Abstract
Potassium inwardly rectifying channel, subfamily J, member 15 (KCNJ15) is a type 2 diabetes–associated risk gene, and Kcnj15 overexpression suppresses insulin secretion in rat insulinoma (INS1) cells. The aim of the current study was to characterize the role of Kcnj15 by knockdown of this gene in vitro and in vivo. Human islet cells were used to determine the expression of KCNJ15. Expression of KCNJ15 mRNA in islets was higher in subjects with type 2 diabetes. In INS1 cells, Kcnj15 expression was induced by high glucose–containing medium. Regulation of Kcnj15 by glucose and its effect on insulin secretion were analyzed in INS1 cells and in normal mice and diabetic mice by the inactivation of Kcnj15 using small interfering RNA. Knockdown of Kcnj15 increased the insulin secretion in vitro and in vivo. KCNJ15 and Ca2+-sensing receptor (CsR) interact in the kidney. Binding of Kcnj15 with CsR was also detected in INS1 cells. In conclusion, downregulation of Kcnj15 leads to increased insulin secretion in vitro and in vivo. The mechanism to regulate insulin secretion involves KCNJ15 and CsR.
Type 2 diabetes is a leading health problem in the developed world, and the disease is becoming increasingly common in developing countries as well (1,2). Clinical studies indicate that type 2 diabetes comprises heterogeneous phenotypes among various ethnic groups. The prevalence of type 2 diabetes in Japan has dramatically increased in the past 40 years, and the same trend was recently reported in other Asian countries, due primarily to genetic factors in combination with behavioral changes, such as high-calorie diets and sedentary lifestyles (3–6). Despite the rise in type 2 diabetes, Asian patients are still characterized as having lower body mass indices and insulin levels compared with European, Mexican American, or African American type 2 diabetic patients (7).
We recently identified potassium inwardly rectifying channel, subfamily J, member 15 (KCNJ15), a member of the potassium inwardly rectifying channel (KIR) family, also called IRKK, KIR1.3, and KIR4.2 (8), as a susceptibility gene for type 2 diabetes (9) in subjects with a mean BMI of ∼23.0 kg/m2 (10). The synonymous single nucleotide polymorphism of KCNJ15 (C566T at rs3746876 in exon 4) increases the risk of type 2 diabetes in Japanese, and the risk allele (T) is associated with increased levels of KCNJ15 mRNA in the peripheral blood of healthy volunteers (9). Clinical observations reveal that subjects with type 2 diabetes carrying the risk allele are more likely to require insulin, indicating that insulin secretion is low in this group. Moreover, Kcnj15 overexpression decreases the insulin response to glucose in rat insulinoma (INS1) cells (9).
In the current study, we examined KCNJ15 expression in human pancreatic islets from normal and type 2 diabetic patients and investigated the effects of Kcnj15 on insulin secretion in vitro and in vivo. Kcnj15 was upregulated by glucose and had a significant inhibitory effect on insulin secretion. We also confirmed that Kcnj15 interacts with Ca2+-sensing receptor (SCARA3, CsR) in INS1 cells, suggesting that KCNJ15 is involved in regulating insulin secretion.
RESEARCH DESIGN AND METHODS
Immunofluorescence staining of KCNJ15 protein in islets of Langerhans.
Immunofluorescence double staining for KCNJ15 and insulin was performed using frozen sections of pancreas derived from humans (Human Tissues & Biofluids Bank, Asterand, Detroit, MI), C57BL/6 mice (10-week-old male), and Sprague-Dawley rats (10-week-old male). When possible, the human tissues obtained from Asterand are obtained with written informed consent and institutional review board approval; see the following web site for details on Asterand’s policies regarding responsible research and informed consent (http://www.asterand.com/Asterand/about/ethics.htm). Human pancreatic tissue was prepared from diabetic patients and nondiabetic control subjects during surgery. Tissue sections (5-μm thick) were cut with a cryotome and mounted on slides. The sections were then dried at room temperature and fixed with acetone for 10 min. The slides were washed three times with PBS and incubated overnight with anti-KCNJ15 goat antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and then anti-insulin rabbit antibody (Santa Cruz Biotechnology) for 1 h. After washing with PBS, the slides were incubated for 40 min with either Alexa-488– or Alexa-633–conjugated secondary antibody (Invitrogen, Carlsbad, CA). After washing with PBS, sections were visualized using a confocal laser scanning microscope (LSM 510 Meta NLO Imaging System; Carl Zeiss, Oberkochen, Germany).
KCNJ15 mRNA expression in the islets of human subjects.
