Abstract
The mechanism responsible for poor reproductive outcomes in type 1 diabetic males is not well understood. In light of new evidence that the Sertoli cells of the testis secrete insulin, it is currently unclear whether diabetic subfertility is the result of deficiency of pancreatic insulin, testicular insulin, or both. In this study, the Akita mouse diabetic model, which expresses a mutant, nonfunctional form of ins2 in testes and pancreas, was used to distinguish between systemic and local effects of insulin deficiency on the process of spermatogenesis and fertility. We determined that Akita homozygous male mice are infertile and have reduced testis size and abnormal morphology. Spermatogonial germ cells are still present but are unable to mature into spermatocytes and spermatids. Exogenous insulin treatment regenerates testes and restores fertility, but this plasma insulin cannot pass through the blood-testis barrier. We conclude that insulin does not rescue fertility through direct interaction with the testis; instead, it restores function of the hypothalamic-pituitary-gonadal axis and, thus, normalizes hormone levels of luteinizing hormone and testosterone. Although we show that the Sertoli cells of the testis secrete insulin protein, this insulin does not appear to be critical for fertility.
Type 1 diabetes is associated with human male reproductive dysfunction, including reports of reduced fertility and poor sperm quality (1–4). Mechanisms for this association are widely debated. Studies in rodent models suggest mechanisms including oxidative stress, DNA damage to sperm, altered hormonal profiles, and abnormal progression through spermatogenesis (5,6). More recently, investigations into human sperm samples from diabetic males show an increase in nuclear and mitochondrial damage (1), suggesting that hyperglycemia may cause oxidative stress and free radical damage to sperm DNA. Clinical data from in vitro fertilization clinics show that sperm from diabetic patients are able to fertilize oocytes at similar rates compared with sperm from nondiabetic patients. Pregnancy rates, however, are significantly lower when these embryos are transplanted, suggesting that a diabetic environment damages sperm cells, causing poor sperm quality (2). It is still unclear whether diabetes affects male fertility at the early stages of spermatogenesis or at the level of mature sperm cells. In light of new data that show insulin expression by cells in the testis (7), it is now unclear whether the effects of diabetes on fertility are mediated through testicular insulin insufficiency or through systemic effects of diabetes.
In this study, we use the Akita mouse model to study the effects of insulin deficiency on gonadal function. The Akita mouse is a model of type 1 diabetes resulting from a mutation in the ins2 gene. Unlike most other organisms, mice and rats have two functional insulin genes located on separate chromosomes. Ins1 arose from a duplication of the ins2 ancestral gene ∼20 million years ago and has since been retained in the mouse and rat genome (8). Murine ins2 is thus orthologous to the human insulin gene. Deletion of both genes results in pup lethality shortly after birth (9). The mutation of the ins2 gene results in a misfolded protein product, which accumulates in the endoplasmic reticulum (ER), causing ER stress and, ultimately, death of the insulin-producing β-cells of the pancreas (10). The diabetic Akita mouse is thus similar to humans with type 1 diabetes, which is caused by an autoimmune destruction of the β-cells of the pancreas (11). The Akita model also displays a severe onset of diabetes, similar to untreated diabetes in human adults, and lacks the drug-induced toxicity of a streptozocin-induced model.
The Akita mouse model provides a unique way to study insulin function in the reproductive tract, given the production of mutant nonfunctional insulin by both the pancreas and the testes. It is the goal of this study to determine the mechanism of infertility in the insulin-deficient Akita mouse and distinguish the requirement of testicular insulin from that of pancreatic insulin in the maintenance of spermatogenesis.
Although both heterozygous and homozygous mice develop hyperglycemia, Akita homozygotes develop severe hyperglycemia by age 3 weeks and, thus, are infertile at age 8–9 weeks, as opposed to Akita heterozygotes, which become infertile at approximately age 6 months. Previous studies on the Akita mice show that sperm from Akita mice fertilize fewer embryos, and those embryos that do fertilize are developmentally impaired at the blastocyst stage, indicating a paternal effect of diabetes on sperm quality (12). Although an association with type 1 diabetes and infertility exists, the significance of insulin and normoglycemia to the reproductive system has not been fully characterized.
More recent studies have begun to characterize the function of insulin and insulin receptor in testes and sperm. Murine testes and sperm have both been shown to produce transcripts of insulin (7,13), suggesting a significant function for insulin in the reproductive tract and an increased potential for diabetes to affect male fertility. While insulin expression in human testes has not yet been examined, human sperm have been shown to release insulin in a dose-dependent manner in response to glucose (13). Insulin receptor has also been detected in the midpiece of sperm cells, implicating an autocrine function for the secretion of insulin by sperm cells and demonstrating the importance of proper insulin signaling in the reproductive tract (14). In this study, we show that Akita mice homozygous for a mutation in the ins2 gene are completely infertile and have a reduced testis size and abnormal testis and sperm morphology. By administering subcutaneous insulin pellets, we are able to rescue Akita homozygous infertility with exogenous insulin pellets. We also see that plasma insulin cannot cross the blood-testis barrier, demonstrating that the exogenous insulin does not directly rescue fertility.
RESEARCH DESIGN AND METHODS
Akita mice.
All mouse studies were approved by the animal studies committee at Washington University School of Medicine and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. FVB.B6-Ins2Akita/MlnJ mice were obtained from The Jackson Laboratory. Ins2Akita is a dominant, spontaneous, point mutation that introduces a Cys-to-Tyr substitution at the seventh amino acid in the A chain of mature insulin (amino acid 96 in the preproinsulin II sequence) and results in a major conformational change in the insulin 2 molecule. The Ins2Akita spontaneous mutation on the C57BL/6 background (The Jackson Laboratory, Stock No. 003548) was backcrossed to FVB/NJ for nine generations. Speed congenic analysis confirmed the N6 generation was <0.2% C57BL/6J and the sex chromosomes were fixed at generations N6 and N7. In 2007, the Type 1 Diabetes Resource received this strain at N9 from Dr. Mary Loeken at the Joslin Diabetes Center. This mouse was mated to FVB/NJ for one generation prior to initiating sibling matings. For this study, heterozygous males and females were mated to obtain homozygous, heterozygous, and wild-type males.
Quantitative PCR of testes.
