Abstract
Type 1 diabetes results from the destruction of β-cells by an autoimmune T-cell response assisted by antigen-presenting B cells producing autoantibodies. CD8+ T-cell responses against islet cell antigens, thought to play a central role in diabetes pathogenesis, can be monitored using enzyme-linked immunosorbent spot (ELISpot) assays. However, such assays have been applied to monitoring of adult patients only, leaving aside the large and increasing pediatric patient population. The objective of this study was twofold: 1) to develop a CD8+ T-cell interferon-γ ELISpot assay for pediatric patients and 2) to determine whether zinc transporter 8 (ZnT8), a recently described target of autoantibodies in a majority of patients, is also recognized by autoreactive CD8+ T cells. Using DNA immunization of humanized mice, we identified nine HLA-A2–restricted ZnT8 epitopes. Among 36 HLA-A2+ children with diabetes, 29 responded to ZnT8 epitopes, whereas only 3 of 16 HLA-A2+ control patients and 0 of 17 HLA-A2− control patients responded. Some single ZnT8 epitopes performed as well as the group of epitopes in discriminating between patients and control individuals. Thus, ZnT8 is a major CD8+ T-cell autoantigen, and ELISpot assays display similar performance in adult and pediatric type 1 diabetes.
Type 1 diabetes results from the destruction of pancreatic β-cells by components of the immune system. It is well established that the adaptive cellular immune response plays a decisive role in islet inflammation and destruction (1). In humans, CD8+ T cells represent a major component of the islet infiltrate (2). At least a portion of islet-infiltrating CD8+ T cells recently have been shown to recognize autoantigenic epitopes in situ (3). Moreover, HLA-A2–restricted autoreactive CD8+ T cells raised in HLA-humanized nonobese diabetic (NOD) mice can kill murine as well as human islets (4,5), providing evidence for a critical role of CD8+ T cells in β-cell destruction. Identifying the antigens recognized by such T cells, therefore, is important not only to understand the pathogenesis of type 1 diabetes but also to identify surrogate markers of the autoimmune response and potential targets for antigen-specific immunointervention (6).
As a result of the efforts of several groups, including ours, a substantial number of—in most cases, HLA-A2–restricted—CD8+ T-cell epitopes derived from islet cell antigens have been identified (reviewed in Babad et al.) (7). HLA-humanized mice have been particularly useful in identifying such epitopes both by active immunization with autoantigens and by screening for spontaneously arising T cells (8). The array of HLA-A2–restricted epitopes available includes peptides from proinsulin, 65 kDa GAD, insulinoma-associated protein 2 (IA-2), and islet-specific glucose-6-phosphatase catalytic subunit (IGRP), which are targeted by spontaneously arising autoreactive and potentially autoreactive (9) CD8+ T cells in both mice and humans. Using epitopes derived from these autoantigens, we have previously established a sensitive enzyme-linked immunosorbent spot (ELISpot) assay measuring interferon (IFN)-γ secretion by peripheral blood lymphocytes (PBLs), which discriminates adult type 1 diabetic patients from control subjects with high accuracy (86% sensitivity, 91% specificity) (10). Assays measuring IFN-γ responses of PBLs to islet cell antigens may be of interest as surrogate markers of islet inflammation and destruction, since both the specificity and the T-cell repertoire use of autoreactive CD8+ T cells in peripheral blood and islet infiltrates have been shown to be highly similar (11,12). However, it is striking that T-cell responses to islet cell antigens have so far been measured exclusively in adult patients. Type 1 diabetes is a mainly pediatric disease with a rapidly increasing incidence among young children (13). Pediatric and adult diabetes differ with respect to not only the acuteness and severity of the clinical presentation but also the frequency of disease-associated HLA alleles and autoantibodies (14,15). As a consequence, autoreactive CD8+ T-cell responses may vary between adult and pediatric patients, which might hamper their potential as diagnostic tools and guideposts for therapeutic intervention.
The most recent major target of autoantibodies in human type 1 diabetes is zinc transporter 8 (ZnT8), which is required for correct insulin processing and β-cell function (16). ZnT8 was identified as a target of autoimmunity through screening of gene products highly expressed in β-cells with sera from type 1 diabetic patients (17). Antibodies to ZnT8 are detected in ∼60% of patients at onset, including 25% of patients testing negative for antibodies to GAD, IA-2, and insulin (17,18). ZnT8 has been shown to be a target of autoreactive CD4+ T cells in pediatric and adult patients (19); however, it is unclear whether this important β-cell antigen also triggers CD8+ T-cell responses. In pediatric diabetes, ZnT8 is of particular interest because antibodies to this autoantigen have been shown to be of high prognostic value with respect to the risk of disease (20).