Optimal cutting temperature compound (Sakura Finetechnical, Tokyo, Japan) was added to fresh-frozen human pancreatic tissues (Human Tissues & Biofluids Bank), and the tissue was sectioned at a thickness of 10 μm. The sections were mounted on membrane-coated slides, and the optimal cutting temperature compound was removed by dipping the slides six times in ice-cold RNase-free water. The sections were then dehydrated in ice-cold 70% ethanol for 3 min. Laser microdissection and pressure catapulting were performed on the caps of microtubes filled with 20 μL lysis buffer (PALM MicroLaser System; Carl Zeiss). Total RNA was extracted from microdissected cells from both nondiabetic control subjects and patients with type 2 diabetes. First-strand cDNA synthesis was performed using a MessageBOOSTER cDNA synthesis kit (Epicentre Biotechnologies, Madison, WI). Relative mRNA expression was compared as described below.
Regulation of Kcnj15 expression by glucose in INS1 cells and HEK293 cells.
Changes in the expression of Kcnj15 mRNA in INS1 cells induced by 5 and 25 mmol/L glucose were compared with changes in the expression of Kcnj11, Glut2, and insulin genes. Changes in the expression of KCNJ15 mRNA in HEK293 cells induced by 5, 25, 45, and 65 mmol/L glucose were also compared. Total RNA was isolated from cultured cells and tissues using TRIzol (Invitrogen). After DNase I treatment (Roche, Lewes, East Sussex, U.K.), cDNA was synthesized using oligo (dT) primers and ImProm-II reverse transcriptase (Promega, Madison, WI). Quantitative real-time PCR was performed using SYBR green (Applied Biosystems, Foster City, CA). The relative expression of those genes was normalized to β-actin. All samples were analyzed in duplicate. The primers are as follows: for human KCNJ15, forward: 5′-GGAATGTCCTCATGCCATCT-3′, reverse: 5′-TTCTGCTTGGTGATGACTGC-3′; for rat Kcnj15, forward: 5′-CCGTTCCATCACAGAGGAGT-3′, reverse: 5′-GCTTTTTGGGTCTTGCAATC-3′; for mouse Kcnj15, forward: 5′-GTGCCAGCTCTCTGGAAAAC-3′, reverse: 5′-AGGGACTCTCGGAAGAGGAG-3′; for rat Kcnj11, forward: 5′-GCCATGCTGTCCCGAAAGGG-3′, reverse: 5′-GGCCAGGGGACATTCCTCTGT-3′; for rat Glut2, forward: 5′-ATGTCAGAAGACAAGATCACCGGA-3′, reverse: 5′-CCCGAGCCACCCACCAAAGAAT-3′; for rat Ins1&2, forward: 5′-TGCCCGGGCTTTTGTCAAAC-3′, reverse: 5′-CTCCAGTGCCAAGGTCTGAA-3′; and for human & rat & mouse B-actin, forward: 5′-CGCACCACTGGCATTGTCAT-3′, reverse: 5′-TTCTCCTTGATGTCACGCAC-3′. All were obtained from Invitrogen. Kcnj15 and actin protein levels in cultured INS1 cell lysates were detected by Western blotting. INS1 cells were incubated for 48 h and harvested.
Inactivation of Kcnj15 and CsR in INS1 cells by small interfering RNA.
To clarify the role of Kcnj15, we knocked down Kcnj15 in INS1 cells using small interfering (si)RNA. A control siRNA (Stealth RNAi Negative Control Med GC), predesigned siRNA against Kcnj15 (NM_001107285.1_stealth_1056), and CsR (NM_001108870.1) were obtained from Invitrogen (Table 1). Cultured INS1 cells were transfected with either control siRNA, Kcnj15 siRNA, and/or CsR siRNA using Lipofectamine 2000 (Invitrogen). Cells were incubated for 72 h to allow knockdown. To determine the extent of the knockdown, the mRNA level in each transfected cell line was examined by quantitative real-time PCR. Kcnj15 protein was detected by Western blotting. Transfected INS1 cells were cultured in 24-well plates and preincubated for 30 min at 37°C in glucose-free Krebs-Ringer buffer, followed by 1-h incubation in 5 or 25 mmol/L glucose to determine the amount of insulin secretion. An ELISA was used to measure insulin secreted into the media (Rat Insulin ELISA kit; Mercodia AB, Uppsala, Sweden).
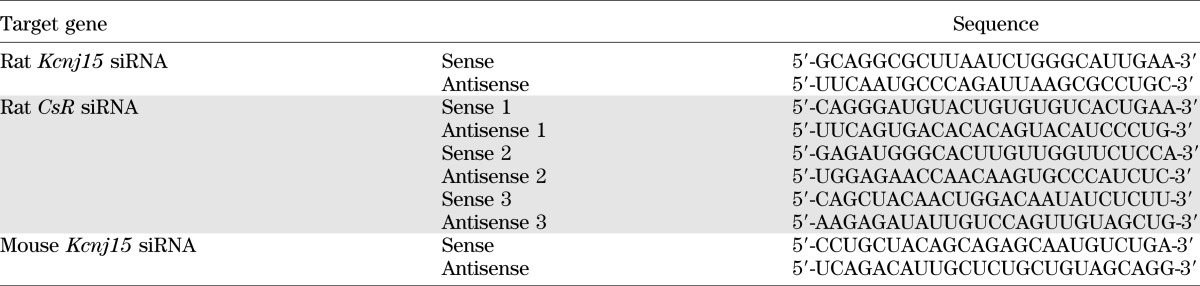
TABLE 1.
siRNA sequences
Inactivation of Kcnj15 in C57BL/6 mice and heterozygote Ins2Akita mice by siRNA.
