Abstract
Preventing activation of diabetogenic T cells is critical for delaying type 1 diabetes onset. The inhibitory molecule lymphocyte activation gene 3 (LAG-3) and metalloprotease tumor necrosis factor-α converting enzyme (TACE) work together to regulate TH1 responses. The aim of this study was to determine if regulating redox using a catalytic antioxidant (CA) could modulate TACE-mediated LAG-3 shedding to impede diabetogenic T-cell activation and progression to disease. A combination of in vitro experiments and in vivo analyses using NOD mouse strains was conducted to test the effect of redox modulation on LAG-3 shedding, TACE enzymatic function, and disease onset. Systemic treatment of NOD mice significantly delayed type 1 diabetes onset. Disease prevention correlated with decreased activation, proliferation, and effector function of diabetogenic T cells; reduced insulin-specific T-cell frequency; and enhanced LAG-3+ cells. Redox modulation also affected TACE activation, diminishing LAG-3 cleavage. Furthermore, disease progression was monitored by measuring serum soluble LAG-3, which decreased in CA-treated mice. Therefore, affecting redox balance by CA treatment reduces the activation of diabetogenic T cells and impedes type 1 diabetes onset via decreasing T-cell effector function and LAG-3 cleavage. Moreover, soluble LAG-3 can serve as an early T-cell–specific biomarker for type 1 diabetes onset and immunomodulation.
In addition to direct cell-mediated killing of β-cells in type 1 diabetes, soluble inflammatory mediators, including cytokines and reactive oxygen species (ROS), often precede the later stages of fulminant β-cell destruction. Regulation of local and systemic redox state affects activation and proliferation of a variety of immune cells and protects tissues/cells from innate and cell-mediated damage (1). On the basis of previous studies showing the importance of ROS in chronic inflammation, our laboratory has used a catalytic antioxidant (CA) to modulate both innate and adaptive immunity in type 1 diabetes. CA is a manganese metalloporphyrin—Mn(III) meso tetrakis (N-alkylpyiridinium-2-yl) porphyrin, MnTE-2-PyP5+—that catalyzes superoxide dismutation, mimicking superoxide dismutase activity (2). CA also scavenges a broad range of ROS, including superoxide, hydrogen peroxide, peroxynitrite, and lipid peroxyl radicals (2–4). CA activity regulates proinflammatory immune processes by decreasing tumor necrosis factor (TNF)-α, interleukin-1β, and ROS synthesis from activated antigen-presenting cells (APCs) (5), likely by inhibition of nuclear factor-κB (NF-κB)–dependent gene transcription and efficient innate immune activation (4). CA also induces CD4+ T-cell antigen–specific hyporesponsiveness (5) and decreases the cytolytic activity of CD8+ T cells (6), delaying islet allograft rejection (7). In the context of type 1 diabetes, diabetogenic BDC-2.5 T-cell clones exhibit impaired diabetes transfer in CA-treated NOD.scid recipient mice (8).
Our previously published work shows that TNF-α secretion is reduced in CA-treated macrophages (5). A disintegrin and metalloproteinase-17, or TNF-α converting enzyme (TACE), is a metalloprotease responsible for cleaving pro–TNF-α from the cell surface. Many metalloproteases, such as TACE, are redox-dependent enzymes, initially formed as latent zymogens that become active upon oxidation of specific Cys residues in their disintegrin/Cys-rich region (9–12). We hypothesize that CA treatment may not only scavenge ROS, decrease proinflammatory cytokine production, and inhibit NF-κB activation but also inhibit TACE, altering the cleavage kinetics of T-cell surface proteins. Support for this hypothesis derives from studies showing that TACE is responsible for the shedding of key transmembrane proteins, such as Notch, epidermal growth factor receptor ligands, CD44, CD62L, and CD223 (lymphocyte activation gene 3 [LAG-3]), making it an essential enzyme in normal immune function (13–18).
LAG-3 is a negative regulator of immune cell activation expressed on activated CD4+ and CD8+ T cells and plasmacytoid dendritic cells (19,20). Upon T-cell receptor (TCR) binding with major histocompatibility complex class II, LAG-3 levels increase on the surface of T cells, resulting in attenuated TCR-dependent T-cell activation and eventual clonal exhaustion (21), possibly by physical competition for major histocompatibility complex interaction (22). LAG-3(−/−) mice have increased T-cell proliferation and interferon (IFN)-γ cytokine production (21), and antibody-mediated LAG-3 blockade results in enhanced CD69 expression and T-cell differentiation (23). Recent studies (24,25) report that LAG-3(−/−) NOD mice demonstrate accelerated spontaneous diabetes, further indicating a potential immunoregulatory function of LAG-3. Soluble LAG-3 (sLAG-3) is a surrogate measure of TACE activity (9,16) and an additional marker of T-cell activation (26,27). Indeed, serum levels of sLAG-3 are considered biomarkers of T-cell activation in breast cancer (26). Therefore, in the context of type 1 diabetes, sLAG-3 could serve as a surrogate marker of autoreactive T-cell activation as well as a predictive biomarker of diabetes progression from preclinical to clinical disease.
In this study, we demonstrate the effects of CA treatment on the TACE redox state, coupled with LAG-3 expression and T-cell activation, to promote autoreactive T-cell hyporesponsiveness and reduce type 1 diabetes onset.
RESEARCH DESIGN AND METHODS
Materials.
NOD.BDC-2.5.TCR.Tg, NOD, and NOD.scid mice were bred and housed under specific pathogen-free conditions in the Animal Facility of Rangos Research Center at Children’s Hospital of Pittsburgh of University of Pittsburgh School of Medicine (UPMC). Female mice aged 4–10 weeks were used in all experiments. All animal experiments were approved by the institutional animal care and use committee of the Children’s Hospital of Pittsburgh and were in compliance with the laws of the U.S. LAG-3-PE (C9B7 W) (eBioscience, San Diego, CA), goat anti-mLAG-3 (R&D Systems, Minneapolis, MN), anti-mTbet (4B10) (Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-mTACE (Abcam, Cambridge, MA) were used for flow cytometry and Western blots. Antibody pairs for IFN-γ enzyme–linked immunosorbent assays (ELISAs) and CD4-APC were purchased from BD Biosciences (San Diego, CA). MnTE-2 CA was a gift from James Crapo, MD, at National Jewish Health. CA was prepared as previously described (5) and used at 68 μmol/L in all in vitro experiments.