Total RNA was extracted from 9-week-old Akita whole testes using TRIzol following the manufacturer’s directions. RNA was DNase treated (Turbo DNAfree; Ambion). RNA (1 μg) was converted into cDNA using QIAGEN Quantitect kit and quantitative (q)RT-PCR was performed using intron-spanning primers. qRT-PCR was performed in triplicate using cDNA from reverse transcription of 100 ng RNA as starting material for ins1 and ins2 reactions and cDNA from 50 ng RNA as starting material for all other reactions. SYBR Green detection system was used for all qRT-PCR assays (Fast SYBR Green; Applied Biosystems). All primers were designed using Integrated DNA Technologies software and validated for efficiency. PCR products were cloned and validated for specificity. All qRT-PCR reactions were performed on an ABI cycler 7500 Fast system, and samples were normalized to actin. The ΔΔCt (cycle threshold) method was used to calculate fold changes (Table 1).
TABLE 1.
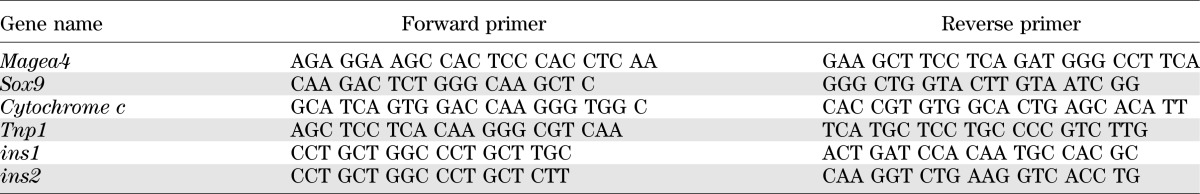
Primer sets for amplicons to determine cell types
Histopathology and immunohistology of testes.
Whole-mouse testes were fixed overnight in 4% paraformaldehyde, after which they were dehydrated in graded series of alcohol and embedded in paraffin. Paraffin sections were processed for immune-peroxidase staining as described (15), using goat anti-GATA4 (sc-1237; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a 1:200 dilution and donkey anti-goat biotinylated IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at a 1:1,000 dilution. The avidin-biotin immunoperoxidase system (VECTASTAIN Elite ABC Kit, Vector Laboratories, Inc., Burlingame, CA) and diaminobenzidine (Sigma-Aldrich, St. Louis, MO) were used to visualize the bound antibody; slides were counterstained with toluidine blue.
Electron microscopy of testes and sperm.
For immunolocalization at the ultrastructural level, samples were fixed in 4% paraformaldehyde/0.05% glutaraldehyde (Polysciences, Inc., Warrington, PA) in 100 mmol/L PIPES/0.5 mmol/L MgCl2, pH 7.2, for 1 h at 4°C. Samples were then embedded in 10% gelatin and infiltrated overnight with 2.3 mol/L sucrose/20% polyvinylpyrrolidone in PIPES/MgCl2 at 4°C. Samples were trimmed, frozen in liquid nitrogen, and sectioned with a Leica Ultracut UCT cryo-ultramicrotome (Leica Microsystems, Bannockburn, IL). Next, 50-nm sections were blocked with 5% FBS/5% normal goat serum for 30 min and subsequently incubated with rabbit anti-Sox9 (1:100) and guinea pig anti-insulin (1:200) followed by anti-rabbit 12-nm and anti–guinea pig 18-nm colloidal gold-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Sections were washed in PIPES buffer followed by a water rinse and stained with 0.3% uranyl acetate/2% methyl cellulose. Samples were viewed with a JEOL 1200EX transmission electron microscope (JEOL USA Inc., Peabody, MA). All labeling experiments were conducted in parallel with isotype primary antibody controls. These controls were consistently negative at the concentration of colloidal gold-conjugated secondary antibodies used in these studies.
Serum hormone enzyme-linked immunosorbent assays.
Blood serum samples were isolated from 8- to 12-week-old individually housed mice. Blood was collected into serum separator tubes (BD Microtainer 365967) and centrifuged at 6000g for 90 s and serum supernatant was frozen at −80°C. Serum hormone levels were assayed for follicle-stimulating hormone (FSH), luteinizing hormone (LH), or testosterone using rodent ELISA kits (ERK R7007, R7010, and R7016; Endocrine Technologies, Newark, CA). Results were read spectrophotometrically on a Molecular Devices VersaMax Microplate reader at a 450-nm wavelength.
Fluorescein isothiocyanate–insulin injection of testes.
Mice were anesthetized with a mixture of xylazine and ketamine (final concentration 86.9 mg/kg ketamine to 13.4 mg/kg xylazine), after which both testicles were exposed and fluorescein isothiocyanate (FITC) insulin (Invitrogen I-13269) was injected just under the tunica albuginea of one testis, whereas the other was injected with PBS. After 30 min, mice were killed and testes were removed and fixed overnight in 4% paraformaldehyde. Testes were then dehydrated in a graded series of alcohol and embedded in paraffin. Next, 5-μm sections were deparaffinized, rehydrated, permeabilized, and blocked in PBS/2% BSA/0.5% Triton X-100 for 1 h at room temperature. Slides were washed three times in PBS/2% BSA and then incubated for 20 min in TO-PRO-3 iodide nuclear dye (1:500; Molecular Probes) in PBS. Slides were washed three times in PBS and mounted with VECTASHIELD. Immunofluorescent images were recovered using a Nikon Eclipse E800 confocal microscope.
Transferase-mediated dUTP nick-end labeling detection in testes.
Testes were fixed in 4% paraformaldehyde overnight. Tissues were then embedded in paraffin and 5-μm sections were cut and dried onto slides. Slides were then deparaffinized in xylazine and rehydrated in a graded series of ethanol. Tissue sections were then permeabilized in 0.5% TritonX-100 in PBS for 20 min, rinsed twice in PBS, and incubated in transferase-mediated dUTP nick-end labeling (TUNEL) reaction mixture for 1 h at room temperature. Next, tissue sections were rinsed twice in PBS and counterstained with TO-PRO-3 Iodide (1:250) (Invitrogen). Confocal immunofluorescent microscopy of stained testes sections was performed with an Olympus laser-scanning microscope.
Computer-assisted sperm analysis.