To investigate the role of Kcnj15 in insulin secretion in vivo, we injected Kcnj15 siRNA into normal and diabetic mice. C57BL/6 mice were used as a normal glucose tolerant model and Ins2Akita (Akita) mice were used as a diabetic model without obesity. Female C57BL/6 mice and female Akita mice (11) were obtained from Japan SLC (Hamamatsu, Japan). Kcnj15 siRNA (MSS205690) was obtained from Invitrogen as annealed, predesigned in vivo siRNA (Table 1). Scrambled siRNA was used as negative control siRNA. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the committee of animal resources of the University of Tokyo. Mice were housed under specific pathogen-free conditions in microisolator cages and maintained on a 12-h light (0800 h)/dark (2000 h) cycle. Using hydrodynamic injections, 400 μg synthetic siRNA dissolved in 0.8 mL PBS was rapidly injected intravenously as previously reported (12,13). To determine the knockdown efficiency, mRNA and protein levels of Kcnj15 in the pancreas were detected by quantitative real-time PCR and immunoblotting on day 3 after treatment. Kcnj15 protein was stained with Alexa-488 fluorescence and visualized using a confocal laser scanning microscope.
Oral glucose tolerance tests in C57BL/6 mice and Akita mice inactivated for Kcnj15.
Oral glucose tolerance tests (OGTTs) (14) were performed in C57BL/6 mice inactivated for Kcnj15 at 3 days after injection of Kcnj15 siRNA. After overnight fasting, whole blood glucose and plasma insulin levels were measured at 0 and 15 min. Blood samples were obtained from the tail vein and collected into heparinized capillary tubes. Glucose concentrations in the whole blood were measured using a blood glucose monitor (Terumo, Tokyo, Japan). Fasting blood samples were obtained after an overnight fast, and casual blood glucose samples were obtained between 0900 and 1000 h. Blood glucose and plasma insulin levels were measured immediately before oral injection of d-glucose (2 g ⋅ 5 mL−1 ⋅ kg−1) and after 15 min. OGTTs in Akita mice inactivated for Kcnj15 were performed at 4 days after injection of Kcnj15 siRNA, as described above.
Morphology of the islets of Langerhans in C57BL/6 mice inactivated for Kcnj15.
Tissue sections isolated from pancreas on the 4th day were fixed with formalin. The tissue was then processed for paraffin embedding, and 3-μm thick sections were cut and mounted on glass slides. The sections were immunostained with anti-insulin antibody and counterstained with Mayer hematoxylin solution. Images of pancreatic tissue were captured on a computer through a microscope connected to a charge-coupled device camera (Olympus). The ratio of the area of insulin-positive cells to the total area of pancreatic tissue was calculated as described previously (15). At least five pancreatic sections from each animal were analyzed, and >100 islets per mouse were scanned.
Immunoblotting and immunoprecipitation of Kcnj15 with CsR.
After 48-h incubation with 5 or 25 mmol/L glucose, INS1 cells were harvested as previously reported (9). In brief, the membrane fraction was collected after centrifuging at 10,000g for 30 min; to increase mobility, we incubated the membranes in the same sample buffer for at least 24 h at room temperature, followed by heating at 50°C for 15 min, at 20°C for 30 min, and at 50°C for 15 min, as previously reported for an SDS-resistant inward rectifier (16). The lysates were separated on a 10–20% gradient SDS-polyacrylamide gel. After the proteins were transferred from the gel to a polyvinylidene difluoride membrane (Amersham Biosciences), immunoblot analysis was performed using Kcnj15, Kcnj11, and actin antibodies (Santa Cruz Biotechnology) and CsR antibody (Abcam, Cambridge, U.K.). For the insulin antibody, whole cell lysates were harvested after 96-h incubation with 25 mmol/L glucose after the knockdown. Analysis was performed with an insulin antibody (Santa Cruz Biotechnology).
The cell lysates were used for coimmunoprecipitation as previously described (17). In brief, anti-CsR or normal IgG (Sigma-Aldrich, Milwaukee, WI) antibodies were separately loaded onto a Dynabead–protein A complex (Invitrogen) and slowly rotated for 2 h. The antibody-loaded Dynabead–protein A complex was rinsed twice; the beads were mixed with the cell lysates and rotated overnight in a cold room. The supernatants were discarded, and the Dynabead–protein A complex was washed. Loading buffer was added, and the mixture was vortexed vigorously. The tubes were placed in DynaMag-2 to collect the sample buffer. The samples were then subjected to immunoblotting.
