incident T2DM in both men and women (Table 2). After multivariate adjustment for age, sex, geographic region, smoking, alcohol intake, physical activity, BMI, Se supplement use, multivitamin use, and dietary risk factors for T2DM, Se levels were inversely associated with incidence of T2DM, with pooled RRs of T2DM across increasing quintiles of Se of 1.0 (reference), 0.91 (95% CI 0.73–1.14), 0.78 (0.62–0.99), 0.72 (0.57–0.91), and 0.76 (0.60–0.97) in both cohorts combined (P for trend = 0.01). Spline analyses suggested monotonic dose-response relationships between Se and the incidence of T2DM in both men and women, although the CIs broadened at the highest Se levels owing to fewer numbers of participants at the extremes (Fig. 1). No significant interactions were observed between toenail Se and incidence of T2DM according to other covariates, including sex, smoking, time, and sample selection status (P for interaction >0.05 for each).
Table 2.
Hazard ratio (95% CI) for T2DM risk according to toenail Se level in the NHS and the HPFS cohorts
Figure 1.
RRs (95% CI) for the relationship between toenail Se level and incidence of T2DM among men in the HPFS (1986–2008) and women in the NHS (1982–2008), evaluated using restricted cubic splines, adjusting for age, sex, future case-control status, geographic region, smoking, alcohol intake, physical activity, BMI, Se supplement use, multivitamin use, and consumption of total energy, ratio of polyunsaturated to saturated fat, trans fat, whole grain, and coffee. Solid lines, RR; dashed lines, 95% CI.
CONCLUSIONS
In these two large prospective U.S. cohorts including both men and women, higher toenail Se levels were associated with a lower risk for development of T2DM. The dose-response relationship appeared generally linear or monotonic at these ranges of toenail Se levels, without evidence for higher risk at higher Se levels, although modestly less benefit at the highest levels could not be excluded.
Prior studies have reported conflicting results for the relationships between Se and T2DM. Three previous cross-sectional analyses have shown mixed results (10–12). Post hoc analyses of two recent randomized clinical trials found no benefit of Se supplementation on incident T2DM. In the Se and Vitamin E Cancer Prevention Trial (SELECT) (14), Se supplementation (Se 200 μg/day l-selenomethionine or mixed form with vitamin E 400 IU/day rac-α-tocopheryl acetate) had no significant effect on incidence of T2DM (2,753 cases; RR 1.07 [95% CI 0.94–1.22]; P = 0.16) during a median follow-up of 5.5 years (range 4.2–7.3 years). In the Nutritional Prevention of Cancer (NPC) trial, 200 μg/day Se supplementation with a bioformed yeast did not prevent T2DM (97 cases; RR 1.50 [95% CI 0.98–2.30]) during 7.7 ± 2.7 years of follow-up (13).
There are several potential explanations for the discrepant findings between our two prospective cohorts and these two Se clinical trials. First, toenail Se levels in our subjects largely represented modest Se exposure from the diet, with very few participants taking Se supplements. In contrast, the trials evaluated the effects of relatively large Se dosages (200 μg/day) from supplements. Approximately 40% of our study populations reported use of a general multivitamin supplement, which in some cases could contain low amounts of Se, but these exposures are small and multivitamin use has not been associated with toenail Se levels in these cohorts (30). Of note, doses of Se in multivitamin supplements used in the 1980s in these cohorts may be different (probably lower) compared with what may be found on the market today. High levels of Se consumption from supplements may have different effects than those of modest dietary doses. For example, in a recent randomized trial, typical supplement doses of antioxidants inhibited the normal pro-oxidant exercise responses of muscles, with consequent inhibition of the beneficial effects of exercise on insulin sensitivity (31). Supradietary doses of antioxidants may therefore inhibit aspects of normal oxidant stress functions, which may be necessary for optimal physiological responses, including at least some responses central to preventing insulin resistance. This may be particularly relevant for Se, in which typical dietary doses may be protective by augmenting antioxidant defenses but with a relatively narrow safety range toward toxicity (32).
The discrepant findings between our prospective cohorts and the two Se clinical trials could also be due to confounding factors in our analyses. For example, other dietary factors in foods that contain Se could protect against T2DM (i.e., the observed protection is due to diet but not to Se per se), or individuals who consume higher levels of Se could have other, nondietary lifestyle behaviors that protect against T2DM. We adjusted for multiple dietary and other lifestyle behaviors independently associated with T2DM risk in these cohorts, and such adjustment actually strengthened the results. Nevertheless, residual confounding due to unmeasured or imperfectly measured factors cannot be fully excluded.
Finally, it is possible that effects of Se on T2DM risk may depend on the specific types of Se consumed or on the underlying population characteristics. The prior trials tested the effects of Se in specific dosage forms (e.g., L-selenomethionine in SELECT, Se–bioformed yeast in NPC) rather than the more diverse types of Se derived from foods. Several lines of evidence suggest that purified nutrients may not always have the same biological effects as nutrients obtained within foods (33,34). The Se trials also enrolled specific high-risk populations (e.g., patients with nonmelanoma skin cancer or at higher risk for prostate cancer), whereas our cohorts included generally healthy men and women, and it is possible that Se may have different effects depending on disease stage or other population characteristics. Such explanations, i.e., biological effect modification by the Se source or by the underlying population characteristics, should generally be considered speculative until confirmed by additional prospective studies, clinical trials, or experiments.