Caudal epididymal sperm was released into HTF EmbryoMax media (Irvine Scientific, Santa Ana, CA) equilibrated overnight in 5% CO2 and 37°C. Sperm were allowed to disperse in a 5% CO2 and 37°C incubator for 15 min, and then sperm cells were loaded into a sperm analysis chamber and inserted into a computer-assisted sperm analysis (CASA) machine (Hamilton-Thorne, Beverly, MA). At least 1,000 cells were counted and sperm cells were evaluated for total motility.
Insulin treatment and unilateral orchiectomy.
Akita homozygous mice were treated with insulin implants (LinShin Canada). At age 9 weeks, Akita homozygous mice were anesthetized and one testis was removed for histological examination and confirmation of spermatogenic arrest. The other testis was left in the mouse for evaluation after insulin treatment. Then, 13-mg insulin pellets were inserted subcutaneously under the dorsal skin. Additional pellets were inserted until blood glucose achieved a level of <200 mg/dL. Mice were treated with insulin for 8 weeks, after which the remaining testis was removed and half was fixed in Bouin solution for histology while the other half was snap frozen in liquid nitrogen for RNA extraction.
Statistical analysis.
Statistical analysis and evaluation were performed using one-way ANOVA with Tukey post tests. Significance was defined as P < 0.05.
RESULTS
Akita mouse model.
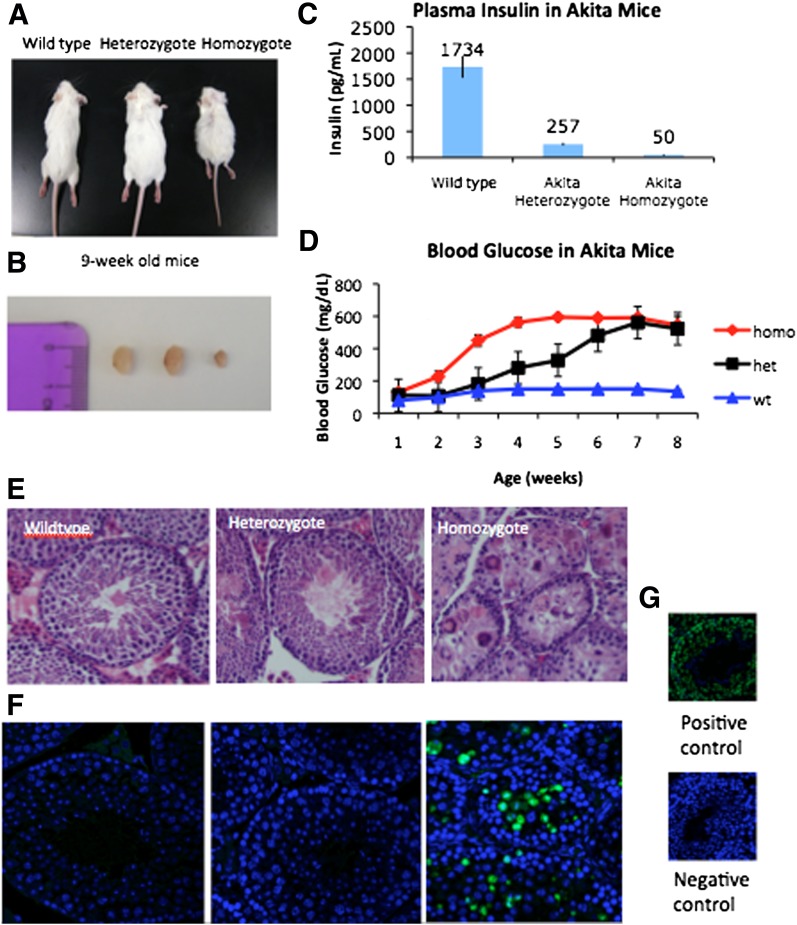
A genetic mutation in the ins2 gene of Akita mice results in a misfolded insulin protein product that is retained in the ER, causing ER stress and subsequent destruction in the insulin-producing β-cells of the pancreas (10). Akita homozygous mice, at age 9 weeks, are smaller than either wild-type or heterozygous Akita mice (Fig. 1A) and have significantly reduced testis size (Fig. 1B). Akita homozygotes develop low plasma insulin levels as well as elevated blood glucose (Fig. 1C and D). Heterozygous males are similar in weight to wild-type mice and develop blood glucose levels >300 mg/dL by age 5 weeks. Akita homozygous males develop blood glucose levels >300 mg/dL by age 3 weeks and have a life span of 8–12 weeks, unless treated with insulin. Although Akita homozygous mice display an accumulation of insulin in the ER within the testes (Supplementary Fig. 1), this tissue does not display an increase in ER stress. Testes were examined for the presence of ER stress by assaying for the presence of spliced XBP-1 as well as an increase in BiP protein levels. In response to ER stress, cells upregulate transcription of chaperone proteins such as BiP/GRP78 to assist in protein folding. XBP-1 mRNA is also spliced in response to the ER stress to produce a functional protein that acts as a transcription factor for many molecules involved in the unfolded protein response (16,17). Neither assay indicated that the testes of Akita mice had an increased level of ER stress (data not shown), suggesting that the testicular phenotype of the Akita homozygous mouse is caused by diabetes and not by ER stress.
FIG. 1.
Testicular atrophy in the male diabetic Akita mouse model. Nine-week-old wild-type, Akita heterozygous, and Akita homozygous mice (A) and corresponding testis decrease in size (B). C: Plasma insulin decreases in Akita heterozygotes and Akita homozygotes (9-week-old mice; n = 6). D: Blood glucose levels in Akita mice. homo, homozygous; het, heterozygous; wt, wild type. E: Hematoxylin-eosin stain of testes section. Akita homozygous testes (9-week-old) lack mature sperm cells and display vacuolization and large multinucleated cells. F: TUNEL analysis on Akita homozygous testes shows a greater degree of DNA damage in the seminiferous tubules. G: Negative and positive controls. (A high-quality digital representation of this figure is available in the online issue.)
Testicular atrophy of insulin-deficient Akita mice.