Statistical analysis.
Data are expressed as the mean ± SEM. Differences between two experimental groups were analyzed using an unpaired t test. Differences between more than two groups were analyzed using a non–repeated measures ANOVA. P < 0.05 was considered significant.
RESULTS
KCNJ15 protein is expressed in the islets of Langerhans in humans, mice, and rats.
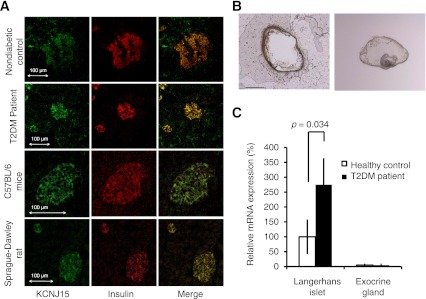
Immunohistochemistry revealed that KCNJ15 was costained with insulin and indicated that KCNJ15 is expressed in β-cells of patients with type 2 diabetes and nondiabetic control subjects, C57BL/6 mice, and Sprague-Dawley rats (Fig. 1A).
FIG. 1.
Expression of KCNJ15 protein and mRNA in the islets of Langerhans. A: Immunofluorescence staining of the pancreas. KCNJ15 was stained with Alexa-488 fluorescence (green), and insulin was stained with Alexa-488 fluorescence (red). Images from the same sections were merged to form an overlapping image (yellow). B: Remaining section after the islet was cut and transferred by laser (left); single islet after it was cut by laser and transferred (right). C: KCNJ15 mRNA expression levels in the islets of Langerhans and exocrine glands. Values are mean ± SEM (n = 5; pooled samples of islets of Langerhans). T2DM, type 2 diabetes mellitus. (A high-quality digital representation of this figure is available in the online issue.)
Expression of KCNJ15 mRNA in islets was increased in patients with type 2 diabetes.
KCNJ15 mRNA levels in the microdissected islets of Langerhans in patients with type 2 diabetes were significantly higher than those in nondiabetic control subjects. KCNJ15 mRNA was not detectable in the exocrine glands of the pancreas in patients with type 2 diabetes or nondiabetic control subjects (P = 0.034, n = 5) (Fig. 1B and C).
Glucose stimulates Kcnj15 expression in INS1 cells.
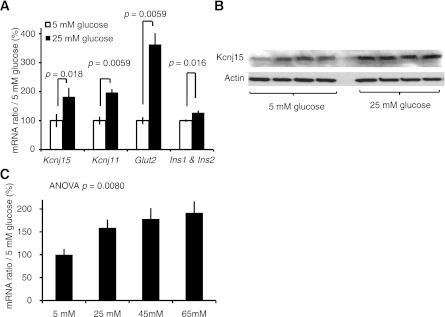
Expression of Kcnj15 mRNA was induced by a high glucose concentration (25 mmol/L) in INS1 cells (P = 0.018, n = 5) (Fig. 2A). Similar levels of induction were observed for Kcnj11 and Glut2, and smaller levels of induction were observed for insulin. The protein expression of Kcnj15 was induced by high glucose concentrations (25 mmol/L; P = 0.013, n = 4) (Fig. 2B). In the human cell line HEK293, the expression of KCNK15 mRNA was induced in proportion to glucose concentrations (P = 0.0080, n = 6) (Fig. 2C)
FIG. 2.
Transcript was activated under high glucose conditions. A: Kcnj15, Kcnj11, Glut2, and insulin mRNA expression levels in cultured INS1 cells. Each value was normalized to the value expressed in 5 mmol/L glucose. Values are mean ± SEM (n = 5). B: Kcnj15 and actin protein levels in lysates of cultured INS1 cells in 5 and 25 mmol/L glucose (n = 4). C: Activation of human KCNJ15 gene transcription in response to different glucose concentrations (5, 25, 45, or 65 mmol/L). HEK293 cells were cultured for 72 h (n = 6).
Inactivation of Kcnj15 in INS1 cells by siRNA.
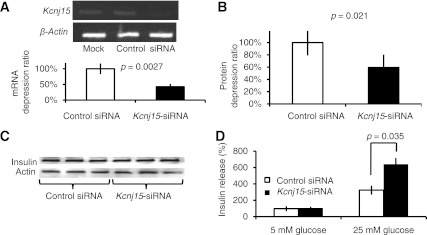
The effectiveness of silencing for Kcnj15 with siRNA was confirmed at both the mRNA and protein levels (Fig. 3A and B). Insulin was detected in the lysates of INS1 cells by Western blotting (Fig. 3C). Insulin concentrations in the lysates of cultured INS1 cells were not changed by the knockdown of Kcnj15 in 5 mmol/L glucose with 1 h of incubation. Insulin concentrations secreted into the culture medium were significantly increased, however, in 25 mmol/L glucose with 1 h of incubation after Kcnj15 knockdown (P = 0.035, n = 5) (Fig. 3D), indicating that Kcnj15 negatively regulates insulin secretion in INS1 cells under high glucose concentrations.
