Abstract
Insulin is secreted from the islets of Langerhans in coordinated pulses. These pulses are thought to lead to plasma insulin oscillations, which are putatively more effective in lowering blood glucose than continuous levels of insulin. Gap-junction coupling of β-cells by connexin-36 coordinates intracellular free calcium oscillations and pulsatile insulin release in isolated islets, however a role in vivo has not been shown. We test whether loss of gap-junction coupling disrupts plasma insulin oscillations and whether this impacts glucose tolerance. We characterized the connexin-36 knockout (Cx36−/−) mouse phenotype and performed hyperglycemic clamps with rapid sampling of insulin in Cx36−/− and control mice. Our results show that Cx36−/− mice are glucose intolerant, despite normal plasma insulin levels and insulin sensitivity. However, Cx36−/− mice exhibit reduced insulin pulse amplitudes and a reduction in first-phase insulin secretion. These changes are similarly found in isolated Cx36−/− islets. We conclude that Cx36 gap junctions regulate the in vivo dynamics of insulin secretion, which in turn is important for glucose homeostasis. Coordinated pulsatility of individual islets enhances the first-phase elevation and second-phase pulses of insulin. Because these dynamics are disrupted in the early stages of type 2 diabetes, dysregulation of gap-junction coupling could be an important factor in the development of this disease.
Insulin secretion from islets of Langerhans is highly dynamic in response to elevated glucose in most species studied. A first phase consisting of a sharp 5- to 10-min peak of insulin secretion is followed by a second phase of sustained elevation of secretion for >30 min. The second phase of insulin secretion is pulsatile, leading to oscillations in plasma insulin with a period of 3–8 min in humans (1), dogs (2), and mice (3). Isolated islets or β-cells from humans and mice also respond to elevated glucose by secreting pulses of insulin (4,5). These pulses are driven by the synchronous oscillations of many variables underlying glucose-stimulated insulin secretion, such as membrane potential and coordinated intracellular free calcium activity ([Ca2+]i) (5–7) or cAMP levels (8). In mice, it has been shown that the pattern of [Ca2+]i oscillations in ex vivo–isolated islets correlates with the pattern of in vivo plasma insulin oscillations (9).
Several studies have suggested a physiological relevance of plasma insulin oscillations by showing that they yield positive effects compared with continuous insulin level. Oscillating insulin levels have been shown to lead to greater glucose-lowering action (10–13), as well as maintenance of peripheral tissue insulin sensitivity (14). Insulin oscillations are also disrupted in patients with type 2 diabetes (15–17) and obese individuals (18). The precise role of pulsatile insulin in its action remains controversial because some other studies have not found that oscillatory insulin significantly enhances insulin action (19,20). However, many studies rely on comparing pulsatile or continuous levels of insulin infusion, either applied to the portal vein or peripheral circulation, and have not measured the impact of altered endogenous insulin pulsatility.
Gap-junction coupling has been shown to be critically important for the coordination of [Ca2+]i oscillations (6,21) and generating pulsatile insulin secretion (6) in isolated islets. However, a deletion of gap-junction coupling alone has minimal impact on the steady-state levels of insulin secretion from isolated islets at both high and low glucose levels (6,22). Because the pattern of oscillatory [Ca2+]i in isolated islets has been linked to the generation of in vivo insulin oscillations (9), we hypothesize that mice lacking Cx36 would show a disruption to the endogenous insulin secretion dynamics, but not steady-state insulin levels. Because pulsatile insulin has been linked to enhancing insulin action, we further hypothesize that mice lacking Cx36 would show altered glucose homeostasis. Therefore, the Cx36 knockout mouse should allow us to understand how the coordinated oscillations in individual islets impact insulin secretion dynamics in vivo, and to understand if these insulin secretion dynamics are important for maintaining glucose homeostasis.
In this study, we characterized the phenotype of Cx36 knockout mice and tested whether there is a defect in glucose homeostasis and a change in insulin levels. By using rapid-sampling glucose-clamp measurements, we measured the dynamics of insulin secretion in Cx36 knockout mice and tested if there is an alteration in two aspects of these dynamics. First, we examined whether the loss of oscillatory insulin secretion seen in isolated islets leads to a disruption in the in vivo insulin oscillations. Second, we measured the amplitudes and timings of first- and second-phase insulin secretion to test whether these values are also affected by a loss of gap-junction coupling, and whether these changes impact glucose tolerance.
RESEARCH DESIGN AND METHODS
Animal care.
All experiments were performed in compliance with the relevant laws and institutional guidelines and were approved by Vanderbilt University Institutional Animal Care and Use Committee. Mice were housed in a temperature-controlled facility with a 12-h light-dark cycle and access to food and water ad libitum. Generation of connexin-36 knockout mice (Cx36−/−), which consist of a truncated Cx36 gene with a LacZ knock-in, has been previously described (23). Mice with at least 98.4% C57Bl/6 background were studied at generation F7–F9, and were genotyped by PCR and gel electrophoresis using published primers (23).
Glucose and insulin tolerance tests.
