Abstract
We evaluated the effects of hyperbaric oxygen therapy (HOT) on autoimmune diabetes development in nonobese diabetic (NOD) mice. Animals received no treatment or daily 60-min HOT 100% oxygen (HOT-100%) at 2.0 atmospheres absolute and were monitored for diabetes onset, insulitis, infiltrating cells, immune cell function, and β-cell apoptosis and proliferation. Cyclophosphamide-induced diabetes onset was reduced from 85.3% in controls to 48% after HOT-100% (P < 0.005) and paralleled by lower insulitis. Spontaneous diabetes incidence reduced from 85% in controls to 65% in HOT-100% (P = 0.01). Prediabetic mice receiving HOT-100% showed lower insulitis scores, reduced T-cell proliferation upon stimulation in vitro (P < 0.03), increased CD62L expression in T cells (P < 0.04), reduced costimulation markers (CD40, DC80, and CD86), and reduced major histocompatibility complex class II expression in dendritic cells (DCs) (P < 0.025), compared with controls. After autoimmunity was established, HOT was less effective. HOT-100% yielded reduced apoptosis (transferase-mediated dUTP nick-end labeling-positive insulin-positive cells; P < 0.01) and increased proliferation (bromodeoxyuridine incorporation; P < 0.001) of insulin-positive cells compared with controls. HOT reduces autoimmune diabetes incidence in NOD mice via increased resting T cells and reduced activation of DCs with preservation of β-cell mass resulting from decreased apoptosis and increased proliferation. The safety profile and noninvasiveness makes HOT an appealing adjuvant therapy for diabetes prevention and intervention trials.
Type 1 diabetes (T1D) is a chronic autoimmune disorder caused by autoreactive T cells, which mediate the destruction of insulin-producing pancreatic β-cells, leading to lifelong dependence on exogenous insulin. Methods to achieve and maintain normoglycemia are currently based on insulin therapy, diet, and exercise. Unfortunately, while able to delay/prevent chronic complications of diabetes, intensive insulin therapy does not always achieve tight daily glycemic control and is associated with increased frequency of severe hypoglycemia.
An ideal treatment for T1D may combine strategies aimed at restoring self immune tolerance with others focused on preservation/restoration of functional β-cell mass. Different approaches have been proposed (1), including prevention studies in high-risk subjects, timely interventions at the time of diabetes onset, delayed interventions to restore self-tolerance and β-cell regeneration, and replacement of β-cell mass via islet or pancreas transplantation (2). Desirable therapeutic regimens should be effective (alone or in combination), readily accessible, and void of severe risks for the patients (1).
Multiple beneficial effects have been recognized for hyperbaric oxygen therapy (HOT), which is clinically used to improve oxygen supply to hypoperfused tissues (i.e., carbon monoxide exposure, embolism and ischemic events, and diabetic ulcers, among other). Anti-inflammatory properties (3–7) and mobilization of bone marrow stem cells (BMSCs) that are involved in tissue repair processes (8–11) have been attributed to HOT. The known safety profile and noninvasive nature of HOT with virtually absent side effects makes its use attractive for the treatment of autoimmune diseases (12,13). In a murine lupus model, HOT was associated with reduced mortality, decreased proteinuria, altered lymphocyte subset redistribution, reduced anti-DNA antibody titers, and amelioration of immune-complex deposition (14).
The nonobese diabetic (NOD) mouse is widely used as a preclinical model of T1D to assess therapeutic approaches able to prevent/halt autoimmune-mediated β-cell loss, although the success in diabetes prevention has been difficult to translate to the clinical arena (15–17). Herein, we report that HOT can prevent/delay the onset of autoimmune diabetes in NOD mice and that this phenomenon is associated with increased β-cell proliferation.
RESEARCH DESIGN AND METHODS
Animals.
Studies were approved by the institutional animal care and use committee. NOD/MrkTac mice (Taconic), NOD.CB17-Prkdcscid/J mice (NOD.scid; The Jackson Laboratory), and Thy1.1-BDC2.5 T-cell receptor (TCR)-transgenic NOD mice (provided by Dr. Zhibin Chen) were housed in virus antibody–free rooms in microisolated cages and exposed to a 12-h light/dark cycle with ad libitum access to autoclaved food and water.
HOT.
Mice were transferred into Plexiglas cages that were placed into small-animal hyperbaric chambers (RSI-B11; Reimers Systems). Control animals were handled in parallel in a similar manner without HOT. The hyperbaric session started by applying increasing pressures during a 5-min period followed by 60-min continuous exposure to either 100% oxygen (HOT-100%), pressurized ambient air (21% oxygen, HOT-21%) or oxygen-depleted air mixture (12% oxygen, HOT-12%, to achieve ∼21% oxygen tension in tissues, that is, normoxia) at 2.0 atmospheres absolute (ATA). The pressure in the chamber was slowly reduced during a 5-min period before opening it.
Diabetes monitoring.
Animals were monitored three times a week for glycosuria (Diastix; Bayer); if positive, glycemic levels were confirmed and tested thereafter on whole blood (tail vein pricking) using portable glucometers (OneTouchUltra2; LifeScan) (18). Diabetes was defined as nonfasting glycemic values ≥300 mg/dL on two consecutive readings.
Experimental models.
Accelerated autoimmune diabetes onset was studied in 10-week-old female NOD mice receiving (day 0) single cyclophosphamide (CyP; 200 mg/kg; Sigma-Aldrich) injection intraperitoneally. Experimental groups received daily HOT-100%, HOT-21%, HOT-12%, or no HOT, starting 1 week before CyP. Since CyP is activated through an oxygen-dependent P450 system, high oxygen tensions may enhance its activity. To avoid this potential bias, HOT was not administered on the day of CyP injection. Animals were monitored for diabetes onset for at least 4 weeks.
Spontaneous autoimmune diabetes onset was evaluated in female NOD mice exposed to either daily HOT-100%, HOT-21%, or no treatment starting at age 4 weeks up to age 35 weeks. In addition, prediabetic 13-week-old female NOD and NOD.scid mice did or did not undergo a 2-week course of HOT-100% to study insulitis and islet cell proliferation.
Intervention at the time of spontaneous diabetes onset in female NOD mice was started after detection of glycosuria and confirmed elevation of nonfasting glycemia ≥250 mg/dL on 2 consecutive days. From one to two insulin pellets (LinShin Canada) were implanted subcutaneously within a few days from diabetes onset. Animals received 2-week glucagon-like peptide-1 analog treatment (1.5 μg/day exenatide [EXN]; Amylin) subcutaneously (osmotic pump; ALZET) with or without HOT-100%. Recurrence of hyperglycemia during insulin therapy and after exhaustion of insulin pellets was assessed.
Recurrence of autoimmunity.
Pancreatic islets isolated from NOD.scid mice were implanted under the kidney capsule of spontaneously diabetic NOD mice treated or not with HOT-100% starting 2 weeks before transplant (day 0) with or without EXN (1.5 μg/day via intraperitoneal osmotic pump for 2 weeks starting on day 0). Nonfasting glycemic values monitored to determine the recurrence of hyperglycemia after transplantation.