CA pellet implantation and spontaneous type 1 diabetes assessment.
NOD female mice were implanted with a 14-day sustain-release CA pellet (2.1 mg/kg/day) subcutaneously at the nape of the neck. Control animals were left untreated. Animals were reimplanted with CA pellets every 2 weeks until age 29 weeks. Spontaneous type 1 diabetes incidence was monitored by blood glucose starting at 12 weeks of age. Overt diabetes was defined as two consecutive readings >300 mg/dL.
In vitro T-cell assay.
BDC-2.5.TCR.Tg splenocytes from mice aged 6–8 weeks were seeded in 96-well round-bottom plates or 12-well plates with 0.5–1 μmol/L BDC-2.5 mimotope (M) (EKAHRPIWARMDAKK) (28) in supplemented Dulbecco’s modified Eagle’s medium (5) (Invitrogen Life Technologies). TAPI-1 (Calbiochem, Darmstadt, Germany) was supplemented daily at 4 μmol/L as indicated. At 24–96 h poststimulation, cells were collected for flow cytometry or for preparation of whole-cell lysates. Supernatants were harvested for ELISA.
Surface staining and flow cytometric analysis.
Cells were stained as previously described (6). Fluorescence was measured on a FACSAria (BD Biosciences). Flow cytometric analysis was done using FlowJo Software version 6.4 (Tree Star, Ashland, OR). All samples were gated on CD4+ cells. Fold change was calculated as (Control/No antigen)/(CA/No antigen).
Cytokine measurements by ELISA.
sLAG-3 ELISAs were performed as described (16). IFN-γ ELISAs were performed according to manufacturer’s instructions (BD Bioscience). IFN-γ and sLAG-3 ELISAs were read on a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA), and data were analyzed using SoftMax Pro version 5.4.2 (Molecular Devices).
Insulin immunization for LAG-3 detection.
One day prior to immunization, 28 NOD mice were treated with CA (10 mg/kg i.p.) or Hanks’ balanced salt solution (HBSS). Mice were injected with 50 μg insulin emulsified in complete Freund’s adjuvant (CFA) subcutaneously at the base of the tail and treated intraperitoneally for 7 days. On days 0.5, 1, 2, 3, 4, 6, and 8, inguinal lymph nodes (LNs) from two mice per group were harvested for flow cytometry of LAG-3. Nonimmunized mice served as negative controls.
Intracellular cytokine staining and ELISPOT assay.
NOD insulin immunization plus or minus CA was conducted as above. Primary intracellular IFN-γ was detected in inguinal LN cells isolated 6 days after insulin immunization. After surface staining for CD4, LN cells were prepared as described (6), stained with APC-labeled mouse anti–IFN-γ (BD Biosciences) or isotype controls, and analyzed by flow cytometry. Antigen recall enzyme-linked immunosorbent spot (ELISPOT) assays were also conducted 6 days after immunization using LN cells (2.5 × 105 in triplicate) seeded in IFN-γ–precoated strips from Mabtech (Cincinnati, OH) with 25 μg insulin. After 2 days of incubation at 37°C in a 5% CO2 humid air chamber, plates were developed following the manufacturer’s instructions. Frequency = 2.5 × 105/average number of spots per treatment.
Preparation of cell lysates and Western blotting.
Whole-cell lysates from BDC-2.5.TCR.Tg splenocytes were prepared as described (29). Membrane lysates were obtained by lysis in 50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl buffer supplemented with inhibitor cocktails, centrifugation at 72,000g for 30’, removal of supernatants, and sonication with Tris buffer plus 1% NP-40. Protein concentration of all lysates was determined by bicinchoninic acid protein assay (Thermo Fisher Scientific, Rockford, IL). Protein lysates were separated on 8% or 4–20% (TACE) SDS–PAGE gels. Western blots were performed as described (4) with antibodies to LAG-3 (1:1,300), Tbet (1:1,000), TACE (1:2,000), and β-actin (1:10,000) in 5% BSA in Tris-buffered saline with Tween. Secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Chemiluminescence was detected using ECL Plus reagent (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). Blots were analyzed using Fujifilm LAS-4000 imager and Multi Gauge software (Fujifilm Life Science, Tokyo, Japan).
sLAG-3 immunoprecipitation.
Supernatants from BDC-2.5.TCR.Tg splenocyte stimulations were concentrated in 30 K Amicon Ultra-15 centrifugal filter units (Millipore). Samples >30 K were immunoprecipitated using 1 μg LAG-3 antibody as described (29). Western blot was performed as above.
In vitro TACE fluorogenic assay.
BDC-2.5.TCR.Tg splenocytes were stimulated for 24 h plus or minus M in the presence of CA or 200 μmol/L TAPI in 96-well black fluorescence plates. TACE-specific fluorogenic substrate (Mca-P-L-A-Q-A-V-Dpa-R-S-S-S-R-NH2, Fluorogenic Peptide Substrate III; R&D Systems) diluted to 10 μmol/L in 50 mmol/L Tris buffer, pH 9.0, was added for 6 h at 37°C. Fluorescence was read at an excitation of 320 nmol/L and emission of 405 nmol/L. The average fold change in activity = stimulated/unstimulated vs. stimulated + CA/unstimulated vs. stimulated + TAPI/unstimulated cells.
Adoptive transfer of diabetes.
One day prior to adoptive transfer, six NOD.scid mice were treated with CA (10 mg/kg i.p.) or left untreated. BDC-2.5.TCR.Tg splenocytes were adoptively transferred (107/mouse) intravenously into NOD.scid recipients on day 0. Mice were treated daily with CA. Serum was collected every 4 days posttransfer for sLAG-3 ELISA. Mice positive for glucosuria after daily urinalysis were monitored by blood glucose levels, and overt diabetes was determined as above. Mice were monitored for disease onset up to 28 days posttransfer, when splenocytes were isolated for in vitro analysis.