To assess glucose tolerance, littermate or age-matched mice (as indicated) were fasted overnight for 16 h prior to glucose tolerance test (GTT). Mice received intraperitoneal injection or oral gavage of 3, 2, 1, 0.5, or 0.2 g/kg body weight of glucose, and blood glucose was measured using a glucose meter (Ascensia Contour; Bayer) on tail vein blood samples at regular intervals (preinjection [0 min] and 15, 30, 60, 90, 120 min after glucose delivery). For plasma insulin and glucagon measurements, littermate mice were fasted overnight for 16 h and received an intraperitoneal injection of 3 g/kg body weight of glucose. Blood samples were taken preinjection (0 min) and 30 min postinjection and centrifuged at 14 krev/min for 10 min, and plasma was assayed for insulin concentration using rat insulin radioimmunoassay. To assess insulin sensitivity, littermate mice were fasted for 6 h prior to insulin tolerance test. Mice received intraperitoneal injection of 0.75 units/kg body weight of human recombinant insulin (Novolin; Novo Nordisk), and blood glucose was measured on tail vein blood samples at regular intervals (preinjection [0 min] and 15, 30, 45, 60, 90 min postinjection).
Hyperglycemic clamps.
Glucose-clamp measurements were performed as described in detail by Satin and colleagues (3,9). In brief, littermate, 16–18-week-old male Cx36−/− and Cx36+/+ mice underwent jugular vein and carotid artery catheterization ≥5 days prior to experiments. Mice were individually housed after surgery. The jugular vein catheter was used for glucose infusion and the carotid artery was used for rapid blood sampling. The conscious mouse model used in these studies contrasts with other mouse models in that restraint is not required and blood is obtained without handling the mouse. It is the only model that has been demonstrated not to elicit a stress response in wild-type mice (24,25). After a 5-h fast, two baseline glucose and insulin samples were taken. A variable infusion of 50% dextrose was then infused to reach constant blood glucose levels of ∼220 mg/dL. After 0 or 40 min, 60-μL blood samples were taken every 1 min for 20–30 min and assayed for blood glucose and plasma insulin. Erythrocytes from a “donor” mouse were infused at a constant rate of 70 μL/min throughout the sampling period to maintain a constant erythrocyte volume.
Islet isolation and secretion measurements.
Islets were isolated as previously described (26) from pancreata of 16- to 20-week-old mice. Islets were maintained in islet medium (RPMI medium, 10% FBS, 11 mM glucose, 100 units/mL penicillin, and 100 μg/mL streptomycin) at 37°C under humidified 5% CO2 for 24 h before perifusion or imaging. The insulin secretion time course from isolated islets was assessed in a cell perifusion apparatus (27) with a flow rate of 1 mL/min with DMEM (plus l-glutamine, Na-pyruvate, and 0.1% BSA) as a perifusate. Batches of Cx36+/+ and Cx36−/− islets were size matched with similar distributions (mean of 48.9 ± 1.1 and 50.5 ± 1.0 islets, respectively, and 49.8 ± 1.0 and 48.8 ± 1.5 islet equivalents [IEQ], respectively) and perifused in parallel for 1 h at 2.8 mM glucose with the column flow-through rejected. Samples were then collected in 2-min windows for 10 min at 2.8 mM, then collected in 2-min windows for 20 min at 11.1 mM, and then collected in 4-min windows for 20 min at 11.1 mM. Insulin content was estimated by ethanol-HCl extraction and sonication on ice. Insulin concentrations were measured by rat insulin radioimmunoassay.
Fluorescence microscopy.
To measure [Ca2+]i dynamics, isolated islets were loaded for 40 min with 4 μM Fluo4-AM (Invitrogen) at 37°C in imaging medium (125 mM NaCl, 5.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES, 11 mM glucose, and 0.1% BSA, pH 7.4). Islets were imaged in a microfluidic flow device (28) on an LSM5Live microscope (Zeiss) with a 20× 0.8NA Fluar objective, using a humidified chamber maintained at 37°C. Images were acquired every 1 s. Fluo4 was excited with a 488-nm diode laser, and fluorescence was detected with a 495-nm long-pass filter.
Insulin pulse analysis.
Pulses of insulin for each data series were identified using the pulse detection algorithm Cluster8 (3,29). Each data series was padded by 3 points at each end and run with the following settings to detect peaks and nadirs: 1-min minimum peak size, 1-min minimum nadir size, no minimum value for peak amplitude, and a t score of 1.0. Hormone half-life was assumed to be 6-min, and a t score of 4.0 was used for identifying outliers in the data series. When analyzing T0 data, the first 5 min was discounted to prevent interference from the first-phase peak. False positives were kept to <5% by using the SD of the duplicates for each data point of a given record of insulin concentration.
RESULTS
Phenotype of connexin-36 knockout mice.
