Type 1 diabetes (T1D) is an organ-specific autoimmune disease characterized by immune-mediated destruction of the insulin-producing β-cells of the islets of Langerhans that results in life-long insulin dependence (1). Given the immunological nature of the disease, numerous antigen-specific as well as nonantigen-specific tolerance induction or immune deviation strategies have been developed as treatments for T1D. Although successful in experimental models, results of studies to translate these strategies to humans have been discouraging (2–5). This has prompted researchers to explore safer, broader, and more effective immunotherapeutic approaches to prevent, treat, and/or revert T1D. In this issue of Diabetes, Faleo et al. (6) show compelling data regarding use of hyperbaric oxygen therapy in autoimmune diabetes using nonobese diabetic (NOD) mice. This model spontaneously develops T1D, a feature that closely mimics human disease. Faleo et al. (6) show that hyperbaric oxygen therapy (HOT) significantly protects from T1D when initiated early in the disease course, but not after its onset, suggesting that this approach could be useful in high-risk individuals.
According to the Undersea and Hyperbaric Medical Society, HOT is a process wherein patients breathe 100% oxygen (atmospheric air contains ~21% oxygen) while inside a treatment chamber at a pressure higher than at sea level (usually 2.5 times greater pressure). In 1662, Henshaw, a British clergyman, first used pressurized atmospheric air to treat certain ailments. Since that time, HOT has been used to treat various conditions such as decompression sickness, arterial gas embolism, and carbon monoxide poisoning (with or without cyanide poisoning), and to facilitate wound healing (7). It should be noted that inhalation of 100% oxygen at 1 normal atmosphere of pressure or exposing isolated parts of the body to 100% oxygen does not constitute HOT.
Faleo et al. (6) exposed prediabetic NOD mice to pressurized 100% oxygen (HOT-100%), 21% oxygen (HOT-21%), or oxygen-depleted air supplemented with pure oxygen (HOT-12%) for 60 min every day for varying periods of time. T1D risk was assessed using different models. In the cyclophosphamide-accelerated model, more than 75% of control, HOT-12%, and HOT-21% mice developed hyperglycemia, compared with less than 50% of HOT-100% mice. The severity of insulitis was also reduced in the HOT-100% group. Even though HOT was not administered on the day of cyclophosphamide therapy, the possibility that HOT could have influenced the metabolism of cyclophosphamide (8), and thereby indirectly influenced the incidence of accelerated hyperglycemia, was not addressed by the authors.
In the spontaneous model, when HOT was initiated at 4 weeks of age, 85% of untreated control NOD mice developed hyperglycemia by 35 weeks compared with 65% of mice in the HOT-100% group. Although this finding was statistically significant, a considerable percentage—65%—of this inbred, homogenous mouse strain still developed hyperglycemia with HOT-100%, an observation that raises questions about the likely effectiveness of HOT among more heterogeneous human T1D populations. Data from a key experiment—the effect of HOT-100% on the incidence of hyperglycemia when administered close to onset of diabetes in NOD mice (13 weeks of age)—were not reported. In this group, HOT-100% attenuated insulitis and significantly delayed the onset of hyperglycemia when administered along with glucagon-like peptide (GLP)-1 analog and exenatide (EXN) delivered by mini-osmotic pumps. Inclusion of a group receiving HOT-100% and vehicle or a scrambled peptide, instead of EXN, would have strengthened the inference that HOT-100% is more effective when combined with EXN. Overall, the findings in this report suggest that only prolonged exposure to HOT-100% that is initiated at a very young age reduced T1D risk in this mouse model.
The mechanisms by which HOT provided its beneficial effects in T1D or even in other models of inflammation are not fully understood. HOT can suppress inflammation by modulating the expression of integrins (9) and probably other adhesion molecules. This would interfere with homing of inflammatory cells to islets, thereby reducing insulitis. In support of this hypothesis, HOT-100%–treated mice had reduced insulitis. Furthermore, expression of CD62L, a lymphocyte homing marker, was altered in the spontaneous, but not cyclophosphamide-accelerated, T1D model.
HOT could modulate inflammation through hypoxia-inducible factor (HIF)-1 (10). Hypoxia increases HIF expression, whereas hyperoxia represses HIF. HIF-1 can induce interleukin (IL)-17 and inhibit Foxp3+ regulatory T-cell (Treg) development (11). Therefore, by reducing the expression of HIF-1 (12,13), HOT could inhibit the Th17 pathway and promote Treg development, thereby protecting from T1D. Although HIF was not investigated in this report, increased Treg in HOT-100% mice supports this theory. However, it remains to be elucidated whether HOT-100% mediates its effects directly through increasing Treg numbers and functions and/or through suppressing IL-17. HIF is also induced rapidly in transplanted islets and promotes islet apoptosis (14,15). Therefore, repression of HIF by HOT may promote β-cell survival and play a beneficial role in diabetes. Reduced apoptosis and increased proliferation of β-cells in HOT-100% mice support this theory. Paradoxically, HOT can also increase HIF expression (16), and HIF can have anti-inflammatory activities in T1D (17). Therefore, the interplay between HOT and HIF in the T1D settings remains to be determined and is a topic ripe for further investigation.
HOT is also known to increase levels of reactive oxygen species (ROS) as well as reactive nitrogen species (RNS) (18). Both ROS and RNS participate in innumerable physiological and pathological processes (19). Of relevance, ROS are known to damage β-cells and participate in inflammatory processes (20), which could be counterproductive in T1D patients receiving HOT. Therefore, the roles of ROS and RNS in HOT need to be thoroughly investigated. Furthermore, this study showed that HOT-100% suppressed the responses of T cells to a potent mitogenic stimulation (anti-CD3) and elevated the levels of IL-10. Induction of such generalized immune suppression by HOT could be a potential drawback in humans, particularly in children, because this might predispose to infections, diminish the ability to clear infections, and mount adequate protective immunity after immunizations.