Statistical analysis.
The difference between mean values was assessed by Student t test, with P < 0.05 considered significant. All experiments were performed at least three times with data obtained in triplicate in each experiment. For LAG-3 flow cytometry analysis, data are representative of at least three independent experiments, and fold change of expression is calculated as indicated. Data are mean ± SEM. Survival analysis was done using the product-limit (Kaplan-Meier) method with the end point defined as disease. Data on animals that did not develop type 1 diabetes were censored. The P values were determined by log-rank test.
RESULTS
CA treatment delays spontaneous diabetes.
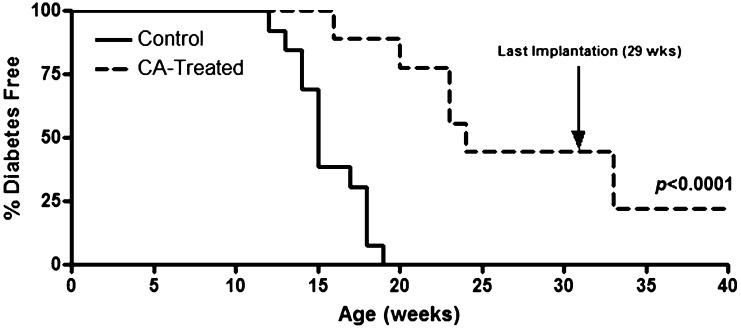
CA treatment disrupts innate immune-mediated proinflammatory signals (5) and delays islet allograft rejection (7), prompting us to determine the effects of its long-term administration on type 1 diabetes onset. NOD females (aged 4 weeks) implanted with CA pellets demonstrated delayed diabetes onset compared with control mice (P < 0.0001). Furthermore, stopping CA pellet implantation at 29 weeks afforded protection against diabetes until 40 weeks of age (Fig. 1), suggesting that redox modulation imparts inhibition of autoreactive processes and delays end-organ autoimmunity.
FIG. 1.
Spontaneous diabetes is reduced upon systemic CA treatment. NOD females (n = 7) were implanted with a 14-day sustain-release CA pellet (2.1 mg/kg/day) biweekly, and control NOD mice (n = 14) were left untreated. Pellet implantation was stopped at 29 weeks of age. Diabetes was monitored by blood glucose, with two consecutive readings of >300 mg/dL indicating overt disease.
Redox modulation decreases TH1 effector function.
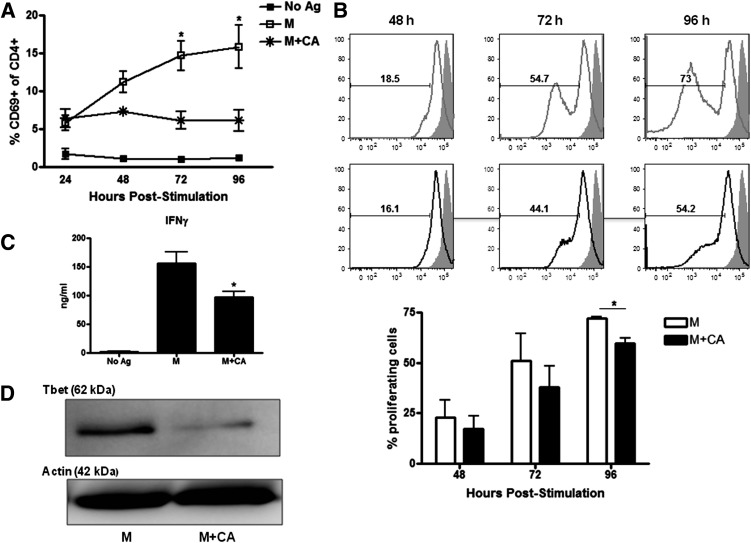
TH1-like T cells play a key role in mediating type 1 diabetes (30,31). To mechanistically determine how modulation of the redox state affects diabetogenic CD4+ TH1 adaptive immune effector responses, BDC-2.5.TCR.Tg splenocytes were stimulated plus or minus M with or without CA in vitro. CA treatment diminished T-cell activation, shown by decreased frequency of CD4+CD69+ cells (P < 0.05 at 72 and 96 h) (Fig. 2A) and reduced CD4+ T-cell proliferation (P < 0.05 at 96 h) (Fig. 2B). In conjunction with previous results (5), redox modulation significantly lowered IFN-γ production (P < 0.05) as well as reduced Tbet protein expression (Fig. 2C and D). These data indicate that CA diminishes T-cell activation and TH1 effector function, likely contributing to the diabetes protection observed above (Fig. 1).
FIG. 2.
Redox modulation promotes a reduction in T-cell activation and effector function. A: BDC-2.5.TCR.Tg splenocytes were left untreated or stimulated with M ± CA. At 24–96 h, cells were stained for and gated on CD4+ cells, and CD69 was analyzed by flow cytometry (n = 3 independent experiments). B: Carboxyfluorescein succinimidyl ester (CFSE)-labeled splenocytes were treated with M ± CA. At 48–96 h, cells were stained and gated on CD4+CFSE+ cells. Gray lines, M; black lines, M + CA; filled histograms, CFSE+ cells at time point 0; % proliferating cells, average of three independent experiments. C: Supernatants from 96-h cultures were used in an IFN-γ ELISA (n = 3 independent experiments performed in triplicate). D: Whole-cell lysates from 96-h cultures were probed for Tbet by Western blot. Actin was probed as a loading control. Data are representative of three independent experiments. *P < 0.05.
CA treatment limits antigen-specific T-cell frequency.