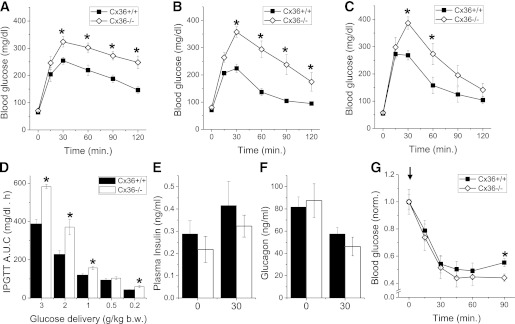
Male and virgin female connexin-36 knockout mice (Cx36−/−) developed healthily, with no differences in body weight at 4–24 weeks. Cx36−/− mice showed no significant difference in fasting blood glucose compared with littermate control mice (Cx36+/+) at 16 weeks (Fig. 1A and B). Both male (Fig. 1A) and female (Fig. 1B) Cx36−/− mice showed significantly greater excursions in blood glucose at 30–120 min after an intraperitoneal glucose challenge (2 g/kg), with blood glucose levels >200 mg/dL at 120 min indicating glucose intolerance (Fig. 1A and B). Under an oral glucose challenge (2 g/kg), significantly greater excursions in blood glucose were also observed in Cx36−/− mice (Fig. 1C). The areas under the curve of the glucose excursion for male Cx36−/− mice challenged with intraperitoneal glucose levels between 3 and 0.2 g/kg are significantly greater than those measured in Cx36+/+ mice (Fig. 1D). Cx36−/− and Cx36+/+ mice showed no significant difference in plasma insulin (Fig. 1E) and plasma glucagon levels (Fig. 1F) both in the fasted state and 30 min after an intraperitoneal glucose challenge. After an intraperitoneal insulin injection, Cx36−/− mice showed no significant difference in blood glucose levels up to 60 min (Fig. 1G), and a small reduction in glucose levels at 90 min compared with control Cx36+/+ mice. Heterozygous knockout mice (Cx36+/−) showed a glucose excursion intermediate of Cx36−/− and Cx36+/+ mice after an intraperitoneal glucose challenge, no significant difference in plasma insulin and plasma glucagon levels compared with Cx36+/+ mice, and no significant difference in blood glucose after an intraperitoneal insulin injection (Supplementary Fig. 1). Therefore, a knockout of Cx36 in mice leads to glucose intolerance despite statistically normal in vivo insulin and glucagon levels and without a substantial change in insulin sensitivity.
FIG. 1.
Phenotype of Cx36−/− mice. A: Intraperitoneal (i.p.) glucose tolerance test (IPGTT) on male Cx36+/+ (■) and Cx36−/− mice (◇), each 16 weeks of age, after 2 g/kg body weight (b.w.) i.p. glucose injection. n = 6 littermate mice in each group. B: IPGTT on female mice 16 weeks of age, as in A. n = 8 littermate mice in each group. C: Oral GTT on male mice 16 weeks of age after 2 g/kg b.w. oral gavage. n = 6 littermate mice in each group. D: Area under the curve (AUC) of the glucose excursion during IPGTT after i.p. injection of variable amounts of glucose. n = 9 age-matched mice studied in parallel in each group. E: Plasma insulin measurements in male mice aged 16 weeks before (0) and 30 min after 3 g/kg b.w. i.p. glucose injection (black bars, Cx36+/+ mice; white bars, Cx36−/− mice). n = 8 littermate mice in each group. F: Plasma glucagon measurements in male mice aged 16 weeks, before (0) and 30 min after i.p. glucose injection, as in D. n = 7 littermate mice in each group. G: Insulin tolerance test after 0.75 units/kg i.p. insulin injection (see arrow). n = 10 littermate mice in each group. *, significant difference (P < 0.05, two-tailed Student t test) at each time point comparing measurements in Cx36+/+ and Cx36−/− mice.
To assess the dynamics underlying glucose-stimulated insulin secretion, we measured the temporal [Ca2+]i responses to glucose in Cx36+/+ and Cx36−/− islets. In Cx36+/+ islets, coordinated oscillatory [Ca2+]i was measured, with individual cells of each islet showing similar periods of 2–5 min (e.g., Supplementary Fig. 2A). In Cx36−/− islets, no coordinated [Ca2+]i was measured, although the majority of individual cells in each islet showed oscillatory [Ca2+]i (6,21) with periods ranging from <5 s up to 8 min (e.g., Supplementary Fig. 2B). Additionally, it has been shown that isolated Cx36−/− islets do not release insulin in regular pulses, unlike isolated Cx36+/+ wild-type islets (6). These data from isolated islets suggest that altered insulin dynamics may also occur in vivo and contribute to the glucose intolerance seen in Cx36−/− mice.
Altered in vivo insulin oscillations in Cx36−/− mice.
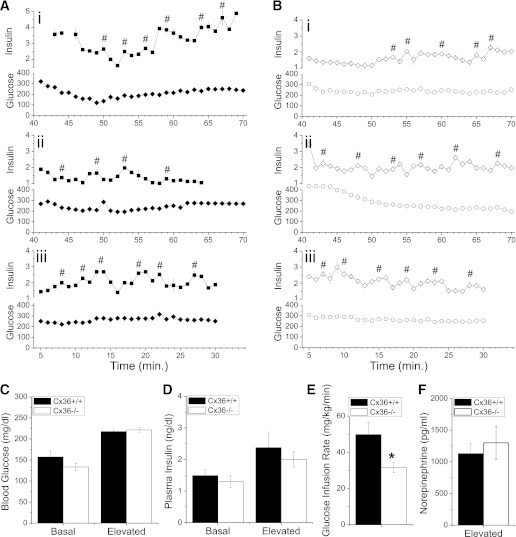
We hypothesized that the lack of coordinated [Ca2+]i oscillations and pulsatile insulin secretion would disrupt in vivo insulin oscillations in Cx36−/− mice, and this disruption could explain the glucose intolerance. To test this concept, rapid-sampling hyperglycemic-clamp measurements were performed following previously established procedures (3,9). Rapid sampling (once per minute) was performed on male Cx36+/+ and Cx36−/− mice in two separate experimental groups: starting 40 min after initiating glucose infusion (T40) or starting immediately after initiating glucose infusion (T0), as previously performed (3,9). Representative time courses of plasma insulin levels and glucose clamps from Cx36+/+ and Cx36−/− mice can be seen in Fig. 2A and B, respectively. Cx36+/+ and Cx36−/− mice were clamped at similar average levels of blood glucose (∼220 mg/dL) in the T40 group (Fig. 2C), as well as in the T0 groups (Supplementary Fig. 3A). No significant difference in the time-averaged plasma insulin levels was observed between Cx36+/+ and Cx36−/− mice in both the T40 and T0 groups (Fig. 2D and Supplementary Fig. 3B, respectively). Cx36+/+ mice required significantly greater glucose infusion compared with Cx36−/− mice to establish the glucose clamp in both T40 and T0 experimental groups (Fig. 2E and Supplementary Fig. 3C, respectively), which further indicates reduced glucose tolerance in the Cx36−/− mice. The mean time course of blood glucose, glucose infusion rate, and plasma insulin is shown in Supplementary Fig. 4. Several points in the mean insulin time course were significantly elevated in Cx36+/+ mice compared with Cx36−/− mice, although the time-averaged levels were not significantly different. Norepinephrine (Fig. 2F) and epinephrine (<15 pg/mL) levels in Cx36+/+ and Cx36−/− mice were not significantly different.