FIG. 3.
Knockdown of Kcnj15 increased insulin secretion in vitro. A: Agarose gel electrophoresis of the products and real-time PCR results generated by RT-PCR using primers specific for rat Kcnj15 and β-actin. INS1 cells were transfected with PBS, control siRNA, or Kcnj15 siRNA. B: Kcnj protein levels in cultured INS1 cell lysates with and without inactivation of Kcnj15 detected by Western blotting. INS1 cells were transfected with PBS, control siRNA, or Kcnj15 siRNA. C: The protein levels of insulin and actin in cultured INS1 cell lysates detected by Western blotting. D: Relative insulin secretion with and without inactivation of Kcnj15 in INS1 cells at 5 and 25 mmol/L glucose. Results are expressed as a percentage of the value secreted with 5 mmol/L (0.98 ± 0.04 mg/mg protein) as a control. Values are mean ± SEM (n = 5).
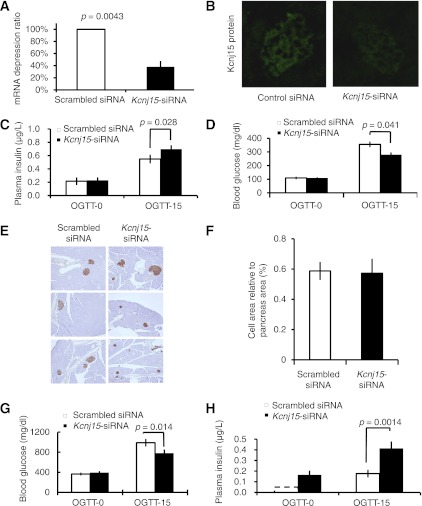
Inactivation of Kcnj15 in nondiabetic mice enhanced insulin secretion.
In the Kcnj15 siRNA–treated group, mRNA levels (Fig. 4A) were reduced by 71.6% (P = 0.0043, n = 3) in the pancreas compared with the scrambled siRNA–treated group. The protein levels were also decreased (Fig. 4B). Three days after injection, OGTTs were performed, and whole blood glucose and plasma insulin levels were measured at 0 and 15 min. Plasma insulin levels were significantly elevated in the Kcnj15 siRNA–treated group compared with the scrambled siRNA–treated group at 15 min (P = 0.028, n = 5) (Fig. 4C). The fasting blood glucose levels did not differ between the groups treated with Kcnj15 siRNA and scrambled siRNA. Blood glucose levels at 15 min were significantly lower in the Kcnj15 siRNA–treated group, however, compared with those in the scrambled siRNA–treated group (355 ± 35 → 279 ± 15 mg/dL, P = 0.041, n = 5) (Fig. 4D). The morphology of the insulin-positive cell mass did not differ between the Kcnj15 siRNA– and scrambled siRNA–treated C57BL/6 mice at 4 days (Fig. 4E and F).
FIG. 4.
Knockdown of Kcnj15 increased insulin secretion in vivo. A: Quantitative real-time PCR data showing Kcnj15 expression levels relative to that of β-actin 3 days after treatment. Values are mean ± SEM (n = 3). B: Representative images of inactivated Kcnj15 protein by siRNA injection in the pancreas. C: Plasma insulin levels before and after OGTT in Kcnj15 siRNA– and scrambled siRNA–treated C57BL/6 mice. Data are mean ± SEM (n = 5). D: Fasting blood glucose and OGTTs in Kcnj15 siRNA– and scrambled siRNA–treated C57BL/6 mice. Each female mouse was fasted overnight. Data are mean ± SEM (n = 5). E: Immunohistochemistry of the islets. Islets were stained for insulin. F: The proportion of β-cells and non–β-cells in the pancreas. Pancreatic sections were stained for insulin. Results are shown in proportion to the pancreas (%) (n = 3). G: Fasting blood glucose and OGTTs in siRNA-treated and control Akita mice. Each female mouse was fasted overnight. Data are mean ± SEM (n = 8). H: Plasma insulin levels before and after OGTTs in siRNA-treated and control Akita diabetic mice. Data are mean ± SEM (n = 4). (A high-quality digital representation of this figure is available in the online issue.)
Inactivation of Kcnj15 in diabetic mice enhanced insulin secretion.
To test whether Kcnj15 could be a therapeutic target, we performed knockdown experiments using Akita mice. Seven-week-old female Akita mice, whose nonfasting plasma glucose was between 300 and 400 mg/dL (325 ± 31 mg/dL), were used. Fasting blood glucose levels were not changed by siRNA treatment. Blood glucose levels at 15 min, however, were decreased compared with those in the siRNA group (938 ± 38 → 774 ± 53 mg/dL, P = 0.014, n = 8) (Fig. 4G). Plasma insulin levels at both fasting and 15 min after OGTTs were significantly higher in the siRNA group than in the control group (P = 0.0014, n = 4) (Fig. 4H).