Adoptive transfer of splenocytes (20 × 106) from recent-onset diabetic NOD mice into NOD.scid mice that were or were not undergoing HOT was performed. In other experiments, >90% fractions of fluorescently labeled (CellTrace; Invitrogen) CD4+Thy1.1+ T cells obtained by positive selection (MACS; Miltenyi) from BDC2.5 TCR-transgenic NOD mice (19) were adoptively transferred (1.2 × 106 cells per mouse) into naïve NOD female mice undergoing or not HOT-100%, and their frequency was assessed by flow cytometry in pancreatic lymph nodes (pLNs) explanted 3 days later.
Cytokine detection.
Serum from experimental animals was assayed for Th1/Th2 cytokines using the Bio-Plex Pro Mouse Cytokine 8-plex Assay on a BioPlex instrument (BioRad).
Flow cytometry analysis.
Phenotype of immune cell subsets from spleens, pLNs and peripheral blood were assessed as described previously (20). Peripheral blood mononuclear cells were purified from whole blood on Ficoll-PaquePlus gradient (GE Healthcare Bio-Sciences AB). Bone marrow cell suspensions were obtained by flushing femurs and tibias (21). Erythrocytes were lysed (ACK Lysis Buffer). Cells (106) were then incubated 20 min at 4°C with rat anti-CD16/32 (clone 24G2) and with specific primary antibodies, were successfully conjugated with different fluorochromes (BD Pharmingen) in fluorescence-activated cell sorter buffer (30 min, 4°C). Samples were acquired using an LSR-II instrument and analyzed with FACSDiva Software (BD Pharmingen).
Immune cell functional assays in vitro.
Enrichment of splenic CD4+ T cells or CD11c+ DCs was achieved by positive selection using specific surface antibodies and magnetic beads (MACS; Miltenyi) followed by cell sorting on a BD FACSAria instrument to ≥95% final purity.
Histopathology.
Pancreata were collected in 10% buffered formalin solution or frozen at −80°C (Tissue-Tek OCT Compound). Formalin-fixed, paraffin-embedded, 4 µm–thick sections (five per organ) were stained with hematoxylin-eosin (H-E) and assessed blindly by light microscopy. Scoring criteria was as follows: 0, no insulitis; 1, peri-insulitis (infiltration restricted to the periphery of islets); 2, mild insulitis (<50% of the islet area infiltrated); 3, severe insulitis (≥50% of the islet area infiltrated); and 4, massive insulitis (≥90% of the islet area infiltrated). Mice received bromodeoxyuridine (BrdU; 1 mg/mL; Sigma-Aldrich) in the drinking water for 2 weeks, and incorporation in proliferating cells was assessed by fluorescence microscopy using specific antibody (1:50; Accurate) (22). To discriminate between endocrine and immune cells, costaining was performed with guinea pig anti-insulin (1:100; Dako) and rat anti–CD45/B220 (1:100; eBioscience). Secondary antibodies used were goat anti–guinea pig AlexaFluor-488 (1:200) and goat anti-rat AlexaFluor-568 (1:200; Invitrogen). Immunohistochemistry was performed on paraffin-embedded tissue sections. Slides were pretreated with 10 mmol/L citrate at pH 6.0 (Biocare Medical) or 1 mmol/L EDTA (pH 8.0) in a steam pressure cooker for antigen retrieval. Primary antibodies used were rabbit anti-CD3 (1:1,500 in EDTA; Cell Marque), rat anti–CD45/B220 (1:100), and rat anti-FoxP3 (1:25; eBioscience). Secondary rabbit anti-rat (1:750; Dako) was used for Foxp3 detection. Hematoxylin and Dako Envision plus system-HRP were used. Images were taken on a Leica DMLB microscope (Leica Application Suite v.3.6.0). Apoptosis was assessed using the DeadEnd Fluorometric TUNEL System (Promega). Digital images were acquired using a Zeiss Apotome microscope. Analysis was performed with Metamorph software.
Statistical analysis.
Survival curves were compared by log-rank test using Prism 5.0 (GraphPad), and data are expressed as median with range. Two-tailed, unpaired Student t test two-group comparison and one-way ANOVA for multiple comparisons were used. All in vitro determinations are means ± SEM from at least three independent conditions. Results were considered statistically significant at P < 0.05.
RESULTS
Prevention of accelerated autoimmune diabetes onset in NOD mice by chronic HOT.
CyP administration leads to accelerated diabetes onset in NOD mice (23–25). A single dose of CyP resulted in diabetes onset in 85.3% of control untreated mice (n = 34; median 13.5 days, range 11–36) (Fig. 1A and B). Application of 2.0 ATA HOT-100% once or twice daily starting 1 week prior to CyP treatment resulted in a sizable reduction of diabetes incidence, with only 48% (n = 25; range 11–34 days; P < 0.005 vs. control) and 40% (n = 10; range 11–14 days; P < 0.05 vs. control) of animals developing the disease, respectively (Fig. 1A). Shorter HOT application (1 week prior to and until 1 week after CyP) yielded only partial reduction of diabetes incidence to 70% (n = 10; median 14 days, range 11–21; P = NS) (Fig. 1A). These results suggested that chronic HOT was required in our setting and that a single daily HOT was as effective as multiple administrations. Thus, subsequent studies used single daily HOT at 2.0 ATA.
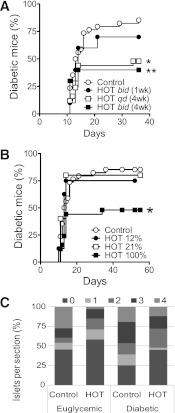
FIG. 1.
Evaluation of HOT on accelerated diabetes onset in NOD mice. Prediabetic 10-week-old female NOD mice were given a single intravenous dose of CyP on day 0. A: Control mice (n = 10) received no HOT (median 13.5 days, range 11–36). Therapy consisted of 60-min sessions of HOT-100% at 2.0 ATA starting 1 week before CyP injection. Animals received HOT twice a day (bid) for 1 week (n = 10; median 14 days, range 11–21) or 4 weeks (n = 10; range 11–14 days) or once a day (qd) for 4 weeks (n = 25; range 11–34 days) after CyP administration. Nonfasting glycosuria and glycemia were monitored to determine the time of diabetes onset. Log-rank test, *P < 0.005; **P < 0.5 vs. control. B: Single daily HOT (60-min session, 2.0 ATA) at the indicated oxygen concentrations was started 1 week before CyP injection and continued daily. Time to diabetes onset after CyP injection in untreated controls (n = 34) and animals exposed to HOT-12% (resulting in tissue oxygen levels comparable with ambient air of ∼21% oxygen; n = 8; median 13 days, range 10–14), HOT-21% (ambient air; n = 10; median 11–14 days, range 11–14), and HOT-100% (n = 25). Log-rank test, *P < 0.05 vs. control. C: Insulitis score on pancreatic sections of long-term euglycemic mice or after diabetes onset in control and HOT-100% groups (six sections per mouse, n = 2–3 mice per group). Score 0 = no insulitis, 1 = polar and/or peri-insulitis, 2 = mild insulitis (<50% of the islet area infiltrated), 3 = severe insulitis (≥50% of the islet area infiltrated), and 4 = massive insulitis (≥90% of the islet area infiltrated). The mean insulitis score was 1.48 ± 0.15 in control euglycemic (n = 125 islets), 0.83 ± 0.19 in HOT euglycemic (n = 37 islets; P < 0.05 vs. control diabetic), 2.18 ± 0.17 in control diabetic (n = 76 islets; P < 0.05 vs. control euglycemic), and 1.32 ± 0.16 in HOT diabetic (n = 84 islets; P < 0.05 vs. control diabetic) mice. One-way ANOVA, P < 0.0001.