Akita male mice are completely infertile. After age 7 weeks, these mice show a reduction in testis size as well as a reduction in seminal vesicle weight (data not shown), suggesting a decrease in testosterone levels. Histological examination of the testes at age 8–9 weeks shows marked atrophy of the seminiferous tubules, characterized by a loss of germ cell populations and an increase in the presence of large, multinucleated cells and vacuolization (Fig. 1E). This phenotype is 100% penetrant and was observed in every Akita homozygote examined at approximately age 9 weeks. The Akita homozygous testes start to undergo atrophy at approximately age 7 weeks (Supplementary Fig. 2). To address the possibility that the testicular atrophy is simply a result of systemic illness of the Akita homozygous mice, a full necropsy was performed on wild-type, heterozygous, and homozygous Akita mice. An outside researcher examined tissue sections, and no severe defects in any organs were observed aside from the testes and seminal vesicles (Supplementary Fig. 3). Pancreatic islets were slightly reduced in size, and the kidneys appeared distended in both Akita heterozygotes and homozygotes.
TUNEL analysis reveals that homozygous Akita testes have an increased amount of DNA damage among the cells in the seminiferous tubules compared with both wild-type and heterozygote testes (Fig. 1D). Akita heterozygous mice also display infertility later in life and cease to sire pups after age 4–6 months.
Akita mice have impaired sperm motility and morphology.
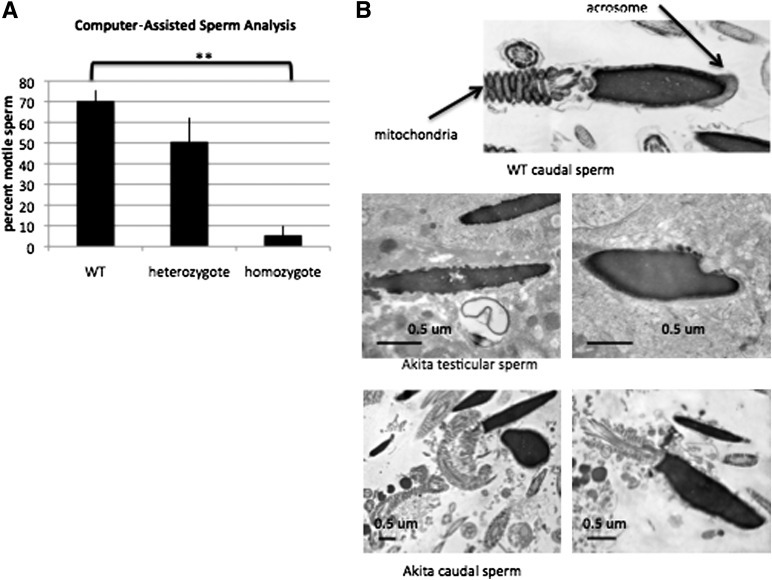
Akita homozygotes have significantly lower motile sperm as well as lower progressively and rapidly moving sperm (Fig. 2A). Akita homozygotes display fewer sperm cells in the epididymis, and sperm cells obtained from the cauda epididymis undergo degeneration. Electron micrograph images of caudal epididymal sperm display sperm lacking acrosomes, some cells with membrane blebbing, and numerous detached sperm heads as well as abnormal mitochondria (Fig. 2B).
FIG. 2.
Akita mice have impaired sperm motility and morphology. A: CASA of caudal sperm from wild-type (WT), Akita heterozygous, and Akita homozygous mice reveals that Akita homozygous sperm has a lower percentage of motile sperm. Akita heterozygous mice also display poorer CASA parameters (n = 3 experiments). **P < 0.01. B: Electron micrographs of wild-type and Akita homozygous sperm. Sperm cells obtained from either testicular sections or the cauda epididymis lack acrosomes and often display head detachment, membrane blebbing, and abnormal mitochondria. um, μm.
Insulin mRNA increases in Akita testes and sperm.
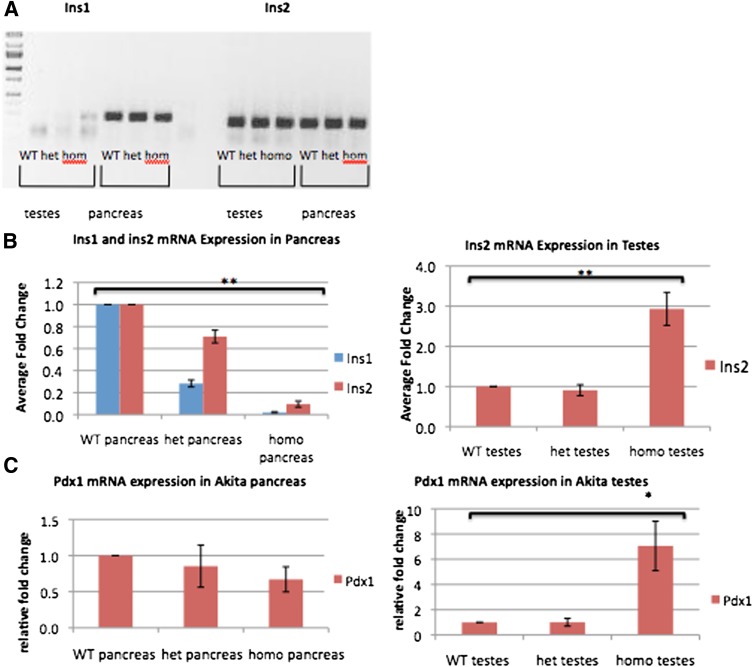
Nonquantitative RT-PCR from 9-week-old Akita mice shows that while ins1 and ins2 mRNA are both present in the pancreas, wild-type testes contain only detectable levels of ins2 and lack any detectable expression of ins1 (Fig. 3A). Insulin expression levels were decreased in Akita pancreata relative to wild-type pancreata, as detected by qRT-PCR. In contrast, Akita homozygous testes show increased insulin expression, suggesting either a compensatory mechanism or an enrichment of the cell types responsible for insulin production in the testes (Fig. 3B). In addition, Pdx-1, a known transcription factor for insulin in the pancreas, is also expressed in the testes, and mRNA expression is significantly increased in the Akita homozygous testes (Fig. 3C).
FIG. 3.
Insulin transcription increases in Akita testes and sperm. A: Differential expression of ins1 and ins2 by RT-PCR. Both transcripts are present in the pancreas, while only ins2 is present in the testes. B: qRT-PCR demonstrates expression of both insulin genes as decreases in the Akita pancreas and increases in Akita homozygous testes. C: mRNA for pdx-1, a transcription factor for both insulin genes, decreases expression in Akita pancreas and yet also increases in Akita homozygous testes, following the same trend as ins2 (9-week-old mice; n = 3). *P < 0.05, **P < 0.01. hom or homo, homozygous; het, heterozygous; WT, wild type. (A high-quality color representation of this figure is available in the online issue.)