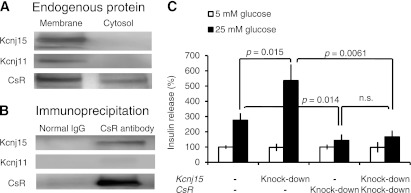
Interactions of Kcnj15 and CsR in INS1 cells.
KCNJ15 inhibits CsR in Xenopus oocytes (18). CsR signals are thought to be necessary for the full insulin secretory response of β-cells (19). Therefore, we hypothesized that CsR might work together with Kcnj15 to regulate insulin secretion. First, to demonstrate Kcnj15-CsR colocalization, we prepared membrane and cytosol fractions and processed them for immunoblotting and immunoprecipitation with antibodies against CsR. Kcnj15, Kcnj11, and CsR were detected predominantly in the membrane fraction (Fig. 5A). A strong band at 130 kDa representing CsR was immunoprecipitated. Bands representing Kcnj15 were clearly detected, whereas a band representing Kcnj11 was not observed (Fig. 5B). To assess the effect of Kcnj15 on CsR-related insulin secretion, we next conducted in vitro double knockdown of both Kcnj15 and CsR. The effectiveness of Kcnj15 and CsR silencing with siRNA was confirmed. As previously reported, CsR inactivation decreased insulin secretion. Under CsR inactivation, however, Kcnj15 inactivation did not increase insulin secretion (Fig. 5C), indicating that CsR is necessary for the effects of Kcnj15 on insulin secretion.
FIG. 5.
Interactions of Kcnj15 and CsR in INS1 cells. A: Coimmunoprecipitation of CsR with Kcnj15 or Kcnj11 from INS1 cell lysate. The INS1 cell membrane and cytosol fractions were processed for immunoblotting with antibodies against Kcnj15, Kcnj11, or CsR to detect the endogenous proteins. B: Cell lysates were used for immunoprecipitation with the anti-CsR antibody. The immunoprecipitated samples were processed for immunoblotting with antibodies against Kcnj15, Kcnj11, or CsR. C: Relative insulin secretion with and without inactivation of Kcnj15 and/or CsR in INS1 cells at 5 and 25 mmol/L glucose. Results are expressed as a percentage of the value secreted with 5 mmol/L as a control. Values are mean ± SEM (n = 5).
DISCUSSION
We performed functional studies of KCNJ15 in a previous study (9). We demonstrated the expression of KCNJ15 in human β-cells and that overexpression of Kcnj15 in INS1 cells decreases insulin secretion in 25 mmol/L but not 5 mmol/L glucose. The results of the current study reveal that the KCNJ15 expression is associated with type 2 diabetes and glucose concentration in both human specimens and a human cell line and that inactivation of Kcnj15 increases glucose-stimulated insulin secretion in vitro and in vivo.
Increased plasma glucose induced KCNJ15 expression at the transcriptional level and inhibited insulin secretion through the KCNJ15 channel. These findings are consistent with our previous observation that the risk allele is associated with increased levels of KCNJ15 mRNA in healthy volunteers, and the prevalence of type 2 diabetic patients treated with insulin is significantly higher among those patients carrying the risk allele. Together, these findings provide the first evidence that KCNJ15 affects glucose homeostasis through the regulation of insulin secretion.
KCNJ15 was cloned from human kidney and is most readily expressed in the kidney and pancreas and less in the lung. The cDNA predicts a 1125-base pair open reading frame that encodes a 375-amino acid polypeptide (8). The importance of KCNJ11 among KIR family members in insulin secretion is well established. Four molecules of KCNJ11 make the pore-forming subunits of the ATP-sensitive K+ (KATP) channel, which is surrounded by the external part of the channel comprising four sulfonylurea receptor (SUR)1 subunits encoded by the ATP-binding cassette transporter, subfamily C, member 8 (ABCC8) gene (20), presenting a hetero-octamer structure. KCNJ15, KCNJ1, and KCNJ10, on the other hand, each form the pore-forming subunit of the KATP channel without an SUR1 subunit (8). On the basis of Xenopus oocyte experiments, inhibition of the KCNJ15 channel functions to stabilize the membrane potential (18). Thus, we speculate that KCNJ15 might be part of an alternative K channel that contributes to the membrane depolarization associated with glucose stimulation. The structure of the human KCNJ15 channel in β-cells, however, is largely unknown.