FIG. 2.
Measuring plasma insulin dynamics. A: Three representative time courses of rapid sampling blood glucose (Glucose, mg/dL) and plasma insulin levels (Insulin, ng/mL) from Cx36+/+ mice during hyperglycemic clamp. Sampling rate is one per minute. The x-axis indicates the time after glucose infusion is started. #, center of each pulse identified during pulse analysis. B: Three representative time courses of rapid sampling glucose and plasma insulin levels from Cx36−/− mice during hyperglycemic clamp, as in A. C: Time-averaged blood glucose in male littermate Cx36+/+ mice (black bars) and Cx36−/− mice (white bars) at 16–17 weeks of age before and during the hyperglycemic clamp, 40 min after the start of glucose infusion. n = 10 and 8 mice in Cx36+/+ and Cx36−/− groups, respectively. D: Time-averaged plasma insulin levels corresponding to measurements made in C. E: Time-averaged glucose infusion rate required to establish glucose clamp in C. F: Norepinephrine levels at the end point of hyperglycemic clamp combined from both T0 and T40 groups, averaged over n = 8 mice in each experimental group. *, significant difference (P < 0.05, Student t test) comparing each measurement in Cx36+/+ and Cx36−/− mice.
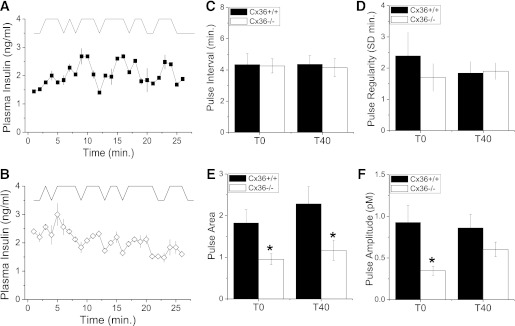
Pulses of insulin were detected in both Cx36+/+ and Cx36−/− mice, with the pattern of these pulses varying between each mouse and each genotype (e.g., Fig. 2A and B). We quantified the pulsatile insulin time course of each mouse using pulse analysis (see RESEARCH DESIGN AND METHODS). Example analyses are displayed in Fig. 3A and B. Average and SD pulse parameters are shown in Supplementary Fig. 5. The mean pulse interval (Fig. 3C) was not significantly different comparing Cx36+/+ and Cx36−/− mice in each experimental group, with mean intervals of 4.3 ± 0.7 (T0) and 4.4 ± 0.6 min (T40) compared with 4.3 ± 0.4 (T0) and 4.2 ± 0.7 min (T40), respectively. The pulse regularity (the SD of pulse intervals in a time course) was also similar for each group (Fig. 3D). In contrast, the mean pulse area (pulse mass) was significantly reduced in Cx36−/− mice (Fig. 3E) for both the T0 group (∼1.9-fold) and T40 group (∼2-fold). The mean pulse amplitude in Cx36−/− mice was also significantly reduced in the T0 group (Fig. 3F). Therefore, the second-phase pulse size is reduced in the absence of Cx36−/−, with changes in pulse size being more significant during the earlier portions of second-phase insulin secretion.
FIG. 3.
Analysis of plasma insulin oscillations. A: Representative time courses of plasma insulin levels from a Cx36+/+ mouse (trace iii in Fig. 2A) together with pulse analysis, which is offset for clarity. B: Representative time courses of plasma insulin levels from a Cx36−/− mouse (trace iii in Fig. 2B) together with pulse analysis, as in A. C: Mean time interval between consecutive pulses for Cx36+/+ and Cx36−/− mice, 0 (T0) or 40 min (T40) after glucose infusion. n = 5 and 10 Cx36+/+ mice and 5 and 8 Cx36−/− mice in the T0 and T40 groups, respectively. D: Pulse regularity, defined by the SD of pulse interval in each time course for Cx36+/+ and Cx36−/− mice, as in C. E: Mean pulse area for Cx36+/+ and Cx36−/− mice, as in C. F: Mean pulse amplitude above basal levels for Cx36+/+ and Cx36−/− mice, as in C. *, significant difference (P ≤ 0.05, Student t test) comparing each measurement in Cx36+/+ and Cx36−/− mice.
First- and second-phase insulin secretion in Cx36−/− mice.