This study was undertaken with the double objective to identify ZnT8 epitopes recognized by autoreactive CD8+ T cells and to examine self-reactive CD8+ T-cell responses in pediatric diabetes. We report that ZnT8 is a major CD8+ T-cell autoantigen recognized by lymphocytes from both pediatric and adult patients and that IFN-γ ELISpot detection of CD8+ T-cell responses to individual ZnT8 epitopes may have an accuracy approaching that of the other major T-cell antigens combined.
RESEARCH DESIGN AND METHODS
ZnT8 cloning and plasmid construction.
A full-length ZnT8 coding sequence was amplified from human pancreas cDNA (Ambion/Life Technologies, Saint-Aubin, France) using Phusion polymerase (Fisher Scientific, Illkirch, France) and the primers (forward) 5′…CCGCG GAGTT GAGTT TCTTG AAAGA ACGTA TCTTG and (reverse) 5′…TCTAG ATCAC TAGTC ACAGG GGTCT TCACA G. The PCR product was cloned in pCR-Blunt (Life Technologies), sequenced to confirm absence of errors, and subcloned into the published vector pVL1392ΔSacII-P3UO (21) using SacII and BamHI restriction sites introduced by the PCR primers. In the resulting construct (pVL1392ΔSacII-P3UhZnT8), the ZnT8 coding sequence replaced that of ovalbumin placed downstream of the triple protein G domain and ubiquitin. The entire cassette (P3UhZnT8) encoding a protein-ubiquitin-ZnT8 fusion protein was transferred into the eukaryotic expression vector pCI (provided by R. Schirmbeck, University of Ulm), in which the NheI and XhoI restriction sites were replaced by new NcoI and NheI sites. The P3UhZnT8 expression cassette was released from pVL1392ΔSacII-P3UhZnT8 using NheI and SpeI and subcloned into modified pCI, resulting in plasmid pCIATG-P3UhZnT8.
Mouse immunizations.
Female or male HHD mice aged 15 or 24 weeks, which express a single chain HLA-A2 molecule in the absence of murine classical major histocompatibility complex class I molecules (22) (provided by F. Lemonnier, Institut Pasteur, Paris), were injected in the tibialis anterior muscle with 50 μL of 10 μmol/L cardiotoxin (Latoxan, Valence, France) in PBS. Five days later, the mice were immunized intramuscularly with plasmid DNA (50 μg in 50 μL PBS per leg, tibialis anterior). From 2 to 4 weeks later, the mice were boosted, using the same sequence of injections. Six days later, the mice were killed and the spleen removed for ELISpot analysis. Animal experimentation was approved by the Comité Régional d’éthique pour l’expérimentation animale (P2.LS.012.06).
IFN-γ ELISpot analysis of murine splenocytes.
Ninety-six–well polyvinylidene difluoride plates (Millipore, Molsheim, France) were permeabilized for 1 min using 35% ethanol, washed five times with PBS, and coated overnight at 4°C with an anti–IFN-γ antibody (U-CyTech, Utrecht, the Netherlands) following the manufacturer’s instructions. Then the plates were blocked for 1 h at 37°C with RPMI-1640 medium containing 10% FCS. Spleens of immunized mice were processed to a single cell solution, suspended in RPMI-1640 containing 10% FCS and 1 unit/mL interleukin-2 (R&D Systems, Lille, France), and distributed together with individual ZnT8 peptides at 7 μmol/L (see Supplementary Table 1) or 1 μg/mL anti-CD3 antibody (BD Pharmingen, Le-Pont-de-Claix, France) or DMSO only at 0.25 × 106 per well in triplicates to ELISpot plates. Peptides were purchased from Proteogenix (Oberhausbergen, France) and were ≥70% pure. After a 40-h incubation at 37°C, plates were washed five times with PBS; biotinylated IFN-γ–detection antibody (U-CyTech) in PBS with 10% FCS was added and incubated for 1 h at 37°C. After extensive washing of both sides of the plate, ExtrAvidin coupled to alkaline phosphatase (Sigma-Aldrich, Saint-Quentin Fallavier, France) and diluted in PBS containing 0.5% FCS was added for a 1-h incubation at 37°C. After another series of washings, 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Sigma-Aldrich) was added to visualize spots. The reaction was stopped 10 min later by washing with water, and plates were air dried for 1 h before spot counting using an ELISpot reader (Autoimmun Diagnostika, Strassberg, Germany).