Other experiments evaluated different oxygen concentrations (Fig. 1B). Diabetes onset occurred in 75 and 80% of mice receiving HOT-12% (n = 8; median 13 days, range 10–14) and HOT-21% (n = 10; median 14 days, range 11–14), respectively (P = NS vs. control) (Fig. 1B). These data indicate that high oxygen concentration is required to observe protection in this model. Pressures >2.6 ATA were not used because of observed discomfort and morbidity (not shown). Histopathology revealed statistically significant reduction of insulitis in HOT-100% mice compared with controls (Fig. 1C). Mean insulitis score was 1.48 ± 0.15 in control euglycemic (n = 125 islets), 0.83 ± 0.19 in HOT euglycemic (n = 37 islets; P < 0.05 vs. control diabetic), 2.18 ± 0.17 in control diabetic (n = 76 islets; P < 0.05 vs. control euglycemic), and 1.32 ± 0.16 in HOT diabetic (n = 84 islets; P < 0.05 vs. control diabetic) mice (one-way ANOVA, P < 0.0001) (Fig. 1C). Evaluation of activation, resting, and regulatory T-cell (Treg) markers in blood, spleens, and pLNs from HOT-100% and untreated controls at days 0, 3, 6, and 9 after CyP administration yielded no remarkable differences (not shown).
Prevention of spontaneous diabetes onset in NOD mice by chronic HOT.
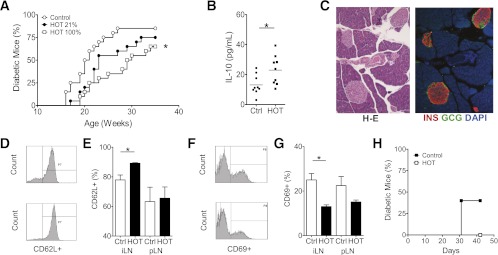
Daily HOT (60-min, 2.0 ATA) was administered chronically to female NOD mice starting at age 4 weeks. Diabetes occurred in 85% of untreated controls (n = 20; median 20.5 weeks, range 16–27). A dose-dependent protection was observed after HOT, with diabetes development in 75% of mice receiving HOT-21% (n = 10; median 23 weeks, range 17–32; P = NS) and in 65% of mice receiving HOT-100% (n = 20; median 29.5 weeks, range 19–33; P = 0.01 vs. control; P = NS vs. HOT-21%) (Fig. 2A). Screening of serum Th1/Th2 cytokine levels at age 10 weeks showed significantly higher circulating interleukin (IL)-10 in HOT-100% mice compared with controls (23.0 ± 3.2 vs. 13.1 ± 2.2 pg/mL, respectively; P = 0.02) (Fig. 2B). Pancreata obtained from euglycemic HOT-100% animals at 25 weeks showed well-preserved islets with intense insulin immunoreactivity and minimal/no immune cell infiltrate (Fig. 2C). Splenocytes from euglycemic HOT-100% mice displayed reduced in vitro proliferative response to mitogenic stimulation with anti-CD3 antibodies compared with controls (not shown). In addition, the proportions of CD4+ T cells expressing resting (CD4+CD62L+) (Fig. 2D and E) and activation (CD4+CD69+) (Fig. 2F and G) phenotypes in LNs were significantly higher and lower, respectively. Adoptive transfer of splenocytes from recently diabetic NOD mice into NOD.scid mice led to diabetes onset in 40% of mice (n = 5), while none of the recipients of cotransfer (1:1 ratio) with splenocytes from euglycemic HOT-100% mice (n = 5) developed diabetes (Fig. 2H).
FIG. 2.
Impact of HOT on spontaneous diabetes onset in female NOD mice. A: Daily HOT (60-min session, 2.0 ATA) at the indicated oxygen concentrations was started at age 4 weeks and continued for the duration of the follow-up. Control animals received no HOT. The graph indicates the time to diabetes onset in untreated controls (n = 20; median 20.5 weeks, range 16–27) and animals exposed to HOT-21% (ambient air; n = 10; median 23 weeks, range 17–32; P = NS) and HOT-100% (n = 20; median 29.5 weeks, range 19–33). Log-rank test, *P < 0.01 vs. control. B: Serum levels of IL-10 were higher in mice undergoing HOT-100% (23 ± 3.2 pg/mL; n = 9) than in untreated controls (13.1 ± 2.2 pg/mL; n = 9) at age 10 weeks (6 weeks since initiation of HOT). Data for each reading and mean (bar) are shown. Unpaired, two-tailed t test, *P = 0.02. C: Representative microscopic images of pancreatic sections from euglycemic mice in the HOT-100% group at age 25 weeks. H-E staining and immunofluorescent confocal microscopy for insulin (INS, red), glucagon (GCG, green), and nuclear staining (DAPI, blue). D and E: Expression of CD62L in CD4+ cells in iLNs and pLNs of control and HOT-100% mice. *P < 0.01. F and G: Expression of activation marker CD69 in CD4+ cells in iLNs and pLNs of control and HOT-100% mice (n = 2–3 per group). Data expressed as mean ± SEM. *P < 0.01. H: Proportion of NOD.scid mice developing diabetes after adoptive transfer of 20 × 106 NOD splenocytes obtained from recently diabetic NOD mice only (40%; n = 5) or in combination (1:1 ratio) with splenocytes from euglycemic HOT-100% (0%; n = 5) at the end of follow-up. Ctrl, control. (A high-quality color representation of this figure is available in the online issue.)
Short-course HOT reduced insulitis and induced changes in immune cell function in prediabetic NOD mice.