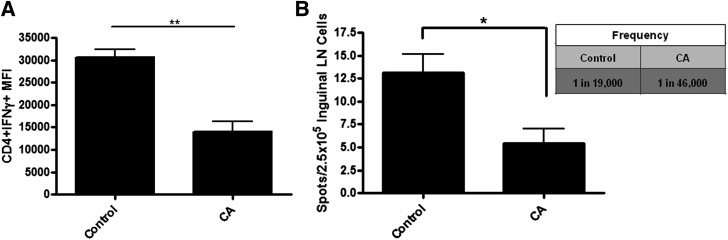
To determine if CA treatment affected the frequency of antigen-specific TH1 cells in vivo, we immunized NOD mice (aged 6–8 weeks) with a known autoantigen, insulin, and used inguinal LN cells on day 6 for primary intracellular IFN-γ detection and recall ELISPOT assay (Fig. 3). IFN-γ–expressing CD4+ T cells were reduced after CA treatment compared with control animals (P < 0.005) (Fig. 3A). Furthermore, LN cells from CA-treated animals displayed decreased IFN-γ–secreting cells compared with control animals (P < 0.05), with a significant reduction in insulin-specific effector function after recall stimulation (Fig. 3B). The frequency of antigen-specific cells in control animals was ∼1 in 19,000, whereas in CA-treated animals, the frequency diminished to 1 in 46,000. These results suggest that redox modulation disrupts insulin-specific T cells, which may lead to delays in autoimmune-mediated β-cell destruction and type 1 diabetes onset.
FIG. 3.
CA treatment reduces insulin-specific T-cell effector function and frequency. NOD mice were treated with CA or HBSS daily. Mice were immunized with insulin in CFA. A: Inguinal LNs were removed at day 6 postimmunization and surface stained for CD4 as well as intracellularly stained for IFN-γ for flow cytometric analysis. Cells were gated on CD4+ cells (n = 3 independent experiments with two mice per group). **P < 0.005. B: Inguinal LNs were isolated on day 6 and stimulated with insulin in a recall IFN-γ ELISPOT. Two days after stimulation, ELISPOT plates were developed, and spots were counted using the Zeiss KS Elispot Imaging system. Frequency = 2.5 × 105/average number of spots per treatment. Graph shows the average of three independent experiments performed in triplicate. *P < 0.05. MFI, mean fluorescence intensity.
CD4+LAG-3+ T-cell frequency is enhanced after CA treatment.
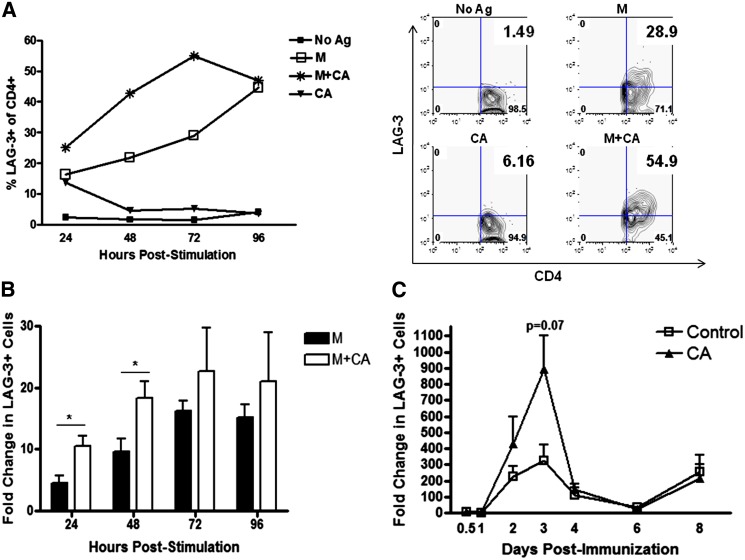
LAG-3 is important in negatively regulating T-cell responses and, thus, may play a role in mediating decreased T-cell activation after CA administration (21,32,33). We first measured LAG-3+ T-cell frequency after M plus or minus CA stimulation of BDC-2.5.TCR.Tg splenocytes in vitro. M plus CA treatment resulted in a higher frequency of LAG-3+ T cells compared with samples stimulated with M alone (Fig. 4A). The mean fluorescence intensity of LAG-3 did not differ between groups (data not shown). Because LAG-3 is not constitutively expressed (34,35), unstimulated cells plus or minus CA treatment expectedly demonstrated low LAG-3+ T-cell frequencies. Upon quantification of the in vitro results, the fold change in LAG-3+ T-cell frequency reached significance at 24 and 48 h (P < 0.05) poststimulation (Fig. 4B), indicating kinetically delayed T cells and decreased TH1 activation.
FIG. 4.
LAG-3+ T-cell frequency is enhanced upon stimulation plus CA. BDC-2.5.TCR.Tg splenocytes were left untreated or stimulated with M ± CA in vitro. A: At 24–96 h, cells were stained and gated on CD4+, and LAG-3 expression was analyzed by flow cytometry. Graph is representative of four independent experiments. Dot blots are representative of 72-h stimulation. B: Fold change in percent CD4+LAG-3+ was calculated as Ag/No Ag vs. Ag+CA/No Ag and averaged from the four in vitro independent experiments represented in A. *P < 0.05. C: NOD mice were split into two groups (n = 14/group) and treated daily with CA or HBSS as a control. On day 0, mice were immunized with insulin in CFA. Inguinal LNs were removed and pooled at the indicated days postimmunization (days 0.5, 1, 2, 3, 4, 6, and 8) from two mice per group and stained for and gated on CD4+ cells, and LAG-3 was analyzed by flow cytometry. Graph shows the average of three independent experiments. Ag, antigen. (A high-quality color representation of this figure is available in the online issue.)
We next looked in vivo for LAG-3 kinetics after insulin immunization and CA administration of NOD mice (aged 6–8 weeks). As shown by others (16,36–39) and similar to our in vitro results (Fig. 4A and B), control-treated animals exhibited a lower peak of LAG-3+ cells by day 3 postimmunization in comparison with CA-treated mice; however, redox modulation demonstrated an enhanced trend toward LAG-3+ cells at day 3 postimmunization (P = 0.07) (Fig. 4C). No difference in LAG-3 mean fluorescence intensity was seen between the groups (data not shown). These data demonstrate CA treatment can affect LAG-3+CD4+ T-cell frequency in vivo, albeit not to significance, suggesting slight obstruction of T-cell activation after autoantigen immunization in the presence of redox modulation.