Patients and control individuals.
All pediatric patients were seen as outpatients or hospitalized in the Endocrinology Department at Necker-Enfants Malades Hospital (Paris) and in the Pediatric Departments at Ambroise Paré (Boulogne-Billancourt), Antoine Béclère (Clamart), and Centre Hospitalier Intercommunal (Créteil) hospitals. Pediatric control individuals were also recruited in the Endocrinology Department at Necker Hospital and treated for various pathologies unrelated to diabetes (see Supplementary Table 2 for details). Adult type 1 diabetic patients were seen in the Diabetes Clinic of the Pitié-Salpetrière Hospital. Informed consent was given by all patients and control subjects and/or their parents. Ethics approval for ELISpot testing of lymphocytes obtained from pediatric and adult type 1 diabetic patients and healthy adult individuals was obtained from the Comité d’Évaluation éthique of INSERM (IRB0000388, FWA00005381; approval of 2 June 2005 extended January 9, 2007), and ethics approval for testing of lymphocytes from pediatric control patients was obtained from the Comité deProtection des Personnes “Ile de France II” (Protocol JJR-2009–01).
Human lymphocyte purification, HLA-A2 typing, and autoantibody determination.
Blood samples were obtained in lithium-heparin tubes stored at room temperature until processing and usually processed within 24 h. Mononuclear cells were isolated from blood using lymphocyte separation medium (PAA, Les Mureaux, France). An aliquot was removed, stained using Alexa Fluor 488–conjugated monoclonal antibody BB7.2 (hybridoma obtained from American Type Culture Collection, Manassas, VA), and analyzed on a FACSCalibur machine. The remaining lymphocytes were frozen in human AB serum containing 10% DMSO and stored in liquid nitrogen until use. Autoantibodies to GAD, IA-2, and ZnT8 were determined using a radioimmunoassay with in vitro–translated 35S-labeled autoantigens, and antibodies to insulin were measured using a competition radioimmunoassay with tritiated insulin. In the Diabetes Autoantibody Standardization Program (DASP) 2009, the performance of our laboratory was 88 and 68% sensitivity and 96 and 99% specificity for GAD and IA-2 antibodies, respectively. Islet cell antibodies were determined by standard methods (23).
IFN-γ ELISpot analysis of human lymphocytes.
Ninety-six well polyvinylidene difluoride plates were prepared as described above, using an anti-human IFN-γ antibody (U-CyTech) and human serum instead of FCS. Lymphocytes were thawed, resuspended in serum-free AIM V medium (Life Technologies) containing 0.5 ng/mL interleukin-7 (R&D Systems), and added at 0.3 × 106 per well in triplicates to coated plates together with 10 μmol/L ZnT8 peptides or anti-CD3 antibody (BD Pharmingen) or DMSO only. We used anti-CD3 antibodies as positive control since some young children will not be immunized against viral peptides used in ELISpot assays with lymphocytes from adult donors. Peptides were obtained from Proteogenix and were ≥90% pure. After culture for 20–24 h at 37°C, the cells were removed by extensive washing, and a secondary anti-human IFN-γ antibody (U-CyTech) was added. Subsequent processing of the plates was identical to the mouse ELISpot protocol described above. All data shown are means of triplicate wells and expressed as spot-forming cells per 106 lymphocytes. Anti-CD3 stimulation resulted in discrete countable spots.
Statistical analysis.
The statistical significance of human ELISpot analyses of human lymphocytes was analyzed by Fisher exact test using GraphPad Prism software.
RESULTS
Identification of CD8+ T-cell epitopes derived from ZnT8.
To identify ZnT8-derived T-cell epitopes, we decided to use DNA immunization of HLA-humanized nonautoimmune mice, which has previously been used successfully by us to identify CD8+ T-cell epitopes derived from type 1 diabetes autoantigens (24). To enhance the efficacy of immunization, we used a strategy pioneered by the Steinman group (25). These researchers showed that immunization with plasmids encoding antigens targeted to professional antigen-presenting cells results in superior cellular immune responses. We used a targeting strategy developed by our laboratory in which antigens are expressed as fusion proteins that can be targeted to a variety of receptors, including Fc receptors (21). Figure 1 shows the components of the fusion proteins. The coding sequence of the antigen chosen is preceded by a signal peptide, three protein G domains with high binding affinity for immunoglobulins, and ubiquitin, which enhances degradation of the fusion proteins by the proteasome. Transfection of muscle cells with a plasmid containing such an expression cassette will result in synthesis and secretion of a fusion protein that will form complexes with circulating or locally secreted antibodies, which in turn will be taken up by infiltrating professional antigen-presenting cells, including dendritic cells.