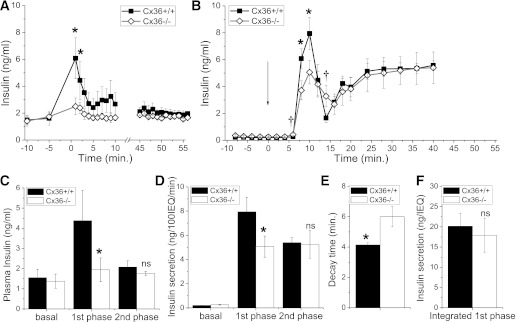
The disruption to pulsatile insulin levels in Cx36−/− mice is greater earlier after glucose infusion. Insulin secretion is biphasic, and these dynamics are missed in static section assays or plasma insulin measurements after a glucose challenge. We analyzed the insulin secretion time course as the hyperglycemic clamp was established. A large first-phase elevation in insulin was measured for the first ∼3 min in Cx36+/+ mice, which was significantly less in Cx36−/− mice (Fig. 4A). However, after this time, the mean insulin levels were not significantly different. To test whether the decreased first phase results from a defect in the islet, we measured the time course of insulin secretion during perifusion of isolated islets from Cx36+/+ and Cx36−/− mice. Upon a step increase in glucose, a characteristic biphasic secretion response was observed in both sets of islets (Fig. 4B). Compared with Cx36+/+ islets, Cx36−/− islets showed reduced insulin secretion within ∼10 min of glucose elevation but similar levels of insulin secretion >30 min after glucose elevation. Insulin content of Cx36+/+ and Cx36−/− islets was similar in each case: 64.7 ± 5.8 and 65.8 ± 6.9 ng/IEQ, respectively, or 66.0 ± 6.6 and 62.2 ± 5.7 ng/islet, respectively (n = 6 sets of aliquots; 47 ± 1 islets/aliquot). Thus, in vivo insulin levels follow a similar pattern as ex vivo–isolated islet secretions, as summarized in Fig. 4C and D, respectively. In isolated islets, the rise time from initial insulin secretion elevation to the first-phase peak was similar between Cx36+/+ and Cx36−/− islets; however, the decay time from the peak elevation was significantly greater in Cx36−/− islets (Fig. 4E). Furthermore, the insulin secretion at the initial elevation of the first phase and at the end of the decay from the first-phase peak was slightly elevated in Cx36−/− islets (Fig. 4B). Thus, the total time-integrated insulin secretion of the first phase is similar between Cx36+/+ and Cx36−/− islets (Fig. 4F). In this way, the absence of Cx36 gap-junction coupling leads not only to a disruption of plasma insulin pulsatility but also to a stretching out and lowering of the first-phase insulin secretion in islets, which correlates with the reduced peak amplitude of the first phase of insulin secretion in vivo.
FIG. 4.
First-phase insulin secretion in vivo and ex vivo. A: Mean time course of plasma insulin levels from Cx36+/+ (■) and Cx36−/− mice (◇) before and immediately after glucose infusion, as well as between 45 and 55 min after glucose infusion. Sampling rate is one per minute. n = 5 littermate mice in each group. B: Mean time course of insulin secretion levels from isolated Cx36+/+ and Cx36−/− islets (per 100 IEQ) after elevated glucose levels. Arrow indicates glucose step from 2.8 to 11.1 mM. Sampling rate is 0.5 per minute until t = 20, then 0.25 per minute thereafter. n = 6 sets of islets from individual mice in each group. *, significant reduction; †, significant elevation (P < 0.05, paired Student t test) in Cx36−/− islets compared with Cx36+/+ islets. C: Summary of mean plasma insulin levels prior to glucose infusion (basal), peak plasma insulin levels 1–3 min after glucose infusion (first phase), and mean plasma insulin levels 45–55 min after glucose infusion (second phase). D: Summary of mean insulin secretion levels during perifusion, prior to glucose elevation (basal), peak insulin secretion at 10 min after glucose elevation (first phase), and mean insulin secretion 40 min after glucose elevation (second phase). E: Mean time from first-phase peak of insulin secretion to first minimum of insulin secretion. F: Mean time-integrated insulin secretion from initial elevation at t = 5 min to first minimum of insulin secretion between t = 10 and 20 min. *, significant difference (P < 0.05, Student t test); NS, no significant difference (P > 0.1, Student t test), comparing each measurement in Cx36+/+ and Cx36−/− mice or islets.
DISCUSSION
In this study, we sought to understand how the dynamics of insulin secretion are regulated in vivo by islet gap-junction coupling, and whether a disruption to islet pulsatile insulin release leads to altered glucose tolerance. Cx36 gap junctions are the sole means of electrical coupling between β-cells in the islet (21), and in the absence of Cx36, isolated islets do not exhibit coordination in [Ca2+]i oscillations (Supplementary Fig. 2) or pulsatile insulin release (6). Many studies indicate that the pulsatile dynamics of insulin secretion are important for insulin action and maintaining insulin sensitivity (10–14), and insulin pulsatility is disrupted at the onset of type 2 diabetes (15–17). Factors that regulate in vivo insulin dynamics may therefore be important for regulating glucose homeostasis. We show here that Cx36−/− mice are glucose intolerant (Figs. 1 and 2 and Supplementary Fig. 3), without any substantial difference in plasma insulin levels (Figs. 1 and 2 and Supplementary Fig. 3), insulin sensitivity (30) (Fig. 1), or catecholamine levels (Fig. 2). Importantly, this glucose intolerance extends to glucose excursions similar to postprandial glucose levels (<200 mg/dL). Overexpression of Cx32 gap junctions in the β-cell also reduces glucose tolerance (31). Therefore, factors affecting the electrical communication between β-cells in the islet are physiologically important in regulating glucose homeostasis.