LAG-3 shedding is reduced upon CA treatment.
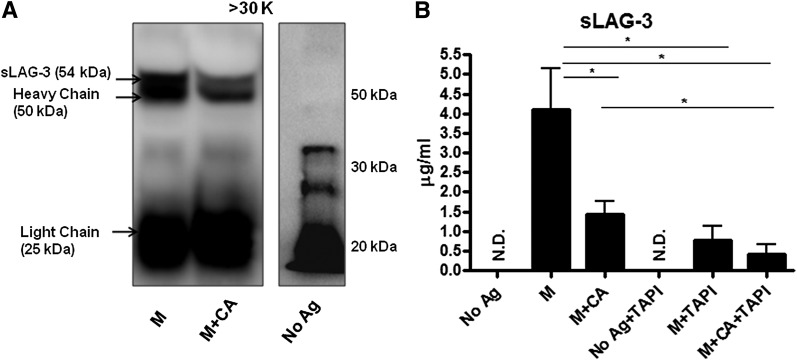
In addition to surface levels, sLAG-3 was also analyzed after M stimulation of BDC-2.5.TCR.Tg splenocytes. LAG-3 contains four extracellular domains (D1–D4), a C-peptide region, a transmembrane domain (16), and a cytoplasmic tail (27). Within the immunological synapse, an antigen-mediated respiratory burst activates TACE by oxidizing Cys522 and Cys600 to release the TACE prodomain (10,12). Active TACE then cleaves the 70-kDa full-length LAG-3 within the C-peptide, shedding D1 through D4 domains, a 54 kDa fragment (16). sLAG-3 shed into the serum can be measured as a marker of T-cell activation (26). Immunoprecipitation of BDC-2.5.TCR.Tg splenocyte culture supernatants demonstrated reduced sLAG-3 after CA treatment compared with control samples (Fig. 5A), illustrating decreased LAG-3 cleavage upon redox modulation. It is notable that sLAG-3 was undetectable in the no antigen sample, which did not undergo antigenic stimulation. From these results, we postulate that CA can reduce LAG-3 shedding by modulating TACE enzymatic function.
FIG. 5.
sLAG-3 protein is decreased after CA treatment. A: Supernatants from BDC-2.5.TCR.Tg splenocytes left untreated or stimulated with M ± CA for 48 h were concentrated using Amicon Ultra Centrifugal Filters at a 30-kDa (K) cutoff. The >30-kDa portion was then immunoprecipitated with anti–LAG-3 antibody, separated on an SDS-PAGE gel, and probed for LAG-3 by Western blot. Data representative of three independent experiments. B: BDC-2.5.TCR.Tg splenocytes were left untreated or stimulated with M ± CA or TAPI-1 for 72 h. Supernatants were collected and used in sLAG-3 ELISAs. Graph shows the average of four independent experiments performed in triplicate. *P < 0.05. Ag, antigen; N.D., none detected.
CA exposure reduces TACE levels and enzymatic activity.
We next wanted to test the dependence, as a result of T-cell activation, of redox-mediated TACE modifications on LAG-3 cleavage. M plus CA decreased sLAG-3 levels in comparison with M alone (P < 0.05) as detected by ELISA (Fig. 5B) and consistent with Fig. 5A. To attribute the reduction of LAG-3 shedding to CA-regulated TACE, we compared M versus M plus CA versus M plus TAPI, a known TACE inhibitor. Both CA and TAPI reduced the amount of detectable sLAG-3 to a similar extent (P < 0.05) in comparison with M alone. Furthermore, a comparison between M plus TAPI and M plus CA and TAPI demonstrated no significant difference in reducing sLAG-3 levels. These data suggest that CA treatment is likely inhibiting TACE-dependent LAG-3 cleavage.
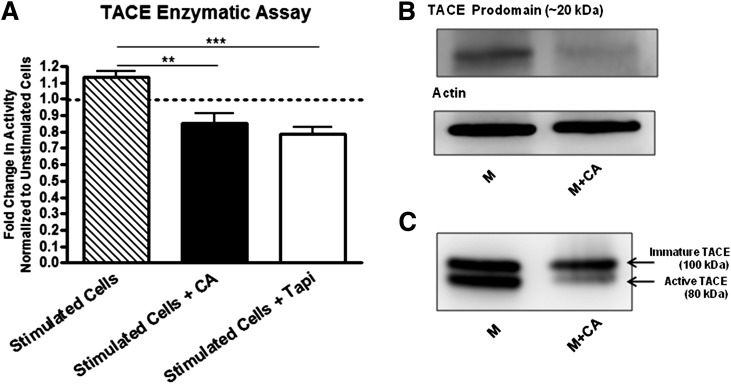
We also wanted to determine whether CA treatment specifically decreased TACE enzymatic activity. Using BDC-2.5.TCR.Tg splenocytes in an in vitro TACE-specific fluorogenic assay, enzymatic activity in CA-treated cells was significantly decreased compared with M-stimulated cells (P < 0.005) (Fig. 6A). As a positive control, TAPI-treated cells also demonstrated a significant reduction in TACE activity (P < 0.0005). To delineate whether the difference in enzymatic activity corresponded with decreased protein levels of TACE, we performed Western blots for the TACE prodomain and active isoforms. TACE is formed as a latent/inactive enzyme containing a disulfide linkage, whereby oxidation of the bond promotes autocatalytic cleavage of the prodomain (20 kDa) from the active subunit (80 kDa) (10,12). Under CA exposure, TACE prodomain was reduced compared with control samples (Fig. 6B), indicating less oxidation of the critical Cys switch and likely resulting in decreased enzymatic activity. Membrane lysates also exhibited diminished levels of active TACE after CA treatment (Fig. 6C). It is interesting that immature TACE was also reduced upon redox modulation, suggesting less overall activation-induced expression of TACE (10,40).
FIG. 6.