FIG. 1.
Fusion protein used as DNA vaccine. Linkers are represented as lines and all other components as boxes. All components are drawn to scale. Sg, signal peptide; PrG, protein G immunoglobulin-binding domain; L, linker; Ubi, ubiquitin.
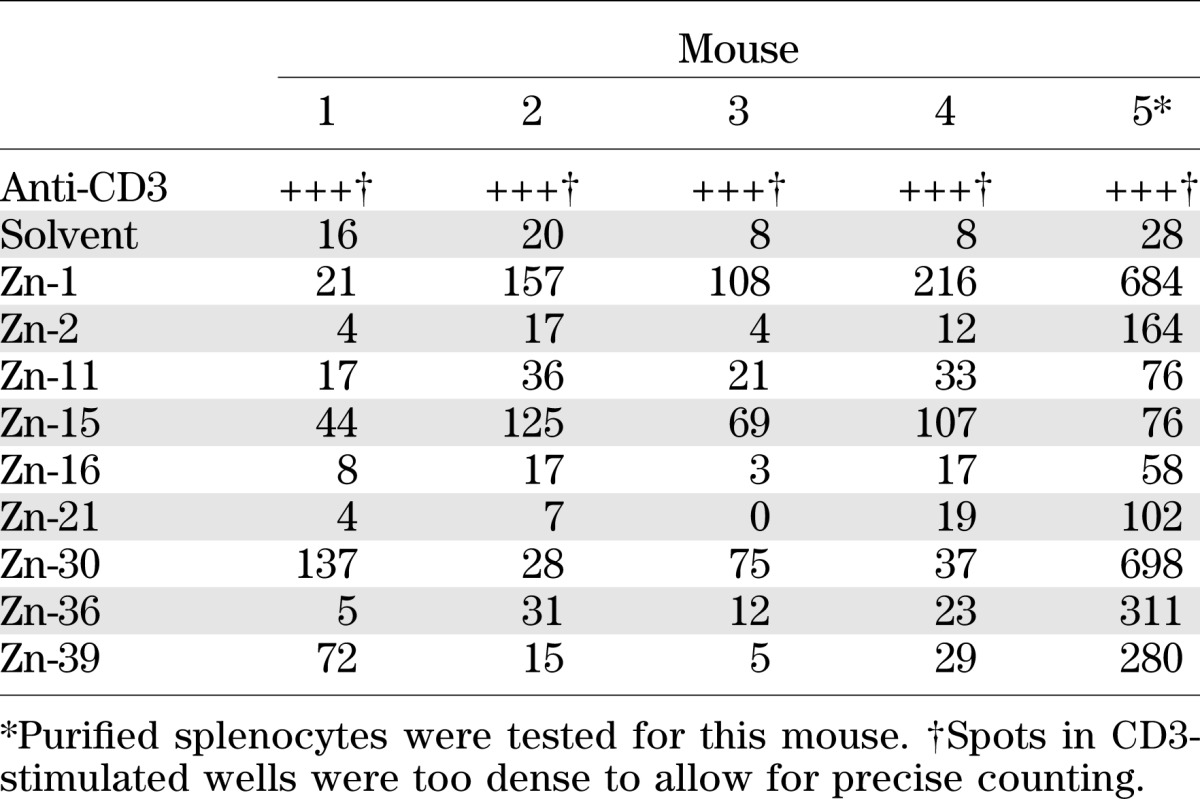
We immunized five HLA-A2–humanized mice with a plasmid encoding a ZnT8 fusion protein and tested hyperimmunized splenocytes for recognition of 32 nonamer and decamer peptides derived from the ZnT8 sequence that were predicted to be presented by HLA-A2 according to the SYFPEITHI algorithm (26) (see peptide list in Supplementary Table 1). Of the 32 peptides, 9 elicited significant IFN-γ secretion by splenic T cells in at least two out of five mice and were retained for further analysis (Table 1). Among these, 4 elicited responses in all animals, 3 in two animals, and 1 each in three or four animals. Most responses were strong (higher than mean of background + 5 SD). It is interesting that there was no bias for “nonself” peptides with sequences differing between human and murine ZnT8 among the immunogenic epitopes: 3 of 9 (33%) immunogenic sequences were identical between the two species vs. 8 of 32 (25%) for the complete group of 32 peptides tested, 4 of 9 (44%) vs. 14 of 32 (44%) contained a single conservative substitution, 1 of 9 (11%) vs. 2 of 32 (6%) contained multiple conservative substitutions, and 1 of 9 (11%) vs. 8 of 32 (25%) contained one or several nonconservative substitutions (Supplementary Table 1).