Cx36 gap junctions enhance the first phase of insulin secretion.
We found a reduction in the amplitude of the first phase of plasma insulin in Cx36−/− mice. Importantly, this reduction in the first phase was also found in isolated islets and indicates that the in vivo reduction is likely due to an islet-specific defect. The peak level of first-phase insulin is reduced in Cx36−/− islets due to the first phase being stretched in time, rather than a loss of overall first-phase output (Fig. 4E and F). This “smearing out” of the first phase and increased decay time from the first-phase peak is similar to the increased insulin secretion decay time measured in Cx36−/− islets after a drop in glucose (32). This altered decay is caused by the lack of coordination in [Ca2+]i changes. Because the average [Ca2+]i levels in Cx36−/− islets are similar to Cx36+/+ islets for the glucose concentrations used (22), the elongation of the first phase and reduction in peak levels are likely due to a lack of coordinated elevations in [Ca2+]i and insulin secretion from different β-cells of the islet.
The reduced first-phase peak of insulin secretion can partly explain the glucose intolerance observed in Cx36−/− mice. Loss of first-phase insulin secretion is generally one of the first secretory defects observed in human patients with type 2 diabetes (see below). This loss diminishes the rapid glucose-lowering action of insulin after glucose delivery and therefore leads to a greater glucose excursion (33). Because the first-phase secretion contributes only a fraction of the overall insulin delivery and the second-phase levels are unchanged, this can also explain the measured differences in steady-state in vivo insulin levels after glucose delivery.
We did not measure a substantial change in fasting plasma insulin levels or steady-state basal islet insulin secretion. Fasting plasma insulin levels have not previously been reported in Cx36−/− mice. Previous studies have reported small elevations in basal islet insulin secretion and larger elevations from a perfused pancreas of Cx36−/− mice (6), which is in disagreement with our ex vivo islet results. However, our present results are supported by previous work that also demonstrated insubstantial changes in islet insulin secretion after a deletion of Cx36, thereby revealing a gap junction–independent means of cell-cell communication that suppresses basal insulin secretion, independent of electrical activity (22). Such a mechanism can explain how in vivo fasting insulin levels, and therefore fasting blood glucose levels, are unchanged after the deletion of Cx36.
Cx36 gap junctions regulate pulsatile in vivo insulin dynamics.
In addition to the reduced first phase, the pulsatile pattern of plasma insulin during the in vivo second phase was also disrupted after a deletion of Cx36. Altered parameters include pulse amplitude and pulse area (pulse mass), which indicates that the coordinated oscillatory behavior observed in wild-type islets is important for amplifying the size of in vivo plasma insulin oscillations.
In humans, there is a strong correlation between insulin pulse mass and hepatic insulin clearance; thus, pulsatile insulin is preferentially extracted compared with the nonpulsatile fraction (13). If this also occurs in mice, then comparing Cx36+/+ and Cx36−/− mice, the difference in the insulin pulse mass delivered to the liver would be greater than the differences we measured in peripheral circulation. The overall level of insulin delivered to the liver would therefore be considerably reduced in Cx36−/− mice compared with Cx36+/+ mice. Reduced hepatic insulin delivery would result in reduced insulin action, reduced glucose clearance, and in turn, glucose intolerance. Therefore, in addition to reduced first-phase amplitude, the lack of coordinated [Ca2+]i dynamics that leads to reduced second-phase insulin pulse mass can also partly explain the glucose intolerance in Cx36−/− mice.
Residual insulin pulsatility is still observed in Cx36−/− mice, despite the fact that isolated Cx36−/− islets lack coordinated pulsatile secretion (6). This suggests that another mechanism of synchronization is still present in vivo. Cholinergic innervation may be such a mechanism because pulses of cholinergic agonist can entrain [Ca2+]i oscillations in isolated islets (34). In wild-type islets, all β-cells show similar regular [Ca2+]i oscillations, whereas β-cells in Cx36−/− islets are very heterogeneous in [Ca2+]i oscillation timing, with periods ranging from a few seconds to several minutes (Supplementary Fig. 2A and B). Therefore in Cx36−/− islets, we expect that only β-cells that have slower oscillations would be entrained to the 4–5-min oscillatory response, and thus, only those β-cells would release insulin in coordinated pulses in vivo. Computer modeling studies suggest that ∼35% of islet mass needs to be entrained to generate in vivo oscillations (35), which is comparable to the 20–30% of Cx36−/− β-cells that show slow oscillations of periods >2 min (unpublished data). Although cholinergic innervation is an attractive explanation, other coupling mechanisms, such as ATP signaling (36) or CO diffusion (37), could also be acting in vivo to coordinate the slowly oscillating β-cells and give rise to the residual pulsatile insulin release.
The insulin pulses measured in this study were qualitatively less clear than those measured in a Swiss-Webster outbred strain (3,9). This may be due to the insulin response being less robust in the inbred C57Bl/6 strain (3,25), but it is also important to consider the technical difficulty of the experiments performed. Nevertheless, significant pulsatility was resolved in all time courses, with pulse-to-pulse intervals and ranges consistent with previous studies. Time courses were of sufficient quality to resolve significant differences in pulsatility between Cx36−/− and control animals.
Relevance to type 2 diabetes.