Insulin localizes to the ER of Sertoli cell nuclei.
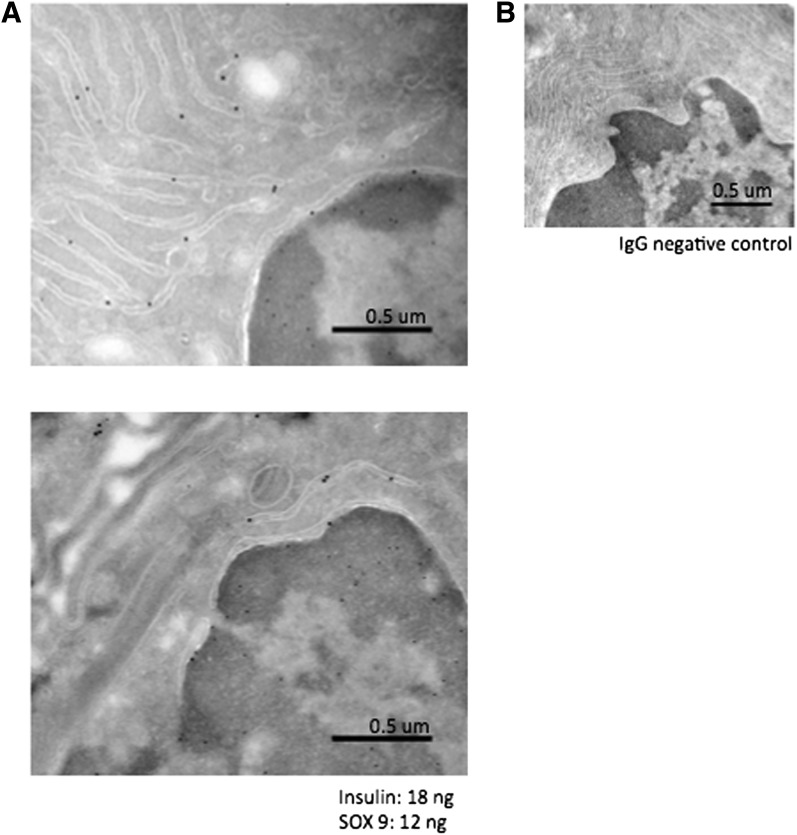
Electron microscopy images of Sertoli cell nuclei of Akita homozygous mice were obtained by immunogold labeling with SOX9, a prominent Sertoli cell nuclear marker (Fig. 4). Since Akita homozygotes accumulate misfolded ins2 protein in the ER, immunogold colabeling with insulin was done in these mice. Insulin particles localize predominantly to the ER surrounding the Sertoli cell nuclei that are labeled with SOX9, showing that Sertoli cells are capable of ins2 production in the testes.
FIG. 4.
Insulin localizes to the ER of Sertoli cell nuclei. A: Akita homozygous mouse testes were fixed for electron microscopy and immunogold labeled for insulin (18-ng large gold particle) and the Sertoli cell nuclear marker Sox9 (12-ng small gold particle). Insulin localizes to the ER surrounding the Sertoli cell nuclei, labeled with Sox9. B: IgG negative control antibody. Bars represent 0.5 μm. um, μm.
Insulin impermeability of the blood-testis barrier.
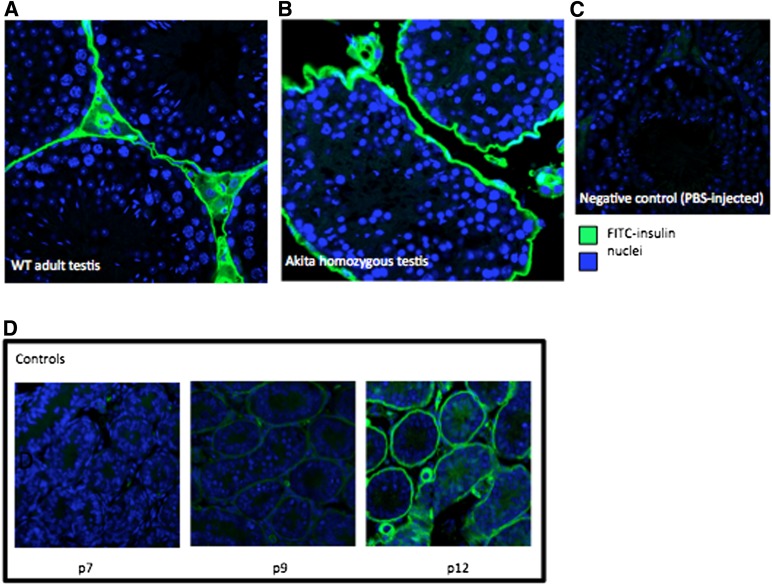
The blood-testis barrier is a dynamic barrier between the interstitium and lumen of the testes that restricts passage of many substances into the lumen of the seminiferous tubule. To determine whether plasma insulin is able to cross the barrier from the bloodstream into the seminiferous tubules, a biologically active FITC-labeled insulin was injected into the interstitium of the testes. The labeled insulin is completely retained in the interstitium of both wild-type and Akita homozygous testes, suggesting that the insulin produced by the testes is the only source in the lumen of the seminiferous tubules (Fig. 5A and B). PBS-injected testes were used as a negative control (Fig. 5C). As a positive control, neonatal male mice were injected with FITC insulin. Since the blood-testis barrier in mice forms at postnatal day (p)12, we injected p7, p9, and p12 testes with FITC insulin, and this experiment showed an increasing restriction to the interstitium as the barrier forms (Fig. 5D). We concluded that the blood-testis barrier was present even in the Akita homozygous mice and prevented systemic insulin from entering the seminiferous tubules.
FIG. 5.