TABLE 1.
ELISpot responses of DNA-immunized HHD mice against ZnT8 peptides
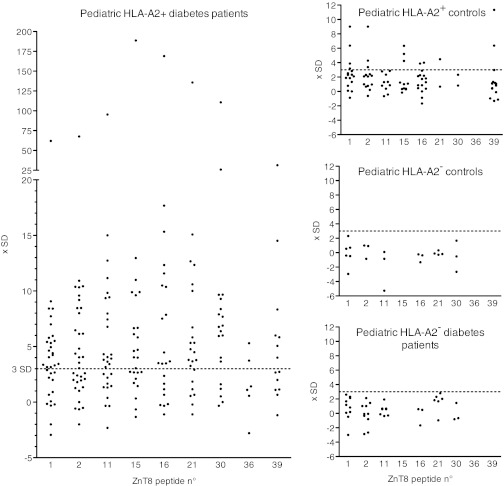
ELISpot responses to ZnT8 peptides in HLA-A2+ children with type 1 diabetes.
To determine whether the ZnT8 peptides immunogenic in HHD mice were also recognized by CD8+ T cells arising spontaneously in pediatric human type 1 diabetes, we tested lymphocytes from HLA-A2+ pediatric patients using our previously established optimized ELISpot assay (27). All ELISpot assays were performed on thawed lymphocytes. Details on all HLA-A2+ patients are shown in Supplementary Table 2. The cohort of A2+ pediatric patients comprised 36 children aged 2 to 15 years (median age 8 years), including 20 (56%) female patients. One patient (2.8%) was negative for all autoantibodies tested, while 42.9% tested positive for ZnT8 antibodies, 72.3% for GAD antibodies, 58.3% for IA-2 antibodies, 30% for insulin antibodies, and 88.9% for islet cell antibodies. With the exception of 1 patient tested 8 months after diagnosis, blood samples were obtained from all patients between the day of diagnosis and day 11 after diagnosis (median day 3).
ELISpot assays were also performed on samples from three pediatric control groups (details in Supplementary Tables 2 and 3). For ethical reasons, only children presenting with a variety of pathologies (rather than healthy children) could be recruited as control subjects. Because of the limited blood volume that could be obtained from the children, lymphocytes from most children could be tested only for a subset of the nine ZnT8 epitopes. The control patients comprised 16 HLA-A2+ (aged 5 to 18, median 12 years) and 7 HLA-A2− children (aged 5 to 17, median 12 years) presenting a variety of diabetes-unrelated pathologies and testing negative for antibodies to the four autoantigens mentioned above. A third group comprised 10 HLA-A2− autoantibody+ children aged 4 to 15 (median 10 years) with type 1 diabetes.
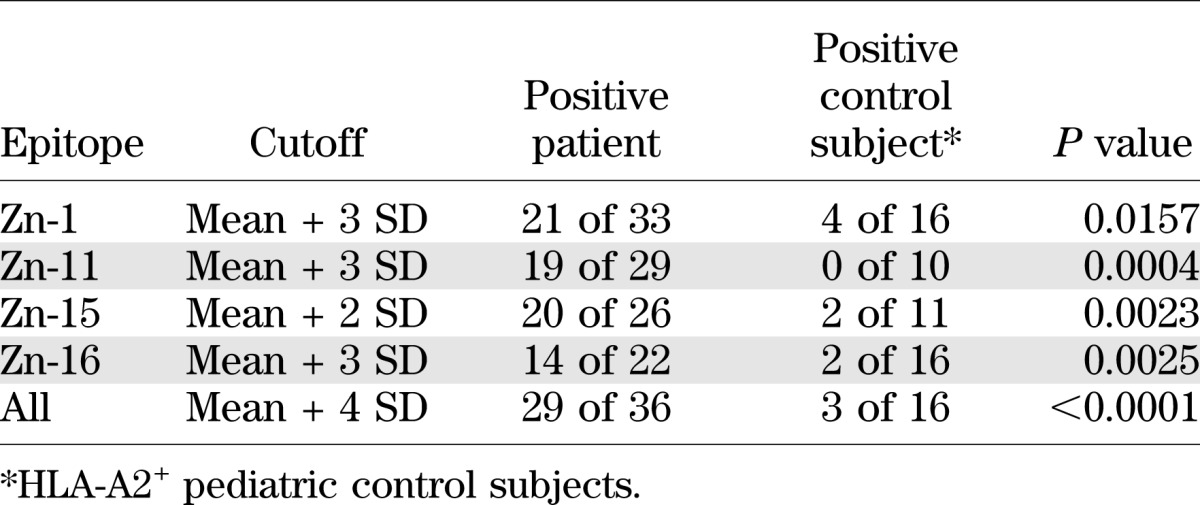
The results obtained in ELISpot assays are shown in Fig. 2, and statistical evaluation of the results for the complete set of ZnT8 peptides and for individual peptides is shown in Table 2. Supplementary Fig. 1 shows individual results for each patient together with basal and CD3-stimulated spot numbers. Lymphocytes from all patients and all control subjects responded to CD3 stimulation with IFN-γ production (Supplementary Fig. 1). However, both background (mean 86.2 vs. 41.6 spots) and anti-CD3–stimulated (mean 985 vs. 557 spots) IFN-γ secretion were higher in diabetic patients than in control subjects, while the ratio between CD3-stimulated and background spot numbers was similar (11.4 vs. 13.4). Given that background values varied between individual patients and control subjects, we used a relative parameter to define positivity in the ELISpot assay. Considering the complete epitope panel and using a cutoff (mean of background triplicate + 4 SD), 29 of 36 HLA-A2+ patients (80.6%) were positive. Note that among the blood samples testing negative, many had very limited volumes so that few peptides could be tested; the average number of peptides studied for samples testing positive was 