In his masterpiece War and Peace (1869), Leo Tolstoy wrote: “The human intellect with no inkling of the immense variety and complexity of circumstances conditioning a phenomenon, any one of which may be separately conceived as the cause of it, snatches at the first and the most easily understood approximation, and says: ‘Oh, here is the cause!’” The field of type 1 diabetes has been plagued by this trend for oversimplification. As a result, individual pathways are often suggested as “the cause” of this complex, multifactorial, and probably heterogeneous disease, thereby leading to therapeutic attempts that nearly always end in failure.
MicroRNAs (miRNAs) are a family of endogenous single-stranded RNA molecules around 22 nucleotides in length (1). They are highly conserved among species and regulate the expression of partially complementary protein-coding genes by either degrading or preventing translation of target messenger RNAs. miRNAs are transcribed from individual genes, located in exons or introns of protein-coding genes or in intergenic regions, and are often clustered (2). Importantly, >50% of human genes may be at least in part regulated by miRNAs (3), adding an extra level of regulation for gene expression. An article by Roggli et al. (4) published in this issue of Diabetes describes a microarray analysis on islets obtained from 4- and 8-week-old female NOD mice (an animal model of autoimmune diabetes, in which 4-week-old animals have little or no immune infiltration whereas 8-week-old mice show insulitis in most islets; hyperglycemia usually starts after 14 weeks of age). They observed a preferential increase in miR-29a/b/c in the islets obtained from 8-week-old animals, a finding confirmed by other methods in islets from 13- to 14-week-old NOD mice and in isolated human and mouse islets exposed to the proinflammatory cytokines interleukin-1β, tumor necrosis factor-α, and interferon-γ, cytokines that probably contribute to β-cell apoptosis in type 1 diabetes (5). Quantitative PCR and in situ hybridization analysis, coupled with immunofluorescence for insulin, confirmed that increased miR-29 expression takes place in β-cells and not in the infiltrating immune cells. In subsequent and elegant mechanistic studies, they showed that overexpression of miR-29a/b/c in insulin-producing MIN6 cells and dissociated mouse islets contributes to impaired glucose-induced insulin release and apoptosis by respectively inhibiting expression of the transcription factor Onecut2 (which leads to a rise in granuphilin, an antagonist of insulin exocytosis [6,7]) and of the antiapoptotic Bcl-2 family member Mcl-1, which plays an important protective role against cytokine-induced β-cell apoptosis (8). These findings, together with previous observations from the same group (confirmed in the current study) showing that prolonged exposure of pancreatic β-cells to proinflammatory cytokines induces expression of three other miRNAs (miR-21, miR-34a, and miR-146, which also affect insulin release and apoptosis) (9), strongly suggest that preferential induction of key miRNAs during islet inflammation constitutes a novel level of regulation of β-cell dysfunction and apoptosis during the early stages of type 1 diabetes.
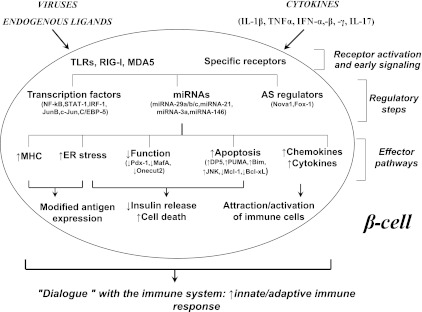
This publication, together with other recent articles suggesting that proinflammatory cytokines modify alternative splicing (AS) in human islets (10,11) and that >60% of the candidate genes for type 1 diabetes may actually act at the pancreatic islet level (11–13), support two conclusions: First, an important part of the action in type 1 diabetes takes place at the pancreatic β-cell level, changing the purely “immune system–centric” vision that has prevailed up to now in the field; and second, the “decision” of the pancreatic β-cell to undergo apoptosis, a central event in type 1 diabetes, depends on several layers of regulation. This regulation includes gene transcription, AS, expression of noncoding messenger RNAs (such as miRNAs and probably also large noncoding RNAs) and posttranslational modifications of proteins secondary to endoplasmic reticulum stress and other mechanisms. As described in Fig. 1, these “checks and balances” may impact β-cell death, expression of neoantigens, and attraction of immune cells.
FIG. 1.
A hypothetical model of the β-cell responses that contribute to insulitis and progressive loss of β-cell mass in type 1 diabetes. Locally produced cytokines (e.g., interleukin [IL]-1β, interferons -α, -β, and -γ, tumor necrosis factor-α, and IL-17) or “danger signals” provided by viruses or endogenous ligands of interferon-induced helicase 1/melanoma differentiation-associated gene 5 (IFIH1/MDA5) and other innate immune response sensors (e.g., retinoic acid–inducible gene I [RIG-I] and Toll-like receptor [TLR] 3) activate transcription factors including signal transducer and activator of transcription-1 (STAT-1) and nuclear factor-κB (NF-κB), miRNAs such as miRNA-29 a/b/c, and regulators of AS such as neuro-oncological ventral antigen 1 (Nova1). Downstream of these and other regulatory factors, there is triggering of endoplasmic reticulum (ER) stress and upregulation of the machinery for antigen presentation. This, together with changes in AS, may generate neoantigens that induce or augment β-cell recognition by the immune system. Additional signals provided by β-cells to the immune system include the production and release of chemokines and cytokines and cell death, which, in the context of local inflammation, may function as “danger signal” for the immune system. β-Cell apoptosis is regulated by key miRNAs and transcription factors and by endoplasmic reticulum stress, culminating in the activation of the intrinsic mitochondrial pathway of apoptosis. Type 1 diabetes candidate genes such as PTPN2 and MDA-5 regulate many of the different steps shown in the figure, besides their effects at the immune system level, providing a link between the genetics of type 1 diabetes and the mechanisms leading to β-cell loss. Activated immune cells, attracted by the local production of chemokines, will produce more cytokines and chemokines, perpetuating the local inflammatory response and changes in miRNAs, transcription factors, and AS. The figure was modified from ref. 5. Additional information, and supporting references, can be found in refs. 5 and 14–16. CEBP-δ, CCAAT/enhancer binding protein-δ; IRF, interferon regulatory factor; JNK, c-Jun N-terminal kinase; Pdx-1, pancreatic and duodenal homeobox 1.