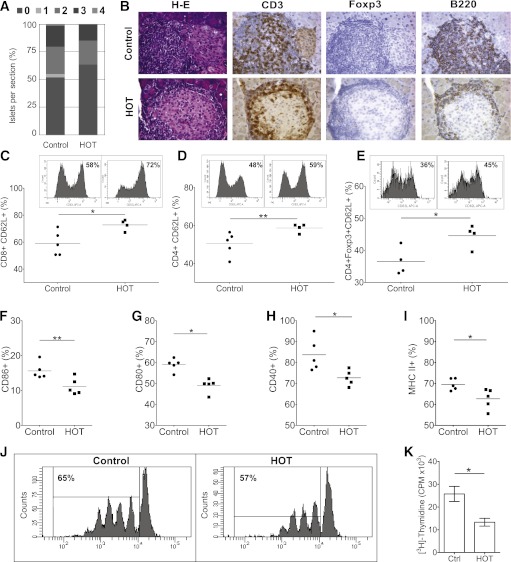
To study the effect of HOT close to the time of diabetes onset, 13-week-old prediabetic female NOD mice were used. A 2-week course of HOT-100% (60-min at 2.0 ATA daily) was associated with significantly improved insulitis scores (0.78 ± 0.07; n = 277 islets; P < 0.012) when compared with control mice (1.01 ± 0.06; n = 400 islets) (Fig. 3A). The mononuclear cells infiltrating islets included CD3+ T cells, a small proportion of Tregs (Foxp3+), and B-cells (B220+) (Fig. 3B). Flow cytometry analysis of splenocytes revealed unremarkable differences between HOT and controls when comparing T cells (percent of CD4+ and CD8+), Tregs (CD4+CD25+Foxp3+), overall proportions of CD11c+ cells, and antigen-presenting cells (not shown). Expression of CD62L on CD8+ (Fig. 3C), CD4+ (Fig. 3D), and CD4+Foxp3+ (Fig. 3E) T cells was significantly higher in HOT than in untreated control mice. Significantly reduced expression of costimulation markers CD86 (Fig. 3F), CD80 (Fig. 3G), and CD40 (Fig. 3H), as well as major histocompatibility complex (MHC) class II expression (Fig. 3I), was observed in DCs of mice receiving HOT compared with controls.
FIG. 3.
Impact of short-course HOT in prediabetic female NOD mice. Single daily HOT (60-min sessions, 2.0 ATA) was given to prediabetic, 13-week-old female NOD mice for 2 weeks. Control animals received no HOT. A: Insulitis score on pancreatic sections in control and HOT-100% groups. The mean insulitis score was 1.01 ± 0.06 in control mice (n = 400 islets) and 0.78 ± 0.07 in HOT mice (n = 277 islets; P < 0.012), respectively (at least three sections per mouse, n = 6 mice per group). Data are mean proportion of islets in a given score per section. B: Pancreatic sections from animals in the two groups: H-E stain and immunohistochemistry with antibodies directed to CD3+ T cells, Tregs expressing Foxp3, and the B-cell marker B220, respectively. C–I: Flow cytometry of splenocytes from HOT-100% or control mice. Increased CD62L expression in HOT-100% mice vs. controls in T cells CD8+ (72.7 ± 4.0 vs. 59.1 ± 9.0; *P < 0.03) (C), CD4+ (58.7 ± 2.3 vs. 50.4 ± 6.1; **P < 0.04) (D), and CD4+Foxp3+ Tregs (44.5 ± 3.5 vs. 36.5 ± 4.2; *P < 0.03) (E). Representative histograms for each cell subset (upper panels) and overall distribution (n = 4–5 mice per condition). Significant reduction in expression of costimulation markers CD80 (49.1 ± 1.5 vs. 59.0 ± 1.3%; **P = 0.001) (F), CD86 (11.1 ± 1.0 vs. 15.6 ± 1.0%; *P = 0.018) (G), and CD40 (72.7 ± 1.6 vs. 83.7 ± 3.5%; *P = 0.020) (H) in CD11b+CD11c+ splenic DCs from HOT-100% mice vs. controls (n = 5 mice per condition), as well as expression of MHC class II in CD11b−CD11c+ splenic DCs (62.7 ± 1.2 vs. 69.5 ± 2.1%; n = 5 mice per condition; *P < 0.025) (I). J and K: In vitro proliferation of CD4+ cells from HOT-100% or control mice exposed to anti-CD3 stimulation. Dilution of CellTrace dye (J) and [3H]-Thymidine incorporation (K). *P < 0.03. CPM, counts per minute; Ctrl, control. (A high-quality digital representation of this figure is available in the online issue.)
Assessment of the efficiency to present an islet peptide to antigen-specific CD4+ T cells from TCR-restricted BDC2.5 mice by CD11c+ DCs obtained from prediabetic NOD mice receiving or not receiving a 2-week course of HOT showed comparable results in vitro (not shown). Upon mitogenic stimulation via TCR engagement with anti-CD3 antibody, significantly reduced proliferation rates were observed in CD4+ cells obtained from HOT-treated mice compared with controls (P < 0.03) (Fig. 3J and K).
Short-course HOT associated with reduced apoptosis and increased proliferation of β-cells in prediabetic NOD mice.
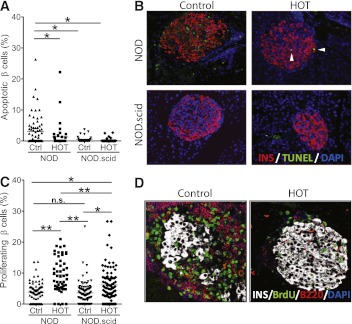
The proportion of transferase-mediated dUTP nick-end labeling (TUNEL)+ insulin+ cells assessed by fluorescence microscopy on pancreatic sections was 3.0 ± 0.6% in untreated mice and 1.3 ± 0.6% in HOT mice (P < 0.01). In NOD.scid mice that lack the autoimmune process, comparable numbers of apoptotic β-cells were observed in both control (0.6 ± 0.2%) and HOT groups (0.3 ± 0.1%), which were significantly lower than immunocompetent NOD mice (Fig. 4A and B), suggesting that the apoptosis is due to the autoimmune attack and that reducing insulitis by HOT in turn reduced cell death.
FIG. 4.
Impact of short-course HOT on β-cell apoptosis and proliferation in prediabetic female NOD mice. Single daily HOT-100% (60-min sessions, 2.0 ATA) was given to prediabetic, 13-week-old female NOD or NOD.scid (age- and sex-matched) mice for 2 consecutive weeks. Control animals received no HOT. A: Proportion of apoptotic β-cells. Data are presented as percent TUNEL+ insulin+ cells per islet and are representative of at least 7–12 islets per animal and six animals per group. Data are presented as mean ± SEM. One-way ANOVA, P < 0.0001; Newman-Keuls multiple comparison test, *P < 0.05; **P < 0.01. B: Representative immunofluorescence micrographs of pancreatic sections from control and HOT groups immunolabeled with anti-insulin antibody (red fluorescence), TUNEL (apoptosis, green fluorescence), and DAPI (nuclei, blue). White arrowheads indicate TUNEL+ nuclei. C: Proportion of proliferating β-cells based on BrdU incorporation during a period of 1 week. Data are presented as percent insulin+ BrdU+ cells per islet and are representative of at least 7–12 islets per animal and six animals per group. Data are expressed as mean ± SEM. One-way ANOVA, P < 0.0001; Newman-Keuls multiple comparison test, *P < 0.01; **P < 0.001. D: Representative immunofluorescence micrographs of pancreatic sections from prediabetic NOD mice undergoing HOT-100% or no treatment immunolabeled with anti-insulin (white color), anti-BrdU (proliferating cell’s nuclei, green), anti-B220 (B cells, red) antibodies, and DAPI (nuclei, blue). Ctrl, control. (A high-quality digital representation of this figure is available in the online issue.)