Insulin is impermeable to the blood-testis barrier. A: FITC-labeled insulin injected into the interstitium of wild-type (WT) mice does not penetrate the blood-testis barrier (n = 3). B: The blood-testis barrier in Akita homozygotes also restricts passage of insulin across the barrier. C: PBS-injected control demonstrates no autofluorescence. D: Experimental controls: FITC-insulin is increasingly restricted to the interstitium as the blood-testis barrier forms in developing mice; interstitial staining is seen at p7 and p9. By p12, the barrier has formed and is intact, thus preventing passage of interstitially injected FITC-labeled insulin. (A high-quality digital representation of this figure is available in the online issue.)
Exogenous insulin treatment rescues testicular phenotype.
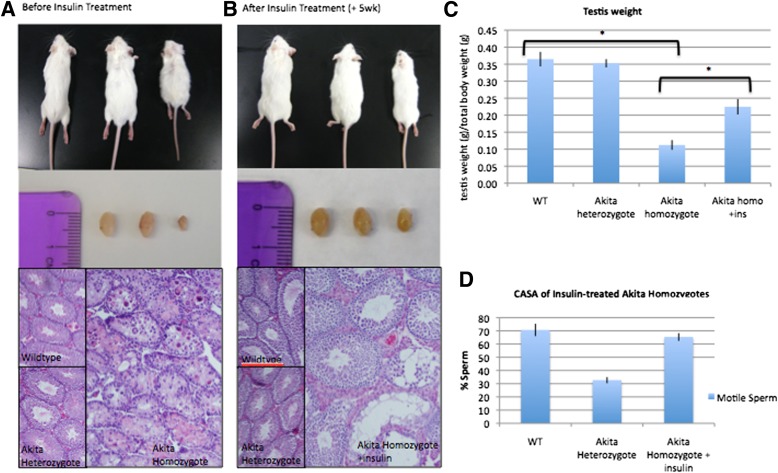
To determine whether exogenous insulin treatment could rescue Akita homozygote infertility, a unilateral orchiectomy was performed prior to insulin treatment. One testis and one epididymis were removed from an anesthetized animal, and the testis was evaluated for histology and qRT-PCR analysis, while cauda epididymal sperm was evaluated for motility. An insulin pellet was then implanted subcutaneously for 8 weeks, after which the remaining testis was evaluated for regeneration. Akita homozygous mice treated with subcutaneous insulin pellets for 8 weeks displayed a regeneration of the seminiferous tubules of the testes (Fig. 6A and B). Testis weight as well as whole-animal weight increase in response to insulin treatment (Fig. 6C; some data not shown). CASA revealed a restoration of motility of sperm from insulin-treated Akita homozygous mice compared with wild-type mice (Fig. 6D). All cell types of the testes were restored upon insulin treatment, detected by qPCR analysis (Fig. 7B). In addition, insulin-treated males were able to successfully sire normal size litters when mated to wild-type females (data not shown).
FIG. 6.
Exogenous insulin treatment rescues testicular phenotype after unilateral orchiectomy. A: Wild-type (WT) and Akita mice before surgery and removal of one testis and insulin pellet treatment (n = 7 experiments with at least three mice in each group). B: Mice after treatment with insulin for 8 weeks. wk, week. Note the regeneration of the seminiferous tubules as well as the presence of all germ cell populations (n = 3 experiments); histology and testes images represent the remaining testis from the same mouse after removal of the first testis and subsequent insulin treatment. C: Mouse testis weight increases after insulin treatment (pre–insulin treatment, n = 7 experiments; post–insulin treatment, n = 3 experiments; *P < 0.01). D: CASA parameters normalize after treatment (n = 3 experiments). (A high-quality digital representation of this figure is available in the online issue.)
FIG. 7.
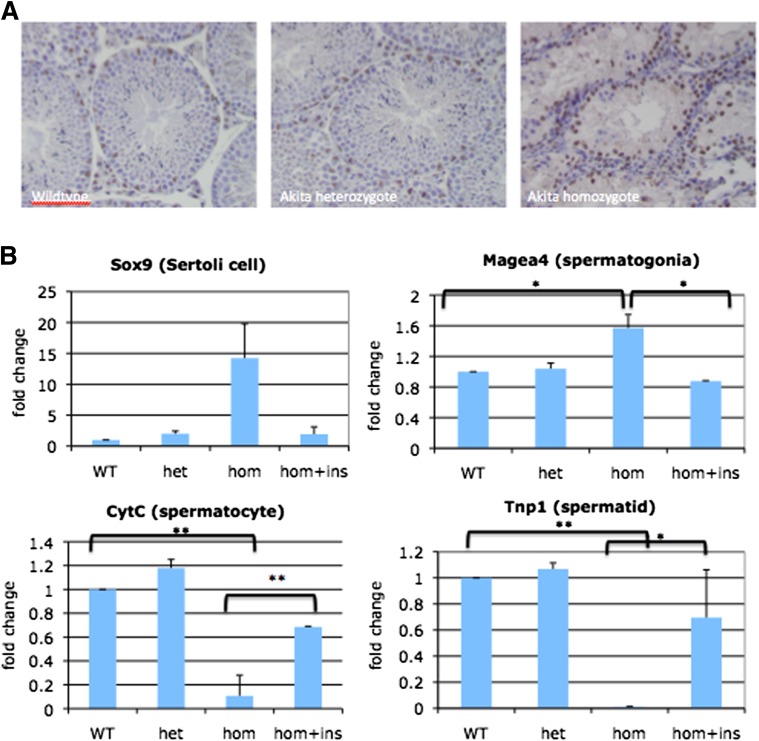
Testicular cell types affected by lack of functional insulin. A: Testes sections from 9-week-old wild-type, Akita heterozygote, and homozygote males. GATA4 staining of Akita homozygous testes reveals a retained population of Sertoli cells (n = 3). B: qRT-PCR analysis of cell types in control and Akita testes suggests increased Sertoli cell and spermatogonia cell populations in homozygous Akita testes, while both spermatocytes and spermatids are virtually absent compared with controls and heterozygotes. Insulin treatment reversed this trend and restored all cell populations in the testes to normal levels. n = 5 for wild-type (WT), Akita heterozygous (het), and Akita homozygous (hom) mice; n = 2 for Akita homozygote + insulin (hom + ins). *P < 0.05, **P < 0.01. (A high-quality digital representation of this figure is available in the online issue.)
Cell types affected by lack of functional insulin.