Although the current and previous studies in the miRNA field (17,18) have yielded a new group of regulatory factors in the natural history of β-cell loss in diabetes, many questions remain to be answered. Do candidate genes for type 1 diabetes impact miRNA expression, as they seem to do for other β-cell gene regulators and effector mechanisms? Which are the transcription factors that regulate expression of the miR-29 family? Is there a cross-talk between key cytokine-modulated transcription factors and miRNAs in the regulation of effector mechanisms of β-cell dysfunction and death? Given that miRNAs also regulate cells from the immune system in type 1 diabetes (17,18), is there similarity or cross-talk between these miRNAs and the ones implicated in β-cell dysfunction/death? Because AS can regulate the effect of miRNAs by removing/adding potential binding sites for these molecules, is there a cross-talk between cytokine-induced AS and miRNAs in pancreatic β-cells? Since MiRNAs are surprisingly stable in circulation and have been suggested as biomarkers for cancer (19), can they be used as early biomarkers for β-cell dysfunction/death and/or activation of the immune system against these cells?
Thanks to recent advances in technology that allow reliable determination of all coding and noncoding transcripts in pancreatic β-cells and other cell types, as well as the development of novel bioinformatics tools that enable organization and interpretation of these “data mountains,” we are for the first time able to face the immense variety and complexity of circumstances conditioning progressive β-cell loss in type 1 diabetes. Integration of these findings with ongoing work focused on understanding the loss of immune tolerance against β-cells in early type 1 diabetes (20) will hopefully point to novel and rational targets to prevent/revert the disease.
ACKNOWLEDGMENTS
Research in the laboratory of D.L.E. is supported by grants from the European Union (projects BetaBat and Naimit in the Framework Programme 7 of the European Community), JDRF, the Fonds National de la Recherche Scientifique: Belgium, and the Communauté Française de Belgique–Actions de Recherche Concertées (ARC).
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1742.
REFERENCES
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–297 [DOI] [PubMed] [Google Scholar]
- 2.Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 2004;23:4051–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roggli E, Gattesco S, Caille D, et al. Changes in microRNA expression contribute to pancreatic β-cell dysfunction in prediabetic NOD mice. Diabetes 2012;61:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 6.Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R. Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell 2002;13:1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol 2005;171:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allagnat F, Cunha D, Moore F, Vanderwinden JM, Eizirik DL, Cardozo AK. Mcl-1 downregulation by pro-inflammatory cytokines and palmitate is an early event contributing to β-cell apoptosis. Cell Death Differ 2011;18:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roggli E, Britan A, Gattesco S, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes 2010;59:978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortis F, Naamane N, Flamez D, et al. Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes 2010;59:358–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet 2012;8:e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore F, Colli ML, Cnop M, et al. PTPN2, a candidate gene for type 1 diabetes, modulates interferon-gamma-induced pancreatic beta-cell apoptosis. Diabetes 2009;58:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergholdt R, Brorsson C, Palleja A, et al. Identification of novel type 1 diabetes candidate genes by integrating genome-wide association data, protein-protein interactions, and human pancreatic islet gene expression. Diabetes 2012;61:954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colli ML, Moore F, Gurzov EN, Ortis F, Eizirik DL. MDA5 and PTPN2, two candidate genes for type 1 diabetes, modify pancreatic beta-cell responses to the viral by-product double-stranded RNA. Hum Mol Genet 2010;19:135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurzov EN, Eizirik DL. Bcl-2 proteins in diabetes: mitochondrial pathways of β-cell death and dysfunction. Trends Cell Biol 2011;21:424–431 [DOI] [PubMed] [Google Scholar]
- 16.Santin I, Moore F, Colli ML, et al. PTPN2, a candidate gene for type 1 diabetes, modulates pancreatic β-cell apoptosis via regulation of the BH3-only protein Bim. Diabetes 2011;60:3279–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guay C, Roggli E, Nesca V, Jacovetti C, Regazzi R. Diabetes mellitus, a microRNA-related disease? Transl Res 2011;157:253–264 [DOI] [PubMed] [Google Scholar]
- 18.Sebastiani G, Vendrame F, Dotta F. MicroRNAs as new tools for exploring type 1 diabetes: relevance for immunomodulation and transplantation therapy. Transplant Proc 2011;43:330–332 [DOI] [PubMed] [Google Scholar]
- 19.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol 2011;8:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virgin HW, Todd JA. Metagenomics and personalized medicine. Cell 2011;147:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]