When β-cell metabolism increases in response to an increase in the plasma glucose concentration, KCNJ11 channels close as a result of increased levels of ATP sensed by SURs. This leads to membrane depolarization and results in the opening of Ca2+ channels with an influx of Ca2+, which stimulates exocytosis of insulin granules (21). Gain-of-function mutations in KCNJ11 result in a larger β-cell KATP current at a given glucose concentration and thereby reduce or stop glucose-dependent depolarization and Ca2+ influx (22,23). Furthermore, hyperglycemic conditions increased Kcnj15 at the transcriptional level in pancreatic β-cells to a magnitude similar to that observed with Kcnj11 (Fig. 2A). SURs cannot control KCNJ15 but can control KCNJ11. An initial increase in the ATP-to-ADP ratio can shut down the K channel with KCNJ11. The K flow produced by KCNJ15 resists depolarization, resulting in a recurring cycle of hypoinsulinemia and hyperglycemia in type 2 diabetes.
To date, in vivo siRNA delivery has succeeded in limited organs. We found only a few reports in the literature of successful in vivo siRNA delivery to the pancreas (12,13). Here we used a hydrodynamic method of transfection to demonstrate the potential effects on insulin secretion in mice. Findings from the siRNA experiments with C57BL/6 mice suggest that downregulation of KCNJ15 decreases glucose levels and increases insulin secretion. Silencing of Kcnj15 may allow pancreatic β-cells to rest and recover their insulin stores by stabilizing the membrane potential. These findings suggest that KCNJ15 is a potential therapeutic target for diabetes as well as persistent hyperinsulinemic hypoglycemia of infancy or nesidioblastosis. We also demonstrated that even in Akita mice, the glucose levels at 15 min after glucose loading were significantly reduced in response to the increased insulin secretion after Kcnj15 knockdown, indicating the clinical importance of KCNJ15 as an alternative key molecule in insulin secretion.
In the current study, insulin secretion increased by Kcnj15 inactivation disappeared with CsR inactivation (Fig. 5). Direct interactions between KCNJ15 and CsR have been reported in kidney (17) and an oocyte expression system (18). Supported by these previous findings, our findings in INS1 cells indicate a clear functional association of Kcnj15 and CsR to secrete insulin. Other than the functional binding of Kcnj15 and CsR, however, the precise mechanism of the insulin secretion has not been elucidated. CsR on β-cells are activated by the divalent cations Ca2+, Mg2+, and Zn2+, which are secreted together with insulin (24,25). Moreover, the CsR signal is necessary for the full insulin secretory response of β-cells (19). Although it is not known how activated CsR signals induce insulin secretion, CsR inhibits Kcnj15 channel function in an oocyte expression system (18). Such an interaction between CsR and KCNJ15 has not been observed for KCNJ11. We observed no effect of Kcnj15 inactivation in a low glucose condition. As previously reported, CsR inactivation alone has no effect on insulin secretion at low glucose concentrations (26). On the basis of these findings, we propose that inhibition of the KCNJ15 channel function is associated with insulin secretion through an interaction with the CsR signal.
In summary, the findings of the current study suggest that KCNJ15 inhibits glucose-stimulated insulin secretion. Thus, increased KCNJ15 as a consequence of hyperglycemia may contribute, at least in part, to the impairment of β-cell function in patients with type 2 diabetes.
ACKNOWLEDGMENTS
This study was partially supported by a grant from the National Center for Global Health and Medicine (21A114), a Grant-in-Aid for Scientific Research (19590338, 22510214), and the Program for Promoting the Establishment of Strategic Research Centers, Special Coordination Funds for Promoting Science and Technology, from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (MEXT; to N.I.) and a Grant-in-Aid for Scientific Research on Priority Area (C) Medical Genome Science from MEXT (to N.I. and K.T.). E.N. is the leading researcher of Science and Technology Research Partnership for Sustainable Development, Japan Science and Technology Agency (JST)/Japan International Cooperation Agency (JICA), Tokyo, Japan. This study was supported by Grants-in-Aid for Scientific Research on Priority Area Comprehensive Genomics from MEXT (to K.T.) and on Innovative Area Genome Science (to K.T.).
No potential conflicts of interest relevant to this article were reported.
K.O. designed the experiments, performed the experimental work and data analysis, and wrote the manuscript. N.I., E.N., and K.T. designed the experiments and supervised the project. K.D. designed the experiments. Y.I., Y.U., and T.F. supervised the project. The manuscript was finalized by K.O. with the assistance of all the authors. K.O. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. G.I. Bell, Departments of Medicine and Human Genetics, University of Chicago, and Dr. M. Kakei, Department of Endocrinology and Metabolism, Jichi Medical University, for scientific discussions. Dr. Itaru Kojima of the Institute for Molecular and Cellular Regulation, Gunma University, kindly provided the INS1 cells.
Footnotes
See accompanying commentary, p. 1659.