To evaluate which cell types in the testis are affected in the Akita mouse, a histological analysis as well as a qPCR approach was used. Histological analysis using GATA4, a Sertoli cell marker, shows that Akita homozygotes still retained a Sertoli cell population (Fig. 7A). qPCR analysis of whole testes shows that relative levels of a Sox9, a Sertoli cell marker, are increased in the Akita homozygotes compared with wild-type mice or Akita heterozygotes (Fig. 7B). In addition, Magea4 (germ cell marker) was increased in Akita homozygotes compared with controls or heterozygotes. Akita homozygous testes, however, display significantly absent populations of both CytC (spermatocyte marker) and Tnp1 (spermatid marker), suggesting that these mice may have either a meiotic block or a targeted apoptosis of postmeiotic germ cells (Fig. 7B). Upon insulin treatment, Sertoli cell, spermatogonia, spermatocyte, and spermatid populations were at least at partially rescued wild-type levels.
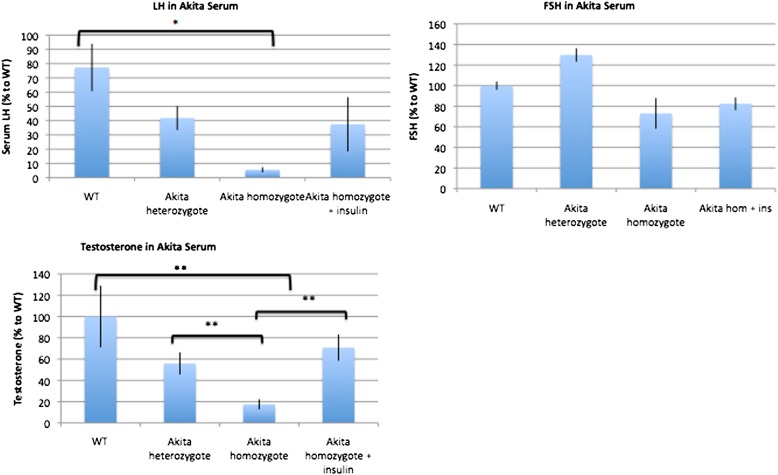
Serum testosterone and LH are decreased in Akita homozygous mice.
Serum levels of both testosterone and LH are severely decreased in Akita homozygous mice compared with wild-type mice, whereas serum levels of FSH are not significantly different (Fig. 8A–C). Exogenous insulin treatment partially rescues both testosterone and LH levels. Given the resumption of spermatogenesis in Akita homozygotes treated with exogenous insulin pellets and the impermeability of the blood-testis barrier to insulin, we conclude that the insulin-mediated rescue is due to normalized levels of LH and testosterone. The increased levels of LH in insulin-treated Akita homozygotes may promote testosterone synthesis in the Leydig cells of the testes, allowing spermatogenesis to resume.
FIG. 8.
Serum testosterone and LH are decreased in Akita mice. LH and testosterone are significantly decreased in Akita heterozygotes and homozygotes. Insulin pellet treatment reversed this trend (n = 4). *P < 0.05, **P < 0.01. WT, wild type; ins, insulin. (A high-quality color representation of this figure is available in the online issue.)
DISCUSSION
The recent discovery that insulin is produced in murine testes adds complexity to the relationship between diabetes and infertility. We sought to elucidate the role of insulin in spermatogenesis using the Akita mouse because it has a loss-of-function ins2 mutation, which leads to a misfolded insulin protein product ultimately causing pancreatic β-cell death and, thus, inhibiting the secretion of insulin from the pancreas. Considering that the murine testes solely produce ins2, as opposed to both ins1 and ins2 by the pancreas, the Akita testis should represent a complete knockout of insulin in the testis. We saw, however, that the application of exogenous insulin was able to rescue the fertility of Akita mice. Given the impermeability of the blood-testis barrier to insulin, we speculate that the fertility-related defects seen in these mice are a result of the systemic effects of diabetes and potentially a less severe and reversible effect of ins2 deficiency within the seminiferous tubules.
Insulin is known to influence the hypothalamic-pituitary axis (18), which can alter serum hormone levels important in spermatogenesis. Brain-specific knockout of the insulin receptor is known to impair spermatogenesis by decreasing circulating amounts of LH (19). LH interacts with the Leydig cells in the interstitium of the testes to promote testosterone production. Indeed, mice lacking the LH receptor display an arrest in spermatogenesis, which is rescued by the administration of exogenous testosterone (20). Our studies indicate that LH and testosterone are significantly decreased in Akita mice. We suspect that the lack of circulating insulin in the Akita mouse reduces LH secretion by the pituitary gland, affecting the ability of the Leydig cells of the testes to produce testosterone and leading to the observed arrest in spermatogenesis.
It is also known that insulin may be able to interact with receptors on the Leydig cells directly to mediate the process of steroidogenesis; thus, we cannot exclude the possibility that plasma insulin also directly interacts with Leydig cells in addition to the indirect signaling through the pituitary. Leydig cells contain insulin receptors (21), and the administration of insulin to Leydig primary cultures causes an increase in testosterone formation (22). This suggests that low plasma insulin levels can significantly decrease testosterone formation, which is known to limit the process of spermatogenesis (23). It is therefore not surprising that administering insulin via the bloodstream should rescue the spermatogenesis process in the Akita mouse model because Leydig cells reside outside the blood-testis barrier and, thus, have access to plasma proteins.
Our data demonstrate that a lack of systemic insulin causes infertility linked to defects in the hypothalamic-pituitary-gonadal axis, though the role of the insulin produced by the testes is still unclear. To determine the function of insulin produced by the Sertoli cells, future studies should be conducted using a complete knockout of ins1 and ins2 specifically in the testes. Given that the Sertoli cells also produce other hormones important in the regulation of spermatogenesis (24), it is reasonable to speculate that these cells might also produce insulin to maintain the process of spermatogenesis. Insulin may be important both as a growth factor/survival signal and as a mechanism for glucose homeostasis in the testes, but further study of Sertoli cell insulin function is necessary.