6.2 versus 4.0 for samples testing negative. Almost all positive samples responded to multiple (up to eight out of nine) epitopes. Consistent with our results obtained previously for adult patients (10), IFN-γ responses to ZnT8 were low, ranging between 1.5- and 5-fold above background. While 3 out of 16 (18.7%) HLA-A2+ control subjects tested positive, 0 out of 17 HLA-A2− diabetic patients and control subjects tested positive for any epitope, consistent with presentation of the ZnT8 epitopes by the HLA-A2 molecule. We also analyzed the discriminative power of individual peptides (Table 2). This showed that several peptides had a discriminative power approaching that of the complete epitope set. For example, at a cutoff of mean + 3 SD, peptides 11 and 16 were positive for 65.5 and 63.6%, respectively, of HLA-A2 diabetic patients, while 0 and 12.5%, respectively, of HLA-A2+ control individuals were positive.
FIG. 2.
ELISpot responses of pediatric type 1 diabetic patients and control subjects to ZnT8 peptides. ELISpot results are represented as multiples of the SD for the individual assay after subtraction of the mean basal spot number measured in the presence of solvent; for example, a value of 10 indicates that the spot number corresponded to (basal mean for the patient tested) + 10 SD. Each dot corresponds to an individual result for the peptide indicated on the x-axis. See text for details on patient and control groups. Raw data are available in Supplementary Fig. 1.
TABLE 2.
Discriminative power of single and combined ZnT8 epitopes
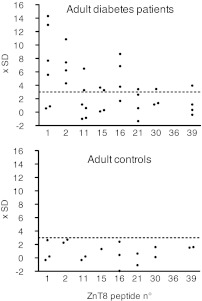
ELISpot responses to ZnT8 peptides in HLA-A2+ adults with type 1 diabetes.
We also could test IFN-γ responses of lymphocytes from a small number of HLA-A2+ adult type 1 diabetic patients and control subjects. Although the limited number of individuals tested does not allow for meaningful statistical evaluation, the data suggest that adult and pediatric patients display similar CD8+ T-cell responses to ZnT8 peptides (Fig. 3). Thus, five out of six patients but no control individuals responded to ZnT8 epitopes. Responses were polyspecific and ranged between 1.5- and 5-fold background levels.
FIG. 3.
ELISpot responses of adult type 1 diabetic patients and control subjects to ZnT8 peptides. ELISpot results are represented as multiples of the SD for the individual assay after subtraction of the mean basal spot number measured in the presence of solvent; for example, a value of 10 indicates that the spot number corresponded to (basal mean for the patient tested) + 10 SD. Each dot corresponds to an individual result for the peptide indicated on the x-axis. Data represent ELISpot results for six HLA-A2+ diabetic patients and three HLA-A2+ healthy control subjects.
DISCUSSION
The two principal findings of this study are that ZnT8 is a major CD8+ T-cell autoantigen in type 1 diabetes and that children with diabetes display similar autoreactive CD8+ T-cell responses as adult patients. Thus, studying autoimmunity to ZnT8 complements assays measuring autoimmune responses to the standard autoantigens, insulin, GAD, and IA-2, not only at the autoantibody level but also at the T-cell level.