The proportion of proliferating (BrdU+ insulin+) β-cells was 3.9 ± 0.4% in untreated NOD mice and significantly increased to 9.4 ± 0.7% after HOT (P < 0.001). In a similar manner, the proportion of BrdU+ insulin+ cells in NOD.scid mice was increased from 3.5 ± 0.4 to 6.2 ± 0.6% after HOT (P < 0.01) (Fig. 4C and D). These data suggest that HOT may induce β-cell proliferation.
Lack of stem cell mobilization by HOT.
BMSC mobilization has been recognized to contribute to HOT-mediated protection in other models through induction of nitric oxide (NO) (8,9,26). We assessed BMSC mobilization in prediabetic NOD mice after 2-week HOT-100%. Lineage-negative cells represented a very small proportion of mononuclear cells (<0.6%), with comparable c-kit+, Sca+, c-kit+Sca+, and Flk-1+ cells in the bone marrow and peripheral blood of HOT and control animals (not shown). No difference in CD34+, CD31+, CD34+CD31+, and CD34+Flk-1+ cells were detected in the marrow (not shown). In addition, in the CyP-accelerated autoimmunity model, treatment with NO synthase inhibitor l-NG-nitro-l-arginine methyl ester (l-NAME) did not preclude attaining a reduction of diabetes incidence in animals receiving HOT (not shown), suggesting that the NO pathway may not be involved in our experimental setting.
Effects of HOT on diabetes progression and recurrence.
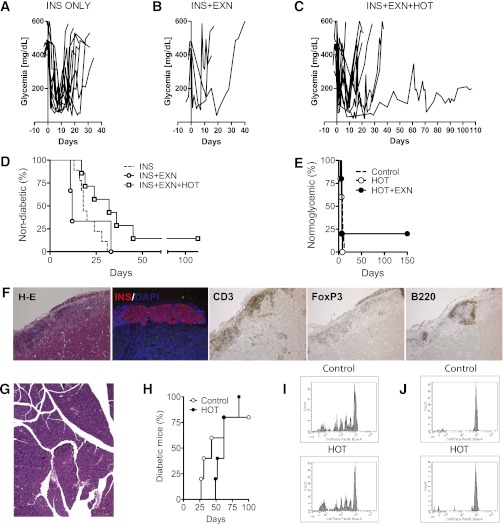
Synergy has been reported after combining glucagon-like peptide-1 agonists with immunotherapy at the time of spontaneous diabetes onset in NOD mice (16,17,27). In our study, insulin therapy alone (n = 12) (Fig. 5A and D) or insulin plus EXN (n = 3) (Fig. 5B and D) resulted in spontaneous diabetes with a median of 18 (range 0–31) and 12 (range 11–33) days, respectively. Mice receiving HOT plus EXN (n = 7) developed hyperglycemia later than controls (median 32 days, range 17–45; P = 0.02 vs. insulin alone) except for one mouse displaying long-term islet function (Fig. 5C and D).
FIG. 5.
Impact of HOT on diabetes progression and recurrence. A–D: Nonfasting blood glucose profile of recently diabetic NOD mice that were treated with either insulin (INS) therapy only by the use of subcutaneous insulin pellets (n = 12) (A); INS+EXN administered at 1.5 ug/day for 14 days via a mini-osmotic pump (n = 3) (B); or INS+EXN+HOT (n = 7) (C). D: Time of recurrence of overt hyperglycemia after initiating therapy. Median survival times were 18 (range 0–31), 12 (range 11–33), and 32 (range 17–45) days (log-rank test, P = 0.02 vs. insulin alone) for groups receiving INS, INS+EXN, or INS+EXN+HOT, respectively. E: Syngeneic islet graft survival in diabetic NOD mice recipients of NOD.scid islets under the kidney capsule. Recurrence of diabetes occurred in untreated and HOT-100% groups with a median of 8 (range 5–12; n = 10) and 7 (range 4–8; n = 5) days, respectively. In the HOT+EXN group, four of five mice developed diabetes promptly (median 7 days, range 6–7) and one maintained graft function for >150 days. F: Histological assessment of islet graft in mouse maintaining syngeneic graft function for >150 days: H-E, immunostaining for insulin (INS/DAPI), T cells (CD3), Tregs (Foxp3), and B cells (B-220). G: Pancreas H-E for same mouse as in F. H: Diabetes incidence in NOD.scid mice treated or not with HOT-100% after transfer of splenocytes from recently diabetic NOD mice. Median survival times were 62 (range 49–85; n = 5) and 43 (range 27–62; n = 5) days for HOT and control mice, respectively. P = NS. I and J: Proliferation of autoreactive cells in vivo. CD4+ cells from BDC2.5-Thy1.1+ were labeled with CellTrace dye and transferred into prediabetic NOD mice. After 3 days, CellTrace dye dilution, evaluated by flow cytometry, was comparable in pLNs of HOT-100% and control mice (n = 5 per group; P = NS) (I), while no proliferation was observed in iLNs (J). (A high-quality digital representation of this figure is available in the online issue.)
Syngeneic NOD.scid islets transplanted into spontaneously diabetic NOD mice invariably lost function in untreated and HOT-100% groups with a median of 8 (range 5–12; n = 10) and 7 (range 4–8; n = 5) days, respectively (Fig. 5E). Hyperglycemia recurred in four of five animals receiving HOT plus EXN (median 7, range 6–7), with one mouse showing sustained function for >150 days. In this mouse, nephrectomy of the graft-bearing kidney promptly restored hyperglycemia. The explanted graft showed well-preserved islet morphology and insulin immunoreactivity in the presence of peri-insular mononuclear cell infiltrate comprising B-cells (B220+) and mostly T cells (CD3+) with numerous Tregs (Foxp3+) (Fig. 5F). The pancreas (overt diabetes for >3 months pretransplant) displayed only a few small-sized islet cell clusters with rare insulin immunoreactivity and minimal mononuclear cells (Fig. 5G).
Administration of HOT-100% to NOD.scid mice starting 1 week before adoptive transfer of splenocytes from spontaneously diabetic NOD mice resulted in hyperglycemia later (n = 5; median 62 days, range 49–85) than controls (n = 5; median 43 days, range 27–62) (Fig. 5H). To assess the effect of HOT on the proliferation of autoreactive T cells in vivo, fluorescently labeled BDC2.5-Thy1.1+CD4+ cells were adoptively transferred into prediabetic female NOD mice receiving or not receiving HOT-100% for 2 weeks prior to inoculum (n = 3/group); comparable degrees of proliferation (measured as fluorescent dye dilution 3 days later) were observed in pLNs (Fig. 5I), while no proliferation occurred in inguinal (i)LNs (Fig. 5J).