REFERENCES
- 1.Office for Lifestyle-Related Disease Control, Ministry of Health and Welfare (Ed.). DiabetesSurvey 1997 Tokyo, Japan, Government of Japan, 1999 [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787 [DOI] [PubMed] [Google Scholar]
- 3.Duval S, Vazquez G, Baker WL, Jacobs DR, Jr, CODA study group The Collaborative Study of Obesity and Diabetes in Adults (CODA) project: meta-analysis design and description of participating studies. Obes Rev 2007;8:263–276 [DOI] [PubMed] [Google Scholar]
- 4.Ehm MG, Karnoub MC, Sakul H, et al. American Diabetes Association GENNID Study Group. Genetics of NIDDM Genomewide search for type 2 diabetes susceptibility genes in four American populations. Am J Hum Genet 2000;66:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong KC, Wang Z. Prevalence of type 2 diabetes mellitus of Chinese populations in Mainland China, Hong Kong, and Taiwan. Diabetes Res Clin Pract 2006;73:126–134 [DOI] [PubMed] [Google Scholar]
- 6.Davis TM, Cull CA, Holman RR, U.K. Prospective Diabetes Study (UKPDS) Group Relationship between ethnicity and glycemic control, lipid profiles, and blood pressure during the first 9 years of type 2 diabetes: U.K. Prospective Diabetes Study (UKPDS 55). Diabetes Care 2001;24:1167–1174 [DOI] [PubMed] [Google Scholar]
- 7.Sone H, Katagiri A, Ishibashi S, et al. JD Study Group Effects of lifestyle modifications on patients with type 2 diabetes: the Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three year-interim report. Horm Metab Res 2002;34:509–515 [DOI] [PubMed] [Google Scholar]
- 8.Shuck ME, Piser TM, Bock JH, Slightom JL, Lee KS, Bienkowski MJ. Cloning and characterization of two K+ inward rectifier (Kir) 1.1 potassium channel homologs from human kidney (Kir1.2 and Kir1.3). J Biol Chem 1997;272:586–593 [DOI] [PubMed] [Google Scholar]
- 9.Okamoto K, Iwasaki N, Nishimura C, et al. Identification of KCNJ15 as a susceptibility gene in Asian patients with type 2 diabetes mellitus. Am J Hum Genet 2010;86:54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwasaki N, Cox NJ, Wang YQ, et al. Mapping genes influencing type 2 diabetes risk and BMI in Japanese subjects. Diabetes 2003;52:209–213 [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997;46:887–894 [DOI] [PubMed] [Google Scholar]
- 12.Bradley SP, Rastellini C, da Costa MA, et al. Gene silencing in the endocrine pancreas mediated by short-interfering RNA. Pancreas 2005;31:373–379 [DOI] [PubMed] [Google Scholar]
- 13.Larson SD, Jackson LN, Chen LA, Rychahou PG, Evers BM. Effectiveness of siRNA uptake in target tissues by various delivery methods. Surgery 2007;142:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest 2000;105:287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terauchi Y, Iwamoto K, Tamemoto H, et al. Development of non-insulin-dependent diabetes mellitus in the double knockout mice with disruption of insulin receptor substrate-1 and beta cell glucokinase genes. Genetic reconstitution of diabetes as a polygenic disease. J Clin Invest 1997;99:861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corey S, Clapham DE. Identification of native atrial G-protein-regulated inwardly rectifying K+ (GIRK4) channel homomultimers. J Biol Chem 1998;273:27499–27504 [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Handlogten ME, Miller RT. Parallel activation of phosphatidylinositol 4-kinase and phospholipase C by the extracellular calcium-sensing receptor. J Biol Chem 2002;277:20293–20300 [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Sindic A, Hill CE, et al. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol 2007;292:F1073–F1081 [DOI] [PubMed] [Google Scholar]
- 19.Gray E, Muller D, Squires PE, et al. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J Endocrinol 2006;190:703–710 [DOI] [PubMed] [Google Scholar]
- 20.Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol 1997;110:655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 1989;54:87–143 [DOI] [PubMed] [Google Scholar]
- 22.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004;350:1838–1849 [DOI] [PubMed] [Google Scholar]
- 23.Sagen JV, Raeder H, Hathout E, et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes 2004;53:2713–2718 [DOI] [PubMed] [Google Scholar]
- 24.Rasschaert J, Malaisse WJ. Expression of the calcium-sensing receptor in pancreatic islet B-cells. Biochem Biophys Res Commun 1999;264:615–618 [DOI] [PubMed] [Google Scholar]
- 25.Squires PE, Harris TE, Persaud SJ, Curtis SB, Buchan AM, Jones PM. The extracellular calcium-sensing receptor on human beta-cells negatively modulates insulin secretion. Diabetes 2000;49:409–417 [DOI] [PubMed] [Google Scholar]
- 26.Kitsou-Mylona I, Burns CJ, Squires PE, Persaud SJ, Jones PM. A role for the extracellular calcium-sensing receptor in cell-cell communication in pancreatic islets of langerhans. Cell Physiol Biochem 2008;22:557–566 [DOI] [PubMed] [Google Scholar]