Next to our desire to explore CD8+ T-cell recognition of the most recently identified autoantigen, our decision to focus on ZnT8 was motivated by reports that ZnT8 autoantibodies have high prognostic value in pediatric patients. In one study, children having multiple autoantibodies, including ZnT8 antibodies, had a much higher diabetes risk (62%) than children having multiple autoantibodies not including ZnT8 specificity (34%) (20). Given the very limited blood volumes that could be obtained from most children in this study, we could not test reactivity to the insulin, GAD, IA-2, and IGRP epitopes that we had previously found to be recognized by CD8+ T cells from adult patients (10).
When comparing the results obtained in this study with our previous study of adult patients (10), ZnT8 appears to be an autoantigen eliciting particularly frequent T-cell responses. Whereas in that study only one epitope (GAD65114–123) triggered responses by 50% of patient samples and all others stimulated responses of 40% or lower, all tested ZnT8 epitopes elicited responses in >63% of patients, with epitope Zn-15 being recognized by >76% of patients vs. 18% of control subjects. Thus, one or two ZnT8 peptides may be sufficient for ELISpot testing, a feature of particular interest for testing of pediatric patients giving limited blood volumes. In comparison with CD8+ T-cell responses to proinsulin in adult patients, ZnT8-specific responses were also particularly broad. While the average number of proinsulin epitopes tested previously in adults (6.1) was similar to that of ZnT8 epitopes in pediatric patients (6.2), the percentage of epitopes eliciting IFN-γ secretion was substantially higher in the latter case: 57.8 vs. 31.6%. However, given that all pediatric control individuals tested in this study presented with diabetes-unrelated pathologies, the potential impact of which on the results of the ELISpot assay is uncertain, the statistical evaluations have to be interpreted with caution.
One notable difference between the results obtained in this study and those in our study of adult patients (10) is the higher background observed with the lymphocytes from pediatric patients. A marked difference was noted when comparing not only adult and pediatric patients but also pediatric patients and control subjects. Relatively high spontaneous secretion of IFN-γ may be related to more severe metabolic perturbation and an associated inflammatory syndrome at disease onset in pediatric patients (28,29); note that almost all children were tested within the first days after diagnosis. Future studies will show whether restoration of metabolic control normalizes spontaneous IFN-γ secretion in pediatric patients.
Another notable observation in this study was the high efficacy of the immunization with plasmid DNA encoding an autoantigen fusion protein, which triggered strong responses in all animals injected. This result corroborates previous conclusions published by others (25) and extends the use of fusion protein DNA vaccines to the triggering of antiself responses. The absence of a bias toward nonself sequences and toward sequences with likely low HLA-A2 binding affinity (i.e., low SYFPEITHI scores) among the peptides triggering strong responses in HLA-A2 transgenic mice suggests that this immunization strategy may be readily able to overcome self-tolerance. However, it is possible that the responding T cells displayed low T-cell receptor avidity as frequently observed at least for autoreactive CD4+ T cells (30).
In summary, we have shown that ZnT8 is a major autoantigen recognized by self-reactive CD8+ T cells in pediatric and probably also adult type 1 diabetes. Frequent recognition of individual ZnT8 by patients suggests that ZnT8 epitopes may be particularly suited for ELISpot assays when limited blood volumes are available. It is important that our results extend the previous demonstration of frequent CD8+ T-cell responses in adult type 1 diabetes to pediatric disease and suggest that IFN-γ ELISpot assays are of interest for monitoring of autoimmune diabetes whatever the age and clinical presentation of the disease.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
É.É. performed ELISpot assays with human PBLs. R.K. and E.B. identified candidate ZnT8 epitopes. J.-B.A., J.B., and J.-J.R. recruited patients and obtained ethical approval. Y.H. and C.M. purified and HLA-typed patient lymphocytes. B.M. and L.C. performed autoantibody testing and helped with patient recruitment. P.v.E. designed and supervised the study and wrote the manuscript. P.v.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to Drs. Caroline Mignot (Ambroise Paré Hospital), Vincent Gajdos (Antoine Béclère Hospital), Sophie Lemerle (Intercommunal Hospital Créteil), and Agnes Hartemann (Pitié-Salpétrière Hospital) for patient recruitment.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0071/-/DC1.