Since the Sertoli cells span the blood-testis barrier, it is conceivable that the insulin produced by these cells may be used by the developing spermatocytes and spermatids that lie beyond the barrier. In addition to indirectly affecting spermatogenesis through interactions with the hypothalamic-pituitary axis, the low levels of plasma insulin in the Akita may also affect the testes directly by interacting with insulin receptors on the Sertoli cell (25). The presence of insulin receptors on Sertoli cells suggests that these cells may be able to directly respond to circulating levels of insulin, considering that the basal compartment of the seminiferous tubule is in contact with the blood and lymph. There is evidence to suggest that insulin has a stimulatory effect on spermatogenesis. Treatment of seminiferous tubule segments in culture with insulin stimulates spermatogonial DNA synthesis in rats (26), and insulin treatment also promotes spermatogonial differentiation of primary spermatocytes in the newt (27). These experiments indicate that in addition to insulin’s effect on the hypothalamic-pituitary axis, insulin may have a direct role in the process of spermatogenesis.
The Akita mouse provides a new model for studying the effects of type 1 diabetes on fertility. The maintenance of proper sperm formation depends on a functional hypothalamic-pituitary-gonadal axis and, thus, depends on proper insulin regulation.
ACKNOWLEDGMENTS
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants R01-HD-040390-07 and 1-R01-HD-065435-01 and a research grant from the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
E.L.S. researched data and wrote the manuscript. G.A. and A.I.F. researched data. K.H.M. reviewed and edited the manuscript. K.H.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The electron microscopy was performed by Dr. Wandy Beatty in the Department of Molecular Microbiology at Washington University School of Medicine. The ins1 and ins2 primers for the PCR were a generous gift from Dr. James Maclean from Southern Illinois University Carbondale.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1527/-/DC1.
See accompanying commentry, p. 1667.
REFERENCES
- 1.Agbaje IM, Rogers DA, McVicar CM, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod 2007;22:1871–1877 [DOI] [PubMed] [Google Scholar]
- 2.Mulholland J, Mallidis C, Agbaje I, McClure N. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod Biomed Online 2011;22:215–219 [DOI] [PubMed] [Google Scholar]
- 3.Pacey AA. Environmental and lifestyle factors associated with sperm DNA damage. Hum Fertil (Camb) 2010;13:189–193 [DOI] [PubMed] [Google Scholar]
- 4.Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 2008;4:46–54 [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Casado L, Juncos-Tobarra MA, Cháfer-Rudilla M, de Onzoño LI, Blázquez-Cabrera JA, Miralles-García JM. Effect of experimental diabetes and STZ on male fertility capacity. Study in rats. J Androl 2010;31:584–592 [DOI] [PubMed] [Google Scholar]
- 6.Cameron DF, Rountree J, Schultz RE, Repetta D, Murray FT. Sustained hyperglycemia results in testicular dysfunction and reduced fertility potential in BBWOR diabetic rats. Am J Physiol 1990;259:E881–E889 [DOI] [PubMed] [Google Scholar]
- 7.Gómez O, Ballester B, Romero A, et al. Expression and regulation of insulin and the glucose transporter GLUT8 in the testes of diabetic rats. Horm Metab Res 2009;41:343–349 [DOI] [PubMed] [Google Scholar]
- 8.Shiao MS, Liao BY, Long M, Yu HT. Adaptive evolution of the insulin two-gene system in mouse. Genetics 2008;178:1683–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duvillié B, Cordonnier N, Deltour L, et al. Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci U S A 1997;94:5137–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 2002;109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther 2005;12:580–591 [DOI] [PubMed] [Google Scholar]
- 12.Kim ST, Moley KH. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction 2008;136:313–322 [DOI] [PubMed] [Google Scholar]
- 13.Aquila S, Gentile M, Middea E, Catalano S, Andò S. Autocrine regulation of insulin secretion in human ejaculated spermatozoa. Endocrinology 2005;146:552–557 [DOI] [PubMed] [Google Scholar]
- 14.Carpino A, Rago V, Guido C, Casaburi I, Aquila S. Insulin and IR-beta in pig spermatozoa: a role of the hormone in the acquisition of fertilizing ability. Int J Androl 2010;33:554–562 [DOI] [PubMed] [Google Scholar]
- 15.Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn 2007;236:203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 2003;23:7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Back SH, Lee K, Vink E, Kaufman RJ. Cytoplasmic IRE1alpha-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J Biol Chem 2006;281:18691–18706 [DOI] [PubMed] [Google Scholar]
- 18.Bucholtz DC, Chiesa A, Pappano WN, et al. Regulation of pulsatile luteinizing hormone secretion by insulin in the diabetic male lamb. Biol Reprod 2000;62:1248–1255 [DOI] [PubMed] [Google Scholar]
- 19.Brüning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 20.Pakarainen T, Zhang FP, Mäkelä S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology 2005;146:596–606 [DOI] [PubMed] [Google Scholar]
- 21.Kimura M, Lukinius A, Ericsson JL, Grimelius L. Distribution of insulin binding sites on Leydig cells of rat testes using insulin-coated gold particles. Histochemistry 1992;97:213–220 [DOI] [PubMed] [Google Scholar]
- 22.Lin T, Haskell J, Vinson N, Terracio L. Characterization of insulin and insulin-like growth factor I receptors of purified Leydig cells and their role in steroidogenesis in primary culture: a comparative study. Endocrinology 1986;119:1641–1647 [DOI] [PubMed] [Google Scholar]
- 23.Spaliviero JA, Jimenez M, Allan CM, Handelsman DJ. Luteinizing hormone receptor-mediated effects on initiation of spermatogenesis in gonadotropin-deficient (hpg) mice are replicated by testosterone. Biol Reprod 2004;70:32–38 [DOI] [PubMed] [Google Scholar]
- 24.Griswold MD. Protein secretions of Sertoli cells. Int Rev Cytol 1988;110:133–156 [DOI] [PubMed] [Google Scholar]
- 25.Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl 2004;25:706–719 [DOI] [PubMed] [Google Scholar]
- 26.Söder O, Bang P, Wahab A, Parvinen M. Insulin-like growth factors selectively stimulate spermatogonial, but not meiotic, deoxyribonucleic acid synthesis during rat spermatogenesis. Endocrinology 1992;131:2344–2350 [DOI] [PubMed] [Google Scholar]
- 27.Nakayama Y, Yamamoto T, Abé SI. IGF-I, IGF-II and insulin promote differentiation of spermatogonia to primary spermatocytes in organ culture of newt testes. Int J Dev Biol 1999;43:343–347 [PubMed] [Google Scholar]