DISCUSSION
Immunomodulatory properties of HOT have been reported. In mice, HOT decreased CD8+CD4+ T cells in thymus and B220+ B cells in spleen (13). In a model of graft-versus-host disease after BMSC transplantation in lethally irradiated mice, HOT ameliorated recipients’ survival that was associated with reduced CD4+ and CD8+ T-cell numbers, as well as adhesion molecule expression (CD11a and CD18) (28). Reduced islet and human fetal pancreas immunogenicity has been reported after pretreatment with high oxygen (29), resulting in indefinite survival upon allo- and xenotransplantation (30,31). Depletion of Langerhans cells in allogeneic murine corneas after HOT resulted in long-term acceptance after transplantation (32). Combinatorial use of cyclosporine and HOT prevented rejection of murine allogeneic skin grafts (33). It also has been proposed that hyperoxia may ameliorate the acute net proinflammatory response generated after ischemia-reperfusion injury via inhibition of polymorphonuclear lymphocyte rolling, adhesion, activation, and transmigration to tissues (34) and/or by ameliorating tissue hypoxia—a key trigger of inflammation (35).
In autoimmune-prone MRLlpr/lpr mice, HOT resulted in marked reduction of cellularity in otherwise enlarged spleens and LNs (13). Significant improvement of immune cell status and clinical outcome was observed after HOT in a murine model of lupus (14). It is interesting that HOT effectively treated a clinical case of severe, mutilating vasculitis refractory to conventional therapy (36). Together, these data make a compelling case for the examination of HOT in the autoimmune diabetes setting.
The autoimmune process underlying T1D is the result of genetic predisposition, immunological defects, and environmental factors concurring to the development of autoreactive T cells. An ideal treatment for T1D should preserve functional β-cell mass from autoimmune attack possibly before (i.e., prevention in high-risk subjects) or at the time of diagnosis. Clinical trials show that preservation of C-peptide can be achieved in recently diagnosed T1D after immunotherapy, but the ultimate goal of a persistent restoration of function remains elusive thus far (1). Therefore, safe interventions able to modulate immune responses and preserve β-cell mass long-term need to be explored.
In our study, HOT was associated with significantly reduced autoimmune diabetes incidence in NOD mice in both spontaneous and accelerated (CyP-induced) experimental models. This phenomenon appeared to be dependent on the duration of HOT and oxygen concentration administered. In the CyP model, diabetes occurred in only 48% of animals receiving HOT-100% and 85.3% of controls. Diabetes incidence in the experimental groups receiving depleted HOT (e.g., HOT-21% and HOT-12%) was comparable with untreated controls, indicating that high oxygen is required to elicit the protective effect of HOT.
Significantly increased IL-10 levels were measured in the sera of NOD mice after 6-week HOT-100%. In this group, spontaneous diabetes onset was significantly reduced/delayed and associated with lower insulitis scores when compared with controls. Long-term euglycemic HOT-100% mice showed increased frequency of resting CD4+CD62L+ and lower activated CD4+CD69+ T cells in LNs than controls. Splenocytes from HOT-100% mice suppressed disease transfer when coimplanted with recent-onset diabetic cells, suggesting the presence of cellular subsets able to suppress autoreactive immune cells. In prediabetic NOD mice, a 2-week course of HOT-100% resulted in significantly higher numbers of well-preserved islets with reduced degrees of insulitis and peri-insulitis compared with controls. Moreover, HOT was associated with significantly increased frequencies of resting T cells expressing CD62L. Previous studies showed that DC-expanded islet-specific (e.g., isolated from BDC2.5 NOD mice) CD4+CD25+CD62L+ Tregs efficiently prevented autoimmunity when adoptively transferred to prediabetic, 13-week-old female NOD mice (37). Inoculum of CD4+CD25+CD62L+ T cells from BDC2.5 NOD mice at the time of diabetes onset resulted in hyperglycemia reversal and sustained normoglycemia in 50% of female NOD mice (37). We observed that CD4+ T cells displayed lower proliferation rates than control in response to polyclonal stimulation in vitro (via anti-CD3 antibody) after HOT. In addition, HOT-100% resulted in a significant reduction in antigen-presenting cell activation measured as lower expression of costimulation markers than controls. Collectively, these data point to decreased immune activation in HOT mice, which may have contributed ultimately to the reduction of insulitis and protection from T1D observed in our study. It is notable that the degree of generalized immunosuppression achieved in our experimental setting did not appear to represent a hazard for the treated mice that despite chronic HOT, did not show increased morbidity and mortality.
HOT appeared less effective when the autoimmune process was already established. Interfering with established autoimmune diabetes has proven quite challenging in recent clinical intervention trials at the time of T1D onset in which immunotherapy resulted in only a transient preservation of C-peptide (1). Recurrence of autoimmunity has been recognized as difficult to control even in chronically immunosuppressed pancreas transplant recipients, in which selective β-cell destruction was associated with the persistence and increased frequency of autoreactive T cells and autoantibody titers refractory to relatively harsh rescue immunotherapies (38).
When administered soon after spontaneous diabetes onset, HOT combined with EXN delayed the progression of the disease that occurred in the majority of the mice. Adoptive transfer of diabetes in NOD.scid mice was delayed by HOT but not prevented. When BDC2.5-Thy1.1+CD4+ cells were adoptively transferred in prediabetic NOD mice treated or not with HOT-100%, comparable rates of proliferation were observed in pLNs. Although the latter model of TCR-restricted islet antigen–specific T cells may be too stringent to observe protection, because of the very acute response (3 days), these data suggest inadequate suppression of already activated autoreactive T cells and point to the need for combinatorial strategies to synergize with HOT after T1D is established. Indeed, long-term function was observed only in a few animals receiving HOT plus EXN at onset and in the transplant setting. Treatments that increase incretin levels are already in clinical use. The use of EXN in combinatorial regimens was shown to be synergistic in T1D intervention trials in NOD mice, possibly through its cytoprotective effects on β-cells (16,17,27). In addition, anti-inflammatory properties of EXN have been recently described (39), which may also contribute to preserving β-cell mass. Nonetheless, the immunomodulating effects of HOT and EXN may be inadequate to counteract the autoimmune process, which could be overcome by combinatorial use of immunotherapy (i.e., T-cell depletion, costimulatory blockade, or modulation of inflammatory pathways, among others) to enhance the success rates, particularly after autoimmunity establishment.
Significant reduction of β-cell death was observed after HOT-100% treatment in prediabetic NOD mice. This is in keeping with the significantly lower insulitis scores observed in animals receiving HOT compared with controls, which, in turn, resulted in preservation of islet β-cells from autoimmune destruction. Unexpectedly, a significant increase in β-cell proliferation was observed after HOT-100%, even in immunodeficient NOD.scid mice that displayed a twofold increase in β-cell proliferation over baseline after HOT. It is interesting that the proportions of proliferating β-cells were significantly lower in NOD.scid than in NOD mice, possibly as the result of a physiological increase in β-cell proliferation occurring during the progression of insulitis in response to increased metabolic demand. Proliferation of β-cells has been demonstrated in pancreatitis models in rodents (40), and it is possible that the inflammatory milieu in islets may activate survival/replication pathways in β-cells as part of local tissue remodeling. Pancreatic islets are richly vascularized endocrine cell clusters representing ∼1% of the pancreas and receiving ∼20% of the blood supply. In mice, the appearance of endocrine cells in the embryonic pancreas coincides with the formation of new blood vessels, suggesting a key role of oxygen in the maturation of endocrine cells, which is supported by the observed enhanced maturation of pancreatic endocrine precursors in vitro into endocrine cells exposed to optimal oxygen concentrations (41), possibly via the modulation of the hypoxia-inducible factor-1α pathway (42). It is conceivable that high oxygen concentrations generated by HOT in the local microenvironment may enhance β-cell mass and/or regeneration in our model. Further studies are needed to understand the mechanisms underlying the increased replicative potential of β-cells exposed to HOT. Beneficial effects of HOT on the metabolic control of patients with diabetes recently have been reported in nonrandomized studies lacking mechanistic data. Improved glycemic control and reduced insulin requirements have been reported in a pilot type 2 diabetes trial in patients receiving HOT and intra-arterial injection of bone marrow mononuclear cells (11). Significant improvement of fasting glycemia, HbA1c levels, and homeostasis model assessment of insulin resistance were reported after HOT for diabetic foot ulcers in patients with type 2 diabetes (43). Improved metabolic function was also described in a large number of subjects with T1D undergoing HOT in addition to conventional therapy (44), and it has been suggested that HOT might ameliorate diabetes complications (45).