REFERENCES
- 1.Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity 2010;32:437–445 [DOI] [PubMed] [Google Scholar]
- 2.Coppieters KT, von Herrath MG. Histopathology of type 1 diabetes: old paradigms and new insights. Rev Diabet Stud 2009;6:85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarchum I, Baker JC, Yamada T, et al. In vivo cytotoxicity of insulin-specific CD8+ T-cells in HLA-A*0201 transgenic NOD mice. Diabetes 2007;56:2551–2560 [DOI] [PubMed] [Google Scholar]
- 5.Takaki T, Marron MP, Mathews CE, et al. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol 2006;176:3257–3265 [DOI] [PubMed] [Google Scholar]
- 6.Stadinski B, Kappler J, Eisenbarth GS. Molecular targeting of islet autoantigens. Immunity 2010;32:446–456 [DOI] [PubMed] [Google Scholar]
- 7.Babad J, Geliebter A, DiLorenzo TP. T-cell autoantigens in the non-obese diabetic mouse model of autoimmune diabetes. Immunology 2010;131:459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serreze DV, Marron MP, Dilorenzo TP. “Humanized” HLA transgenic NOD mice to identify pancreatic beta cell autoantigens of potential clinical relevance to type 1 diabetes. Ann N Y Acad Sci 2007;1103:103–111 [DOI] [PubMed] [Google Scholar]
- 9.Roep BO, Peakman M. Diabetogenic T lymphocytes in human type 1 diabetes. Curr Opin Immunol 2011;23:746–753 [DOI] [PubMed] [Google Scholar]
- 10.Mallone R, Martinuzzi E, Blancou P, et al. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes 2007;56:613–621 [DOI] [PubMed] [Google Scholar]
- 11.Enée E, Martinuzzi E, Blancou P, Bach JM, Mallone R, van Endert P. Equivalent specificity of peripheral blood and islet-infiltrating CD8+ T lymphocytes in spontaneously diabetic HLA-A2 transgenic NOD mice. J Immunol 2008;180:5430–5438 [DOI] [PubMed] [Google Scholar]
- 12.Wong CP, Stevens R, Long B, et al. Identical beta cell-specific CD8(+) T cell clonotypes typically reside in both peripheral blood lymphocyte and pancreatic islets. J Immunol 2007;178:1388–1395 [DOI] [PubMed] [Google Scholar]
- 13.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 14.Caillat-Zucman S, Garchon H-J, Timsit J, et al. Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest 1992;90:2242–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pipeleers D, In't Veld P, Pipeleers-Marichal M, Gorus F. The beta cell population in type 1 diabetes. Novartis Foundation Symp 2008;292:19–24; discussion 24–31, 122–129, 202–203 [DOI] [PubMed]
- 16.Wijesekara N, Dai FF, Hardy AB, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010;53:1656–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeulen I, Weets I, Asanghanwa M, et al. Belgian Diabetes Registry Contribution of antibodies against IA-2β and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care 2011;34:1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang M, Rockell J, Wagner R, et al. Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol 2011;186:6056–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009;52:1881–1888 [DOI] [PubMed] [Google Scholar]
- 21.Kratzer R, Mauvais FX, Burgevin A, Barilleau E, van Endert P. Fusion proteins for versatile antigen targeting to cell surface receptors reveal differential capacity to prime immune responses. J Immunol 2010;184:6855–6864 [DOI] [PubMed] [Google Scholar]
- 22.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Pérarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med 1997;185:2043–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groop LC, Bottazzo GF, Doniach D. Islet cell antibodies identify latent type I diabetes in patients aged 35–75 years at diagnosis. Diabetes 1986;35:237–241 [DOI] [PubMed] [Google Scholar]
- 24.Blancou P, Mallone R, Martinuzzi E, et al. Immunization of HLA class I transgenic mice identifies autoantigenic epitopes eliciting dominant responses in type 1 diabetes patients. J Immunol 2007;178:7458–7466 [DOI] [PubMed] [Google Scholar]
- 25.Nchinda G, Kuroiwa J, Oks M, et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest 2008;118:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 1999;50:213–219 [DOI] [PubMed] [Google Scholar]
- 27.Martinuzzi E, Scotto M, Enée E, et al. Serum-free culture medium and IL-7 costimulation increase the sensitivity of ELISpot detection. J Immunol Methods 2008;333:61–70 [DOI] [PubMed] [Google Scholar]
- 28.Planas R, Pujol-Borrell R, Vives-Pi M. Global gene expression changes in type 1 diabetes: insights into autoimmune response in the target organ and in the periphery. Immunol Lett 2010;133:55–61 [DOI] [PubMed] [Google Scholar]
- 29.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 30.Gebe JA, Falk BA, Rock KA, et al. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur J Immunol 2003;33:1409–1417 [DOI] [PubMed] [Google Scholar]