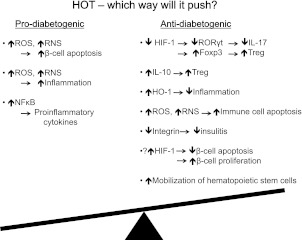
In conclusion, this descriptive work by Faleo et al. (6) addresses the potential benefits of HOT in T1D. Molecular oxygen is a fundamental component of various biochemical processes, and its use as a therapeutic agent could have diverse effects (21), both positive and negative. A detailed investigation is warranted to understand its mechanisms of actions as well as possible side effects (Fig. 1). In T1D, HOT is ineffective once the autoimmunity has progressed to a prediabetic stage, making its utility in patients with existing T1D questionable. When started early in the disease course, HOT reduced the incidence of T1D only by ~20% in NOD mice. However, in humans, this could be a significant number. Therefore, given the established safety and limited side effects of HOT in humans, its translation to a pilot human clinical trial, either alone or with other immunomodulatory agents, at least in high-risk individuals (those with a first-degree relative with T1D or who express susceptible HLA alleles, etc.), is a possibility.
FIG. 1.
Potential role of HOT in T1D. In individuals at high risk of developing T1D or in patients with T1D, the balance is tilted toward the prodiabetogenic process. HOT could push the balance either toward the prodiabetogenic pathway or toward the antidiabetogenic pathway. The outcome may be determined by multiple factors. NFκB, nuclear factor κB; RORγT, retinoid-related orphan receptor γ T; HO-1, hemeoxygenase 1.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1769.
REFERENCES
- 1.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev 2010;9:A355–A365 [DOI] [PubMed] [Google Scholar]
- 2.Waldron-Lynch F, Herold KC. Immunomodulatory therapy to preserve pancreatic β-cell function in type 1 diabetes. Nat Rev Drug Discov 2011;10:439–452 [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med 2012;366:433–442 [DOI] [PubMed] [Google Scholar]
- 4.Orban T, Bundy B, Becker DJ, et al. Type 1 Diabetes TrialNet Abatacept Study Group Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi GY, Murad MH, Flynn DN, et al. Immunotherapeutic agents in type 1 diabetes: a systematic review and meta-analysis of randomized trials. Clin Endocrinol (Oxf) 2008;69:244–252 [DOI] [PubMed] [Google Scholar]
- 6.Faleo G, Fotino C, Bocca N, et al. Prevention of autoimmune diabetes and induction of β-cell proliferation in NOD mice and hyperbaric oxygen therapy. Diabetes 2012;61:1769–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med 1996;334:1642–1648 [DOI] [PubMed] [Google Scholar]
- 8.Rump AFE, Siekmann U, Kalff G. Effects of hyperbaric and hyperoxic conditions on the disposition of drugs: theoretical considerations and a review of the literature. Gen Pharmacol 1999;32:127–133 [DOI] [PubMed] [Google Scholar]
- 9.Labrouche S, Javorschi S, Leroy D, Gbikpi-Benissan G, Freyburger G. Influence of hyperbaric oxygen on leukocyte functions and haemostasis in normal volunteer divers. Thromb Res 1999;96:309–315 [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 2012;148:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang EV, Barbi J, Yang H-Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011;146:772–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvert JW, Cahill J, Yamaguchi-Okada M, Zhang JH. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1alpha and its downstream target genes. J Appl Physiol 2006;101:853–865 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Zhou C, Calvert JW, Colohan ART, Zhang JH. Multiple effects of hyperbaric oxygen on the expression of HIF-1 alpha and apoptotic genes in a global ischemia-hypotension rat model. Exp Neurol 2005;191:198–210 [DOI] [PubMed] [Google Scholar]
- 14.Miao G, Ostrowski RP, Mace J, et al. Dynamic production of hypoxia-inducible factor-1α in early transplanted islets. Am J Transplant 2006;6:2636–2643 [DOI] [PubMed] [Google Scholar]
- 15.Sakata N, Chan NK, Ostrowski RP, et al. Hyperbaric oxygen therapy improves early posttransplant islet function. Pediatr Diabetes 2010;11:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu G-J, Li Y-P, Peng Z-Y, et al. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1alpha and erythropoietin in rats. J Appl Physiol 2008;104:1185–1191 [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan S, Bolick DT, Lukashev D, et al. Sphingosine-1-phosphate reduces CD4+ T-cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes 2008;57:484–493 [DOI] [PubMed] [Google Scholar]
- 18.Thom SR: Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg 127:131S-141S DOI: 10.1097/PRS.1090b1013e3181fbe1092bf, 2011 10.1097/PRS.1090b1013e3181fbe1092bf [DOI] [PMC free article] [PubMed]
- 19.Pourova J, Kottova M, Voprsalova M, Pour M. Reactive oxygen and nitrogen species in normal physiological processes. Acta Physiol (Oxf) 2010;198:15–35 [DOI] [PubMed] [Google Scholar]
- 20.Thayer TC, Delano M, Liu C, et al. Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes. Diabetes 2011;60:2144–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain K. Physical, physiological, and biochemical aspects of hyperbaric oxygenation. In Textbook of Hyperbaric Medicine 5th ed. Jain K, Ed. Cambridge, MA, Hogrefe Publishing, 2009 [Google Scholar]