HOT may enhance tissue regeneration (46,47) and repair processes (i.e., wound healing) (48,49) as a result of the mobilization of BMSCs via induction of increased NO levels (8,9,26). No significant differences were detected in our model to support this mechanism. This might reflect intrinsic differences in BMSC function in NOD mice, when compared with nondiabetes-prone mouse strains. In addition, our preliminary data suggest that the NO pathway is not involved in the beneficial effects observed after HOT, since treatment with the NO synthase inhibitor l-NAME did not prevent the ability to reduce diabetes incidence in animals receiving HOT (not shown). Thus, other mechanisms may be operational in our model.
Our data suggest that HOT may reduce the incidence of autoimmune diabetes in NOD mice via reduction of insulitis, possibly through the modulation of T-cell and DC functions, which in turn result in preservation of β-cell mass via reduction of apoptosis and enhanced proliferation. HOT is a safe therapy that may be considered as part of combinatorial strategies to preserve/restore β-cell mass and halt autoimmunity in future clinical trials. Since our results showed a stronger effect of HOT in prevention of T1D rather than after disease onset, we envision its use for the treatment of subjects at high risk of developing T1D. In addition, the lack of side effects makes HOT an ideal candidate to be used in combinatorial therapies aimed at halting the progression of insulitis, for instance, in conjunction with biologics that target immune cell function and/or agents that may enhance functional β-cell mass. Furthermore, in-depth understanding of the molecular pathways involved in the beneficial effects of HOT in T1D could help identify potential targets for novel drugs in the future.
ACKNOWLEDGMENTS
This work was supported by a grant from the Diabetes Research Institute Foundation (DRI; www.DiabetesResearch.org) to C.R. and A.P. The study relied heavily on the availability of shared infrastructures at the DRI, including the Preclinical Cell Processing and Translational Models, the Imaging and Flow Cytometry Cores (all partially supported by the Juvenile Diabetes Research Foundation International), and the University of Miami (UM) Miller School of Medicine’s Animal Care and Use Committee and Division of Veterinary Resources. The UM has an Animal Welfare Assurance on file (A-3224-01, effective 12/4/02) with the Office of Laboratory Animal Welfare, National Institutes of Health, as well as full accreditation by the Association for Assessment and Accreditation of Laboratory Animal Care.
This work also was supported in part by Converge Biotech, Inc., Miami, Florida. C.R. and A.P. are cofounders and members of the scientific advisory board of Converge Biotech, Inc. R.D.M., C.R., and A.P. are stock option holders in Converge Biotech, Inc. There are no patents, products in development, or marketed products to declare. No other potential conflicts of interest relevant to this article were reported.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
G.F., C.F., N.B., and R.D.M. contributed to study design, researched data, and wrote the manuscript. E.Z.-A., J.M., S.V., and O.U. researched data. J.S.S. and A.L.B. contributed to study design and reviewed the manuscript. C.R. and A.P. conceived and designed the studies, analyzed data, and wrote the manuscript. A.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors are grateful to Drs. Alberto Pugliese (UM-DRI), Eckhard Podack (UM), Luca Inverardi (UM-DRI), Ricardo L. Pastori (UM-DRI), Rodolfo Alejandro (UM-DRI), Christopher A. Fraker (UM-DRI), Juan Dominguez-Bendala (UM-DRI), George McNamara (UM-DRI), Pramod K. Srivastava (University of Connecticut), and Mark Anderson (University of California–San Francisco) for invaluable discussions. Special thanks to Yelena Gadea (UM-DRI), Irayme Labrada (UM-DRI), Maite Lopez-Cabezas (UM-DRI), and Kevin Johnson (UM-DRI) for outstanding technical assistance.
Footnotes
See accompanying commentary, p. 1664.
REFERENCES
- 1.Skyler JS, Ricordi C. Stopping type 1 diabetes: attempts to prevent or cure type 1 diabetes in man. Diabetes 2011;60:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant 2008;8:1990–1997 [DOI] [PubMed] [Google Scholar]
- 3.Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol 2009;106:988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlodavsky E, Palzur E, Soustiel JF. Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model of traumatic brain injury. Neuropathol Appl Neurobiol 2006;32:40–50 [DOI] [PubMed] [Google Scholar]
- 5.Wilson HD, Toepfer VE, Senapati AK, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment is comparable to acetylsalicylic acid treatment in an animal model of arthritis. J Pain 2007;8:924–930 [DOI] [PubMed] [Google Scholar]
- 6.Wilson HD, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Res 2006;1098:126–128 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Chang Q, Cox RA, Gong X, Gould LJ. Hyperbaric oxygen attenuates apoptosis and decreases inflammation in an ischemic wound model. J Invest Dermatol 2008;128:2102–2112 [DOI] [PubMed] [Google Scholar]
- 8.Gallagher KA, Goldstein LJ, Thom SR, Velazquez OC. Hyperbaric oxygen and bone marrow-derived endothelial progenitor cells in diabetic wound healing. Vascular 2006;14:328–337 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein LJ, Gallagher KA, Bauer SM, et al. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells 2006;24:2309–2318 [DOI] [PubMed] [Google Scholar]
- 10.Milovanova TN, Bhopale VM, Sorokina EM, et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J Appl Physiol 2009;106:711–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrada EJ, Valacchi F, Nicora E, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant 2008;17:1295–1304 [DOI] [PubMed] [Google Scholar]
- 12.Lee AK, Hester RB, Coggin JH, Gottlieb SF. Increased oxygen tensions influence subset composition of the cellular immune system in aged mice. Cancer Biother 1994;9:39–54 [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Yi H, Kato M, et al. Differential sensitivities to hyperbaric oxygen of lymphocyte subpopulations of normal and autoimmune mice. Immunol Lett 1997;59:79–84 [DOI] [PubMed] [Google Scholar]
- 14.Chen SY, Chen YC, Wang JK, et al. Early hyperbaric oxygen therapy attenuates disease severity in lupus-prone autoimmune (NZB x NZW) F1 mice. Clin Immunol 2003;108:103–110 [DOI] [PubMed] [Google Scholar]