Our study has several strengths. We evaluated the longitudinal associations between toenail Se concentrations, an objective biomarker of long-term Se consumption, and future development of T2DM in two large well-established cohorts including both men and women. This represents the first prospective investigation of this relationship. Thus, our results provide the best evidence to date of how habitual Se intake from dietary sources may influence the onset of T2DM. Low loss to follow-up and centralized adjudication of outcomes reduced the likelihood of missed or misclassified T2DM outcomes. We adjusted for multiple demographic, lifestyle, and dietary covariates, which were assessed using validated questionnaires to minimize residual confounding.
Potential limitations should be considered. Participants were mostly Caucasian and of higher education and income, which reduced confounding due to race or socioeconomic status but could limit generalizability to other populations if the biological effects of Se on T2DM vary substantially among different races or socioeconomic classes. While toenail Se is a valid measure of dietary Se, there is no accepted single optimal metric for assessing Se status overall, in the circulation, or in specific tissues. Other potential metrics of Se status were not available in these cohorts, and the functional relevance of toenail Se concentrations is uncertain. While we adjusted for demographic, lifestyle, and dietary characteristics, residual confounding effects from unmeasured or incompletely measured factors cannot be excluded. Se levels were assessed at baseline, and changes in consumption over time would result in exposure misclassification and attenuate results toward the null.
In conclusion, our findings suggest that higher toenail Se, a biomarker of dietary Se consumption, is associated with lower incidence of T2DM among generally healthy U.S. men and women. Our findings do not exclude the possibility of no benefit or even harm of larger supplement doses, which may, for example, block normal oxidant stress pathways essential to optimal physiological function. Our results support the need for further investigations into the effects of Se, at both dietary and higher doses, on metabolic pathways and risk factors for T2DM.
Acknowledgments
This study was supported by National Institutes of Health (NIH) Grant R01-ES014433 (National Institute of Environmental Health Studies and National Heart, Lung, and Blood Institute) and the Harvard School of Public Health Genes and Environment Initiative, with additional support from NIH grants CA55075 and HL35464.
No potential conflicts of interest relevant to this article were reported.
K.P. developed the study design, conducted the analysis, wrote the draft of the manuscript, and had primary responsibility for the final content. E.B.R., D.S.S., and D.S. assisted with obtaining funding and acquiring data, contributed to discussion, and reviewed and edited the manuscript. J.E.M. contributed to discussion and reviewed and edited the manuscript. J.S.M. assisted with obtaining funding and acquiring data, contributed to discussion, and reviewed and edited the manuscript. F.B.H. contributed to discussion and reviewed and edited the manuscript. D.M. developed the study design, assisted with obtaining funding and acquiring data, contributed to discussion, and reviewed and edited the manuscript. K.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2136/-/DC1.
References
- 1.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003;52:1–8 [DOI] [PubMed] [Google Scholar]
- 2.Shah S, Iqbal M, Karam J, Salifu M, McFarlane SI. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: pathophysiological insights. Antioxid Redox Signal 2007;9:911–929 [DOI] [PubMed] [Google Scholar]
- 3.Park K, Gross M, Lee DH, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care 2009;32:1302–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973;179:588–590 [DOI] [PubMed] [Google Scholar]
- 5.Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem 1993;268:2571–2576 [PubMed] [Google Scholar]
- 6.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463–466 [DOI] [PubMed] [Google Scholar]
- 7.Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem 2002;277:30010–30018 [DOI] [PubMed] [Google Scholar]
- 8.Simmons RA. Developmental origins of diabetes: the role of oxidative stress. Free Radic Biol Med 2006;40:917–922 [DOI] [PubMed] [Google Scholar]
- 9.Longnecker MP, Stampfer MJ, Morris JS, et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr 1993;57:408–413 [DOI] [PubMed] [Google Scholar]
- 10.Rajpathak S, Rimm E, Morris JS, Hu F. Toenail selenium and cardiovascular disease in men with diabetes. J Am Coll Nutr 2005;24:250–256 [DOI] [PubMed] [Google Scholar]
- 11.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care 2007;30:829–834 [DOI] [PubMed] [Google Scholar]
- 12.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003-2004. Environ Health Perspect 2009;117:1409–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:217–223 [DOI] [PubMed] [Google Scholar]
- 14.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002;287:1815–1821 [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Ascherio A, Hu FB, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005;111:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longnecker MP, Stram DO, Taylor PR, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology 1996;7:384–390 [DOI] [PubMed] [Google Scholar]
- 18.Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr 2009;89:2025S–2039S [DOI] [PubMed] [Google Scholar]
- 19.Morris JS, Stampfer MJ, Willett WC. Dietary selenium in humans toenails as an indicator. Biol Trace Elem Res 1983;5:529–537 [DOI] [PubMed] [Google Scholar]
- 20.Morris JS, Spate VL, Ngwenyama RA. Determinants of selenium in the toenail biomonitor. J Radioanal Nucl Chem 2006;269:283–290 [Google Scholar]
- 21.Garland M, Morris JS, Rosner BA, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev 1993;2:493–497 [PubMed] [Google Scholar]
- 22.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 23.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 25.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 26.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 27.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 28.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473 [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA 2000;283:2961–2967 [DOI] [PubMed] [Google Scholar]
- 30.Park K, Rimm E, Siscovick D, Spiegelman D, Morris JS, Mozaffarian D. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr Res Pract 2011;5:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 2009;106:8665–8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan AM, Kizer KW. Selenium. Nutritional, toxicologic, and clinical aspects. West J Med 1990;153:160–167 [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs DR, Jr, Tapsell LC. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev 2007;65:439–450 [DOI] [PubMed] [Google Scholar]
- 34.Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. J Hum Hypertens 2010;24:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]