Redox modulation diminishes active TACE levels and enzymatic function. A: BDC-2.5.TCR.Tg splenocytes were stimulated with M ± CA ± TAPI for 24 h and supplemented with TACE-specific fluorogenic substrate. Fluorescence was measured at 6 h post substrate addition. The fold change in activity was calculated by stimulated/unstimulated vs. stimulated + CA/unstimulated vs. stimulated + TAPI/unstimulated cells. Graph shows the average of three independent experiments performed in triplicate. **P < 0.005, ***P < 0.0005. B and C: BDC-2.5.TCR.Tg splenocytes were stimulated with M ± CA for 72 h and probed for TACE by Western blot. Whole-cell lysates were used in B. Actin was probed as a loading control. Membrane lysates were used in C. Data are representative of three independent experiments.
CA treatment prevents diabetes transfer in correlation with reduced sLAG-3 serum levels.
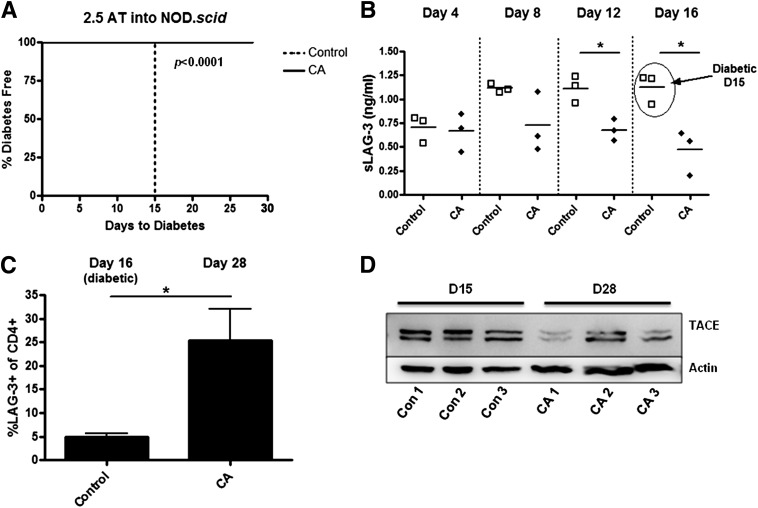
Lastly, we monitored LAG-3 in conjunction with diabetes progression upon adoptive transfer of disease. Measurement of sLAG-3 has been used as an index of breast cancer prognosis, with greater levels corresponding to better antitumor cytotoxic T-cell responses and patient survival (26). Underlying these observations is an increase in T-cell activation and, therefore, we propose that diabetogenic T-cell activation can be indirectly ascertained by serum sLAG-3. To assess this possibility, NOD.scid mice (aged 10 weeks) were adoptively transferred with BDC-2.5.TCR.Tg splenocytes and treated daily with CA. Control animals all developed diabetes by day 15 posttransfer, whereas CA-treated mice remained disease free until the end of the study at day 28 (P < 0.0001) (Fig. 7A). Furthermore, serum levels of sLAG-3 steadily increased in control animals over time, yet sLAG-3 from CA-treated mice was significantly lower at days 12 and 16 posttransfer (P < 0.05) (Fig. 7B). Splenocytes were isolated either at diabetes onset (day 16) or at the end of the experiment (day 28) and stained for LAG-3. CA-treated animals had a higher frequency of LAG-3+CD4+ T cells versus control animals (P < 0.05) (Fig. 7C). In addition, in vivo CA treatment decreased active TACE protein levels compared with control animals (Fig. 7D). sLAG-3, therefore, serves as a biomarker of type 1 diabetes progression and correlates with enhanced LAG-3+ T cells, decreased active TACE levels, and inhibition of disease after CA treatment.
FIG. 7.
Redox modulation delays diabetes onset, which correlates with decreased sLAG-3 and enhanced LAG-3+ cells. NOD.scid mice (n = 3/group) were treated on day −1 through day 28 intraperitoneally with or without CA. BDC-2.5.TCR.Tg splenocytes were transferred intravenously on day 0. A: Mice were monitored by glucosuria for the onset of type 1 diabetes, with two consecutive blood glucose readings of >300 mg/dL indicating diabetes. AT, adoptive transfer. B: Blood was collected retro-orbitally every 4 days to day 16, and serum was isolated for sLAG-3 ELISA. *P < 0.05. C: On days 16 and 28 posttransfer, splenocytes were stained for and gated on CD4+ cells. LAG-3 was detected by flow cytometry. Graph shows the average of three mice per group. *P < 0.05. D: Lysates were made from splenocytes and ran on an SDS-PAGE gel. Western blots were performed to measure TACE expression.
DISCUSSION
Because CA treatment directly affects innate immune cells and proinflammatory third signal synthesis (5) as well as NF-κB and NF-κB–dependent gene transcription (4), we sought to understand how modulating redox balance could influence activation and function of diabetogenic TH1 cells. In particular, we hypothesized that CA administration would decrease TACE-dependent LAG-3 shedding, leading to autoreactive T-cell hyporesponsiveness and reduced type 1 diabetes.
In this study, long-term modulation of the redox state resulted in significantly delayed type 1 diabetes onset, illustrating the importance of ROS in promoting autoreactive immune responses. However, stopping the CA treatment at 29 weeks does not seem to afford absolute enduring protection against disease onset. This may be due to blood clearance of the modulator as a result of troughing of the CA level below the effective concentration and, consequently, loss of therapeutic efficacy. Under this circumstance, CA treatment alone may require chronic administration to inhibit diabetes onset; however, CA administration in combination with an antigen-specific therapeutic approach targeting self-reactive T cells might afford long-lasting protection by inducing T-cell–specific ignorance. Nonetheless, CA treatment has marked effects on early T-cell responses, resulting in a delay in diabetes onset.