- 15.Zhang YC, Pileggi A, Agarwal A, et al. Adeno-associated virus-mediated IL-10 gene therapy inhibits diabetes recurrence in syngeneic islet cell transplantation of NOD mice. Diabetes 2003;52:708–716 [DOI] [PubMed] [Google Scholar]
- 16.Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes 2004;53:1700–1705 [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Chen M, Carter JD, et al. Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun 2006;344:1017–1022 [DOI] [PubMed] [Google Scholar]
- 18.Molano RD, Berney T, Li H, et al. Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes 2001;50:270–276 [DOI] [PubMed] [Google Scholar]
- 19.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell 1993;74:1089–1100 [DOI] [PubMed] [Google Scholar]
- 20.Molano RD, Pileggi A, Berney T, et al. Prolonged islet allograft survival in diabetic NOD mice by targeting CD45RB and CD154. Diabetes 2003;52:957–964 [DOI] [PubMed] [Google Scholar]
- 21.Li H, Inverardi L, Molano RD, Pileggi A, Ricordi C. Nonlethal conditioning for the induction of allogeneic chimerism and tolerance to islet allografts. Transplantation 2003;75:966–970 [DOI] [PubMed] [Google Scholar]
- 22.Zahr E, Molano RD, Pileggi A, et al. Rapamycin impairs in vivo proliferation of islet beta-cells. Transplantation 2007;84:1576–1583 [DOI] [PubMed] [Google Scholar]
- 23.Augstein P, Elefanty AG, Allison J, Harrison LC. Apoptosis and beta-cell destruction in pancreatic islets of NOD mice with spontaneous and cyclophosphamide-accelerated diabetes. Diabetologia 1998;41:1381–1388 [DOI] [PubMed] [Google Scholar]
- 24.Kim YH, Kim S, Kim KA, et al. Apoptosis of pancreatic beta-cells detected in accelerated diabetes of NOD mice: no role of Fas-Fas ligand interaction in autoimmune diabetes. Eur J Immunol 1999;29:455–465 [DOI] [PubMed] [Google Scholar]
- 25.Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia 1984;27:604–606 [DOI] [PubMed] [Google Scholar]
- 26.Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol 2006;290:H1378–H1386 [DOI] [PubMed] [Google Scholar]
- 27.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 2007;148:5136–5144 [DOI] [PubMed] [Google Scholar]
- 28.Song XY, Sun LN, Zheng NN, Zhang HP. Effect of hyperbaric oxygen on acute graft-versus-host disease after allogeneic bone marrow transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2008;16:623–626 [PubMed] [Google Scholar]
- 29.MacKenzie DA, Sollinger HW, Hullett DA. Decreased immunogenicity of human fetal pancreas allografts following hyperbaric oxygen culture. Transplant Proc 2003;35:1499–1502 [DOI] [PubMed] [Google Scholar]
- 30.Bowen KM, Andrus L, Lafferty KJ. Successful allotransplantation of mouse pancreatic islets to nonimmunosuppressed recipients. Diabetes 1980;29(Suppl. 1):98–104 [DOI] [PubMed] [Google Scholar]
- 31.Sullivan FP, Ricordi C, Hauptfeld V, Lacy PE. Effect of low-temperature culture and site of transplantation on hamster islet xenograft survival (hamster to mouse). Transplantation 1987;44:465–468 [DOI] [PubMed] [Google Scholar]
- 32.He YG, Niederkorn JY. Depletion of donor-derived Langerhans cells promotes corneal allograft survival. Cornea 1996;15:82–89 [PubMed] [Google Scholar]
- 33.Erdmann D, Roth AC, Hussmann J, et al. Hyperbaric oxygen and cyclosporine as a combined treatment regimen to prevent skin allograft rejection in immunohistoincompatible mice. Ann Plast Surg 1996;36:304–308 [DOI] [PubMed] [Google Scholar]
- 34.Tjärnström J, Wikström T, Bagge U, Risberg B, Braide M. Effects of hyperbaric oxygen treatment on neutrophil activation and pulmonary sequestration in intestinal ischemia-reperfusion in rats. Eur Surg Res 1999;31:147–154 [DOI] [PubMed] [Google Scholar]
- 35.Nathan C. Immunology: oxygen and the inflammatory cell. Nature 2003;422:675–676 [DOI] [PubMed] [Google Scholar]
- 36.Jacobs P, Wood L, Van Niekerk GD. Therapy: hyperbaric oxygen as the only effective treatment in mutilating and resistant systemic vasculitis. Hematology 2000;5:167–172 [DOI] [PubMed] [Google Scholar]
- 37.Tarbell KV, Petit L, Zuo X, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med 2007;204:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vendrame F, Pileggi A, Laughlin E, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes 2010;59:947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cechin SR, Pérez-Álvarez I, Fenjves E, et al. Anti-inflammatory properties of exenatide in human pancreatic islets. Cell Transplant. 7 June 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Cano DA, Rulifson IC, Heiser PW, et al. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes 2008;57:958–966 [DOI] [PubMed] [Google Scholar]
- 41.Fraker CA, Alvarez S, Papadopoulos P, et al. Enhanced oxygenation promotes beta-cell differentiation in vitro. Stem Cells 2007;25:3155–3164 [DOI] [PubMed] [Google Scholar]
- 42.Heinis M, Simon MT, Ilc K, et al. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 2010;59:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karadurmus N, Sahin M, Tasci C, et al. Potential benefits of hyperbaric oxygen therapy on atherosclerosis and glycaemic control in patients with diabetic foot. Endokrynol Pol 2010;61:275–279 [PubMed] [Google Scholar]
- 44.Efuni SN, Kakhnovskiĭ IM. The role of hyperbaric oxygenation in the complex treatment of patients with type 1 diabetes mellitus. Vestn Akad Med Nauk SSSR 1989;(5):70–76 [in Russian] [PubMed] [Google Scholar]
- 45.Ostashevskaia MI, Afanas’eva NB, Valuĭskova RP, Serebriakova AD, Shirenkov IuA. Experience in the use of hyperbaric oxygenation to treat diabetes mellitus in children. Probl Endokrinol (Mosk) 1986;32:16–19 [in Russian] [PubMed] [Google Scholar]
- 46.Muralidharan V, Christophi C. Hyperbaric oxygen therapy and liver transplantation. HPB (Oxford) 2007;9:174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori H, Shinohara H, Arakawa Y, et al. Beneficial effects of hyperbaric oxygen pretreatment on massive hepatectomy model in rats. Transplantation 2007;84:1656–1661 [DOI] [PubMed] [Google Scholar]
- 48.Khan M, Meduru S, Mohan IK, et al. Hyperbaric oxygenation enhances transplanted cell graft and functional recovery in the infarct heart. J Mol Cell Cardiol 2009;47:275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan HC, Chin CS, Yang DY, et al. Human amniotic fluid mesenchymal stem cells in combination with hyperbaric oxygen augment peripheral nerve regeneration. Neurochem Res 2009;34:1304–1316 [DOI] [PubMed] [Google Scholar]