Abstract
Cardiovascular autonomic neuropathy (CAN) is associated with increased mortality in diabetes. Since CAN often develops in parallel with diabetic nephropathy as a confounder, we aimed to investigate the isolated impact of CAN on cardiovascular disease in normoalbuminuric patients. Fifty-six normoalbuminuric, type 1 diabetic patients were divided into 26 with (+) and 30 without (−) CAN according to tests of their autonomic nerve function. Coronary artery plaque burden and coronary artery calcium score (CACS) were evaluated using computed tomography. Left ventricular function was evaluated using echocardiography. Blood pressure and electrocardiography were recorded through 24 h to evaluate nocturnal drop in blood pressure (dipping) and pulse pressure. In patients +CAN compared with −CAN, the CACS was higher, and only patients +CAN had a CACS >400. A trend toward a higher prevalence of coronary plaques and flow-limiting stenosis in patients +CAN was nonsignificant. In patients +CAN, left ventricular function was decreased in both diastole and systole, nondipping was more prevalent, and pulse pressure was increased compared with −CAN. In multivariable analysis, CAN was independently associated with increased CACS, subclinical left ventricular dysfunction, and increased pulse pressure. In conclusion, CAN in normoalbuminuric type 1 diabetic patients is associated with distinct signs of subclinical cardiovascular disease.
Cardiovascular autonomic neuropathy (CAN) is a severe complication of diabetes associated with increased morbidity and mortality (1,2). CAN results from damage to the autonomic nerve fibers to the heart, and an early manifestation is a decrease in heart rate variation (HRV) during deep breathing (3). CAN is present in 25% of type 1 diabetic patients but is often overlooked (1,3).
The increased mortality associated with CAN appears to be partly explained by coexistence with other long-term complications of diabetes (4), and since especially nephropathy and CAN generally develop in parallel, the relative contribution of CAN on mortality has been difficult to identify (5). However, some studies have found CAN to be associated with sudden cardiac death (6) and to be an independent predictor of mortality (7,8). Different mechanisms through which CAN may promote mortality have been suggested including silent myocardial ischemia, silent myocardial infarction, impaired respiratory response to hypoxia, intraoperative cardiovascular lability, and fatal arrhythmia due to QT prolongation (2,5,9–11). However, the mechanism remains unclear (12,13).
A noninvasive evaluation of coronary artery stenosis can be performed with high diagnostic accuracy using multislice computed tomography (MSCT) coronary angiography (14). MSCT also allows assessment of coronary artery calcification, which can be quantified as a coronary artery calcium score (CACS). Coronary artery calcium deposit has been shown to be a strong predictor of future cardiovascular events (15–17). Other markers of increased cardiovascular risk have recently been introduced: left ventricular dysfunction evaluated with tissue Doppler imaging (TDI) (18,19), a reduction in nighttime blood pressure drop (20,21), and increased arterial pulse pressure (22) are all independently associated with increased cardiovascular risk.
The aim of this study was to investigate the potential association between CAN and subclinical coronary atherosclerosis in addition to other prognostic markers of cardiovascular disease. For avoidance of the confounding effect of diabetic nephropathy, only patients with normal albumin excretion rates (albuminuria) were included.
RESEARCH DESIGN AND METHODS
Patients with long-lasting type 1 diabetes, persistent normoalbuminuria, and no history or symptoms of cardiac disease (23) were recruited from the outpatient clinic cohort of type 1 diabetic patients at Steno Diabetes Center and the Diabetes Unit, Rigshospitalet. Inclusion criteria were type 1 diabetes according to American Diabetes Association criteria of at least 10 years’ duration, age between 18 and 75 years, and HbA1C <10%. Exclusion criteria were albuminuria (urinary albumin-to-creatinine ratio >30 mg/g; elevated S-creatinine >120 μmol/L), untreated hypertension (>140/85 mmHg), and electrocardiographic signs or clinical symptoms of heart disease.
In the study population registry of 3,000 type 1 diabetic patients, autonomic testing had previously been performed in 350 patients owing to clinical suspicion of autonomic neuropathy, and 123 of these patients were eligible according to inclusion/exclusion criteria. CAN testing was repeated in all patients included in the current study.
From this group, informed consent to participate in the study was obtained in 60 randomly selected patients during their visit to the outpatient clinic. Patients were divided into two groups according to the outcome of four autonomic function tests: HRV during deep breathing, Valsalva ratio, lying-to-standing test, and blood pressure response to standing up. CAN was defined as two or more abnormal tests (24), and age-normative values were used to define abnormality (25,26). Of the included 60 patients, 26 had CAN, 30 were without CAN, and 4 had inconclusive tests.
All patients underwent MSCT and transthoracic echocardiography (TTE). In order to decrease heart rate for optimized image quality, all patients were given 5 mg ivabradine orally on the evening before and on the morning of cardiac imaging. Ivabradine was selected to reduce heart rate because β-adrenoceptor blockade was considered relative contraindicated in this population. MSCT and TTE were performed on the same day, and 24-h blood pressure and Holter monitoring was performed simultaneously after a few days. Information on retinopathy was retrieved from the records of the patients, as retinopathy status is registered at least once a year. Vibratory perception threshold (V) was evaluated using biothesiometry (Bio-Medical Instrument Company, Newbury, OH) as an assessment of distal neuropathy. Blood and urine samples were collected and analyzed with standardized clinical chemistry methods including measurements of N-terminal pro-brain natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein, and cystatin in addition to routine blood tests to exclude signs of other diseases or neuropathies.
Coronary artery disease evaluated with MSCT was the primary outcome measure. Other parameters were included as confounding factors in the analysis.