BDC-2.5.TCR.Tg T cells demonstrated decreased activation, proliferation, and effector function upon CA treatment, which correlated with enhanced LAG-3+CD4+ T cells in vitro and a trend toward significant increases in vivo. Absence of T-cell activation coupled with greater LAG-3+CD4+ T-cell frequency indicates two possible consequences of redox modulation: 1) less activation/progression to effector function of antigen-specific autoreactive T cells and/or 2) obstruction of LAG-3 shedding. It is notable that T cells from insulin-immunized mice exhibited reduced TH1 effector responses (decreased IFN-γ synthesis and a lower frequency of antigen-specific T cells), suggesting that regulation of LAG-3 may be responsible for this phenomenon. With recent reports showing accelerated diabetes in LAG-3(−/−) NOD mice (24,25), our redox modulation results may be reflective of T-cell ignorance (41,42). Diabetogenic T cells in NOD mice already have an advantage of efficiently expanding from a greatly reduced precursor pool (43). If CA delays or prevents the autoantigen-specific T-cell pool necessary for reaching the threshold at which a break in tolerance to self-antigen occurs, disease onset should be reduced.
LAG-3 abates T-cell responses and is regulated through cleavage via redox-dependent TACE (16,21–23,32,38). CA was able to affect TACE activation and enzymatic activity, thereby leading to decreased sLAG-3. Immature TACE levels were also reduced after treatment. Because oxidants activate signaling kinases and GTPases to drive the expression of metalloproteases (10,40), scavenging of oxidants by CA (2–4) may inhibit proper signal transduction and cause decreased expression of immature TACE. Another metalloprotease, a disintegrin and metalloproteinase-10, unlike TACE, is responsible for constitutive LAG-3 shedding (16) and may also be partially inhibited by CA treatment (9–11,15). However, our experiments using TAPI blocked LAG-3 shedding by both metalloproteases, ruling out enzyme-specific differences (44). Both TAPI and CA demonstrated similar reductions in sLAG-3 versus M alone, supporting the notion that CA can directly modify TACE-dependent cleavage.
In addition to LAG-3, TACE, and IFN-γ, we have previously identified NF-κB as another target of redox modulation (4). Of note, redox-dependent NF-κB is a predicted transcription factor responsible for LAG-3 expression (SABiosciences’ Text Mining Application). Therefore, it is tempting to speculate that new LAG-3 protein synthesis is retarded as a result of CA-mediated NF-κB inhibition and, hence, contributes to the reduction in sLAG-3 observed. Furthermore, it may be possible that redox-modulated cells are functioning as suppressive T cells that retain their surface expression of LAG-3 (39,45,46). Collectively, our results also propose that an overall delay in T-cell activation kinetics may be the main cause of these anomalies. Impaired TACE activity, via LAG-3, offers new insights into a novel mechanism of autoreactive T-cell activation thus far unknown, and redox-dependent modifications together can contribute and feed forward to prevent diabetogenic immune responses.
sLAG-3 shedding coincides with in vivo staphylococcal enterotoxin B–mediated T-cell activation (27) as well as T-cell activation in breast cancer screens (26). At present, there is a paucity of serum biomarkers that measure T-cell activation before overt diabetes (47), and little is known about the endogenous role of LAG-3 in autoimmune settings. Therefore, we wanted to determine whether we could monitor diabetes progression via serum sLAG-3. Redox modulation prevented type 1 diabetes onset in the rapid adoptive transfer model, similar to previous reports (8), but more importantly, sLAG-3 directly correlated with T-cell activation and autoimmunity. Serum sLAG-3 was enhanced in control animals before and upon disease onset, suggesting greater activation of diabetogenic T cells. sLAG-3, therefore, may serve as a T-cell–specific diagnostic marker for initiation of β-cell destruction, and along with surface LAG-3 expression, may additionally function as surrogates of immunomodulation. Therapies that use prophylactic drugs as well as attempts at reversal of autoimmunity may result in the modification of adaptive immune responses, and measurement of sLAG-3 would allow for determination of treatment efficacy on autoreactive T cells in a noninvasive manner.
Taken together, our data suggest that redox modulation arrests LAG-3 shedding by impeding expression kinetics and decreasing TACE activity. Through redox manipulation, LAG-3 surface expression is maintained at levels adequate to attenuate TCR-mediated TH1 cell activation and effector function. This would be beneficial for maintaining diabetogenic effector cells in a quiescent state or in preventing their activation entirely. In concert with the recently reported mechanisms of direct and indirect actions of CA on immune cells and diabetes progression (1,4–8), the identification of LAG-3–mediated immunoregulation adds another layer to the control of autoimmunity. Our discovery also supports the use of sLAG-3 as a novel surrogate marker of type 1 diabetes progression in preclinical situations and possibly as a means to monitor the effectiveness of T-cell–directed immunotherapy.
ACKNOWLEDGMENTS
This work was supported by funding from the following grants and organizations: National Institutes of Health (NIH; 1T32-AI-089443-01 and 5T32-AI-089443-02 to M.M.D.), American Diabetes Association (CDA 7.07 CD-16 to J.D.P.), NIH (AI-39480 to D.A.A.V.), National Cancer Institute Comprehensive Cancer Center Support CORE Grant (CA-21765 to D.A.A.V.), and the America Lebanese Syrian Associated Charities (to D.A.A.V.).
D.A.A.V. and C.J.W. have submitted patents that are pending and are entitled to a share in net income generated from licensing of these patent rights for commercial development. No other potential conflicts of interest relevant to this article were reported.
M.M.D. researched data and wrote the manuscript. A.J.S. researched data. M.M.T. and D.A.A.V. contributed to discussion and reviewed and edited the manuscript. C.J.W. contributed to discussion. J.D.P. contributed to the design of the experiments and reviewed and edited the manuscript. J.D.P., an established investigator in modulating redox balance to combat type 1 diabetes, is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the Children’s Hospital of Pittsburgh of UPMC’s Immunogenetics Flow Cytometry Core for helping with flow cytometry collection and analyses. The authors also thank Gina M. Coudriet, PhD, and Meghan L. Marré, PhD (Children’s Hospital of Pittsburgh of UPMC) and Hubert M. Tse, PhD, University of Alabama at Birmingham, for critical review of the manuscript.