Reduced first-phase insulin secretion (38) and disrupted pulsatile insulin (15–17) are found in patients with type 2 diabetes, with the second-phase pulse size and amplitude, but not the pulse timing, specifically disrupted (17). This is consistent with the changes we observe in mice lacking Cx36. Isolated islets from obese and diabetic mouse models also exhibit reduced coordination of [Ca2+]i oscillations and insulin pulsatility (39), again correlating with our observations in islets from Cx36−/− mice. Because Cx36 gap junctions are present in human islets (40), we speculate that a reduction of Cx36 gap-junction conductance may occur in the islets of patients with type 2 diabetes, which could explain the disrupted insulin oscillations and reduced first-phase insulin secretion.
Clearly, type 2 diabetes is a complex disease, but a disruption to islet gap-junction coupling would be expected to exacerbate glucose intolerance due to its impact on insulin dynamics. Indeed, a role for islet gap-junction coupling in the development of type 2 diabetes has previously been proposed (30,41). From studies describing how the synchronized calcium activity varies with gap-junction coupling conductance (21) (Supplementary Fig. 2), we predict that a partial loss (>50%) of Cx36 could lead to glucose intolerance approaching that in Cx36−/− mice. In a similar manner, pulsatile insulin infusion has been implicated for use in patients with type 2 diabetes with beneficial results (42). Therefore, we also speculate that gap-junction coupling and the coordinated pulsatile insulin release from islets may be a factor that is important to protect against the development of hyperglycemia and diabetes.
To summarize, Cx36 gap junctions are important for coordinating and enhancing both islet first-phase insulin secretion and pulsatile second-phase insulin secretion. This enhances the amplitude of the first-phase and pulsatile second-phase insulin levels in vivo. Defects in these parameters correlate with glucose intolerance, without changes in other measured defects such as insulin sensitivity or steady-state insulin levels. Therefore, islet electrical coupling is critical for regulating in vivo insulin secretory dynamics and glucose homeostasis.
ACKNOWLEDGMENTS
This study was primarily supported by a MicroMouse Pilot and Feasibility award (U24-DK-076169 to R.K.P.B.) and National Institutes of Health grants K99-DK-085145 (to R.K.P.B.), R01-DK-053434 (to D.W.P.), and R01-DK-46409 (to L.S.S.). The Metabolic Pathophysiology Core is supported by the Mouse Metabolic Phenotype Center (U24-DK-59637). The Islet Procurement and Analysis Core is supported by the Vanderbilt Diabetes Research and Training Center (P30-DK-20593). Experiments on the LSM5Live were performed in part through the use of Vanderbilt Cell Imaging Shared Resource (CA-68485, DK-20593, DK-58404, HD-15052, DK-59637, and EY-08126). Radioimmunoassays for insulin concentration were performed within the Vanderbilt University Hormone Assay Core (DK-20593).
No potential conflicts of interest relevant to this article were reported.
W.S.H. and M.L.O. researched data. C.S.N. researched data and reviewed and edited the manuscript. L.S.S. designed experiments and reviewed and edited the manuscript. D.W.P. reviewed and edited the manuscript. R.K.P.B. designed experiments, researched data, and wrote the manuscript. D.W.P. and R.K.P.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank David Wasserman (Vanderbilt University) and Owen McGuinness (Vanderbilt University) for helpful comments and suggestions in performing this study and for critical reviewing of this manuscript, Tasneem Ansari (Vanderbilt University) and Carlo Malabanan (Vanderbilt University) in the Metabolic Pathophysiology Core for performing the complex procedures to rapidly measure insulin levels in the conscious mouse, and Anastasia Golvin (Vanderbilt University) in the Islet Procurement and Analysis Core for performing islet perifusion measurements.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1312/-/DC1.
†Deceased.
See accompanying commentary, p. 1656.
REFERENCES
- 1.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med 1979;301:1023–1027 [DOI] [PubMed] [Google Scholar]
- 2.Jaspan JB, Lever E, Polonsky KS, Van Cauter E. In vivo pulsatility of pancreatic islet peptides. Am J Physiol 1986;251:E215–E226 [DOI] [PubMed] [Google Scholar]
- 3.Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am J Physiol Endocrinol Metab 2006;290:E523–E529 [DOI] [PubMed] [Google Scholar]
- 4.Song SH, Kjems L, Ritzel R, et al. Pulsatile insulin secretion by human pancreatic islets. J Clin Endocrinol Metab 2002;87:213–221 [DOI] [PubMed] [Google Scholar]
- 5.Bergsten P. Slow and fast oscillations of cytoplasmic Ca2+ in pancreatic islets correspond to pulsatile insulin release. Am J Physiol 1995;268:E282–E287 [DOI] [PubMed] [Google Scholar]
- 6.Ravier MA, Güldenagel M, Charollais A, et al. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005;54:1798–1807 [DOI] [PubMed] [Google Scholar]
- 7.Nunemaker CS, Dishinger JF, Dula SB, et al. Glucose metabolism, islet architecture, and genetic homogeneity in imprinting of [Ca2+](i) and insulin rhythms in mouse islets. PLoS ONE 2009;4:e8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyachok O, Idevall-Hagren O, Sågetorp J, et al. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab 2008;8:26–37 [DOI] [PubMed] [Google Scholar]
- 9.Nunemaker CS, Zhang M, Wasserman DH, et al. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes 2005;54:3517–3522 [DOI] [PubMed] [Google Scholar]
- 10.Bratusch-Marrain PR, Komjati M, Waldhäusl WK. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes 1986;35:922–926 [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes 1983;32:617–621 [DOI] [PubMed] [Google Scholar]
- 12.Paolisso G, Sgambato S, Torella R, et al. Pulsatile insulin delivery is more efficient than continuous infusion in modulating islet cell function in normal subjects and patients with type 1 diabetes. J Clin Endocrinol Metab 1988;66:1220–1226 [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;54:1649–1656 [DOI] [PubMed] [Google Scholar]
- 14.Goodner CJ, Sweet IR, Harrison HC., Jr Rapid reduction and return of surface insulin receptors after exposure to brief pulses of insulin in perifused rat hepatocytes. Diabetes 1988;37:1316–1323 [DOI] [PubMed] [Google Scholar]
- 15.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med 1988;318:1225–1230 [DOI] [PubMed] [Google Scholar]
- 16.Pørksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes 2002;51(Suppl. 1):S245–S254 [DOI] [PubMed] [Google Scholar]
- 17.Menge BA, Grüber L, Jørgensen SM, et al. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes 2011;60:2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peiris AN, Stagner JI, Vogel RL, Nakagawa A, Samols E. Body fat distribution and peripheral insulin sensitivity in healthy men: role of insulin pulsatility. J Clin Endocrinol Metab 1992;75:290–294 [DOI] [PubMed] [Google Scholar]
- 19.Grubert JM, Lautz M, Lacy DB, et al. Impact of continuous and pulsatile insulin delivery on net hepatic glucose uptake. Am J Physiol Endocrinol Metab 2005;289:E232–E240 [DOI] [PubMed] [Google Scholar]
- 20.Kerner W, Brückel J, Zier H, et al. Similar effects of pulsatile and constant intravenous insulin delivery. Diabetes Res Clin Pract 1988;4:269–274 [DOI] [PubMed] [Google Scholar]
- 21.Benninger RKP, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J 2008;95:5048–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benninger RKP, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol 2011;589:5453–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degen J, Meier C, Van Der Giessen RS, et al. Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol 2004;473:511–525 [DOI] [PubMed] [Google Scholar]
- 24.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 2007;56:1025–1033 [DOI] [PubMed] [Google Scholar]
- 25.Berglund ED, Li CY, Poffenberger G, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 2008;57:1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koster JC, Remedi MS, Flagg TP, et al. Hyperinsulinism induced by targeted suppression of beta cell KATP channels. Proc Natl Acad Sci USA 2002;99:16992–16997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Lacík I, Brissová M, et al. An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol 1997;15:358–362 [DOI] [PubMed] [Google Scholar]
- 28.Rocheleau JV, Remedi MS, Granada B, et al. Critical role of gap junction coupled K-ATP channel activity for regulated insulin secretion. PLoS Biol 2006;4:e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 1986;250:E486–E493 [DOI] [PubMed] [Google Scholar]
- 30.Bavamian S, Klee P, Britan A, et al. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes Metab 2007;9(Suppl. 2):118–132 [DOI] [PubMed] [Google Scholar]
- 31.Charollais A, Gjinovci A, Huarte J, et al. Junctional communication of pancreatic beta cells contributes to the control of insulin secretion and glucose tolerance. J Clin Invest 2000;106:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces beta-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 2007;56:1078–1086 [DOI] [PubMed] [Google Scholar]
- 33.Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992;326:22–29 [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Fendler B, Peercy B, et al. Long lasting synchronization of calcium oscillations by cholinergic stimulation in isolated pancreatic islets. Biophys J 2008;95:4676–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fendler B, Zhang M, Satin L, Bertram R. Synchronization of pancreatic islet oscillations by intrapancreatic ganglia: a modeling study. Biophys J 2009;97:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellman B, Dansk H, Grapengiesser E. Pancreatic beta-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab 2004;286:E759–E765 [DOI] [PubMed] [Google Scholar]
- 37.Lundquist I, Alm P, Salehi A, Henningsson R, Grapengiesser E, Hellman B. Carbon monoxide stimulates insulin release and propagates Ca2+ signals between pancreatic beta-cells. Am J Physiol Endocrinol Metab 2003;285:E1055–E1063 [DOI] [PubMed] [Google Scholar]
- 38.Poitout V, Robertson RP. An integrated view of beta-cell dysfunction in type-II diabetes. Annu Rev Med 1996;47:69–83 [DOI] [PubMed] [Google Scholar]
- 39.Ravier MA, Sehlin J, Henquin JC. Disorganization of cytoplasmic Ca(2+) oscillations and pulsatile insulin secretion in islets from ob/ obmice. Diabetologia 2002;45:1154–1163 [DOI] [PubMed] [Google Scholar]
- 40.Serre-Beinier V, Bosco D, Zulianello L, et al. Cx36 makes channels coupling human pancreatic beta-cells, and correlates with insulin expression. Hum Mol Genet 2009;18:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamelin R, Allagnat F, Haefliger JA, Meda P. Connexins, diabetes and the metabolic syndrome. Curr Protein Pept Sci 2009;10:18–29 [DOI] [PubMed] [Google Scholar]
- 42.Mirbolooki MR, Taylor GE, Knutzen VK, Scharp DW, Willcourt R, Lakey JRT. Pulsatile intravenous insulin therapy: the best practice to reverse diabetes complications? Med Hypotheses 2009;73:363–369 [DOI] [PubMed] [Google Scholar]