REFERENCES
- 1.Delmastro MM, Piganelli JD. Oxidative stress and redox modulation potential in type 1 diabetes. Clin Dev Immunol 2011;2011:593863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batinić-Haberle I, Benov L, Spasojević I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem 1998;273:24521–24528 [DOI] [PubMed] [Google Scholar]
- 3.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic Biol Med 1999;26:730–736 [DOI] [PubMed] [Google Scholar]
- 4.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med 2004;36:233–247 [DOI] [PubMed] [Google Scholar]
- 5.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol 2007;178:908–917 [DOI] [PubMed] [Google Scholar]
- 6.Sklavos MM, Tse HM, Piganelli JD. Redox modulation inhibits CD8 T cell effector function. Free Radic Biol Med 2008;45:1477–1486 [DOI] [PubMed] [Google Scholar]
- 7.Sklavos MM, Bertera S, Tse HM, et al. Redox modulation protects islets from transplant-related injury. Diabetes 2010;59:1731–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piganelli JD, Flores SC, Cruz C, et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 2002;51:347–355 [DOI] [PubMed] [Google Scholar]
- 9.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med 2008;29:258–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 2004;37:768–784 [DOI] [PubMed] [Google Scholar]
- 11.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A 1990;87:5578–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J Immunol 2009;182:2449–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becherer JD, Blobel CP. Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs). Curr Top Dev Biol 2003;54:101–123 [DOI] [PubMed] [Google Scholar]
- 14.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997;385:729–733 [DOI] [PubMed] [Google Scholar]
- 15.Black RA, White JM. ADAMs: focus on the protease domain. Curr Opin Cell Biol 1998;10:654–659 [DOI] [PubMed] [Google Scholar]
- 16.Li N, Wang Y, Forbes K, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J 2007;26:494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss ML, Jin SL, Becherer JD, et al. Structural features and biochemical properties of TNF-alpha converting enzyme (TACE). J Neuroimmunol 1997;72:127–129 [DOI] [PubMed] [Google Scholar]
- 18.Moss ML, White JM, Lambert MH, Andrews RC. TACE and other ADAM proteases as targets for drug discovery. Drug Discov Today 2001;6:417–426 [DOI] [PubMed] [Google Scholar]
- 19.Bruniquel D, Borie N, Hannier S, Triebel F. Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics 1998;48:116–124 [DOI] [PubMed] [Google Scholar]
- 20.Workman CJ, Wang Y, El Kasmi KC, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol 2009;182:1885–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol 2003;33:970–979 [DOI] [PubMed] [Google Scholar]
- 22.Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol 1995;25:2718–2721 [DOI] [PubMed] [Google Scholar]
- 23.Maçon-Lemaître L, Triebel F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology 2005;115:170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazaki T, Okazaki IM, Wang J, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 2011;208:395–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bettini M, Szymczak-Workman AL, Forbes K, et al. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. J Immunol 2011;187:3493–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triebel F, Hacene K, Pichon MF. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett 2006;235:147–153 [DOI] [PubMed] [Google Scholar]
- 27.Li N, Workman CJ, Martin SM, Vignali DA. Biochemical analysis of the regulatory T cell protein lymphocyte activation gene-3 (LAG-3; CD223). J Immunol 2004;173:6806–6812 [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K, Martin T, Yamamoto K, et al. Evidence for shared recognition of a peptide ligand by a diverse panel of non-obese diabetic mice-derived, islet-specific, diabetogenic T cell clones. Int Immunol 2002;14:1439–1447 [DOI] [PubMed] [Google Scholar]
- 29.Coudriet GM, He J, Trucco M, Mars WM, Piganelli JD. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: implications for inflammatory mediated diseases. PLoS ONE 2010;5:e15384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol 2005;87:123–162 [DOI] [PubMed] [Google Scholar]
- 31.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145–173 [DOI] [PubMed] [Google Scholar]
- 32.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol 2004;172:5450–5455 [DOI] [PubMed] [Google Scholar]
- 33.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol 2002;169:5392–5395 [DOI] [PubMed] [Google Scholar]
- 34.Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 2007;117:3383–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur J Immunol 2002;32:2255–2263 [DOI] [PubMed] [Google Scholar]
- 36.Scala E, Carbonari M, Del Porto P, et al. Lymphocyte activation gene-3 (LAG-3) expression and IFN-gamma production are variably coregulated in different human T lymphocyte subpopulations. J Immunol 1998;161:489–493 [PubMed] [Google Scholar]
- 37.Woo SR, Li N, Bruno TC, et al. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. Eur J Immunol 2010;40:1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol 2005;174:688–695 [DOI] [PubMed] [Google Scholar]
- 39.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity 2004;21:503–513 [DOI] [PubMed] [Google Scholar]
- 40.Thomas P, Khokha R, Shepherd FA, Feld R, Tsao MS. Differential expression of matrix metalloproteinases and their inhibitors in non-small cell lung cancer. J Pathol 2000;190:150–156 [DOI] [PubMed] [Google Scholar]
- 41.Adelstein S, Pritchard-Briscoe H, Anderson TA, et al. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science 1991;251:1223–1225 [DOI] [PubMed] [Google Scholar]
- 42.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol 2010;11:21–27 [DOI] [PubMed] [Google Scholar]
- 43.Serreze DV, Johnson EA, Chapman HD, et al. Autoreactive diabetogenic T-cells in NOD mice can efficiently expand from a greatly reduced precursor pool. Diabetes 2001;50:1992–2000 [DOI] [PubMed] [Google Scholar]
- 44.Tousseyn T, Thathiah A, Jorissen E, et al. ADAM10, the rate-limiting protease of regulated intramembrane proteolysis of Notch and other proteins, is processed by ADAMS-9, ADAMS-15, and the gamma-secretase. J Biol Chem 2009;284:11738–11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamura T, Fujio K, Shibuya M, et al. CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci U S A 2009;106:13974–13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haudebourg T, Dugast AS, Coulon F, Usal C, Triebel F, Vanhove B. Depletion of LAG-3 positive cells in cardiac allograft reveals their role in rejection and tolerance. Transplantation 2007;84:1500–1506 [DOI] [PubMed] [Google Scholar]
- 47.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 [DOI] [PubMed] [Google Scholar]