All measurements and analysis were performed with the investigator blinded to the CAN status of the patients. The study was conducted in accordance with the Declaration of Helsinki II and approved by the local ethics committee (protocol number H-4-2009-091). All patients gave written informed consent.
Cardiovascular autonomic neuropathy tests.
Age-normative values were used to define abnormality in all tests (25,26). HRV was assessed in previously trained patients who were asked to breathe deeply at a rate of six breaths per minute while being monitored on a (50 mm/s) 12-lead electrocardiography. The maximum and minimum heart rates during each breathing cycle were measured, and the mean difference of six cycles was calculated.
The lying-to-standing heart rate ratio was determined after at least 5 min rest in the supine position, and HRV was determined by calculating the maximal-to-minimal heart rate ratio: the longest R-R interval, measured around the 30th beat after standing up, to the shortest R-R interval, measured around the 15th beat after standing up.
The Valsalva test consisted of forced exhalation into a mouthpiece with a pressure of 40 mmHg for 15 s, and the ratio of the maximum R-R after the maneuver to the minimum R-R during the maneuver was calculated. The test was performed three times, and the mean value of the ratios was used.
Orthostatic hypotension was defined as a decrease in systolic blood pressure (SBP) of 30 mmHg when changing from supine to the upright position. Measurements were taken every minute for at least 3 min.
MSCT.
All examinations were performed using a Toshiba Aquillon One 320 Volume scanner (Toshiba Medical Systems, Tokyo, Japan) according to the recommendations of the vendor and analyzed on dedicated software (Vitrea 2; Vital Images).
First, a prospective low-dose calcium scan without contrast enhancement was performed followed by coronary angiography using a prospective protocol. For the calcium score, slices of 3 mm were acquired using prospective electrocardiogram-gated axial scanning (27). We infused 80–100 mL intravenous contrast agent (Visipaque 320; GE Healthcare) (according to body weight) with a flow rate of 5 mL/s, followed by a saline chaser (50 mL). Image acquisition was initiated automatically at a density threshold of 180 Hounsfield units (HU) in the descending aorta. The scan parameters were 320 × 0.5 mm detector collimation and 100–120 kV tube voltage depending on BMI (threshold 30 kg/m2). Rotation time was between 350 and 375 ms depending on the heart rate. Owing to the relatively high heart rate (25 patients with heart rate >65 bpm), the scanners (sureCardio software) frequently required two gantry rotations. Median radiation dose for nonconstrast and for angiography scans was 1.2 mSv (range 0.9–2.6) and 4.3 mSv (1.9–15.3), respectively. For the coronary computed tomography angiography, 0.5-mm slices were obtained. The images were reconstructed at 0.5-mm slices with an increment of 0.25 mm, thereby giving true isotropic voxels of 0.5 mm (28).
Coronary calcium scoring.
Coronary calcium deposit was identified as a dense area in the coronary artery exceeding the threshold of 130 HU. Areas were automatically registered by the software and were manually assigned the corresponding coronary arteries. Total mass of the coronary calcium deposit and the Agatston score were determined for each patient. CACS was compared with reference values on an individual level according to age and sex (29,30).
Coronary stenosis and plaque composition.
Based on computed tomography coronary angiography recordings, the presence of coronary atherosclerosis was evaluated by visual inspection of all coronary arteries. Degree of coronary stenosis was classified according to previously published guidelines (31): absence of plaque; minimal, plaque with <25% stenosis; mild, 25–49% stenosis; moderate, 50–69% stenosis; severe, 70–99% stenosis; and occluded.
Echocardiography.
Each patient underwent examination with a Philips IE with a S5 transducer with two-dimensonal and TDI in the left lateral decubitus using standard parasternal short axis and apical four-chamber, two-chamber, and long-axis views. Data were analyzed with commercially available software (Excelera; Philips).
Evaluation of the left ventricular ejection fraction was performed by the modified Simpsons biplane method. Left ventricular mass index was calculated as the anatomic mass divided by body surface area of the patient.
Pulsed-wave Doppler at the apical position was used to record mitral inflow between the tips of the mitral leaflets. Peak velocities of early (E) and atrial (A) transmitral flow velocities and deceleration time of the early transmitral flow were measured, and the E-to-A ratio was calculated. All valves were examined to exclude significant valvular disease.
With TDI, peak systolic (s′), early diastolic (e′), and late diastolic (a′) velocities were measured in all six mitral annular positions. Ratios of E to e′, e′ to a′, and e′ to (a′ × s′) (eas index), were calculated as measures of left ventricular filling pressures, diastolic performance, and combined systolic and diastolic performance (19).
Ambulatory 24-h blood pressure recording.
Measurements were performed on the nondominant arm with a properly calibrated Blood Pressure Monitor System 90217 from Space Laboratories (Washington, DC). Blood pressure measurements were validated with an automatic oscillometric device. The examination was not considered valid if >30% of the measurements were missing. The SBP, diastolic blood pressure (DBP), and heart rate were measured automatically every 20 min during daytime (between 0600 and 2200 h) and once every hour during nighttime (between 2200 and 0600 h) for 24 consecutive hours. Participants were instructed to go about their normal daily conditions; however they were advised not to rest in bed during the day or exercise heavily and to abstain from caffeine beverages and tobacco.
Analyses were performed with dedicated software (Spacelabs ABP 92506 report management system). Blood pressure dipping was defined as an average reduction in SBP and DBP ≥10% from day to night (32).
Ambulatory 24-h electrocardiography.
Ambulatory electrocardiography recordings were obtained with a 12-lead Rozinn RZ153+. The electrocardiography recordings were analyzed with dedicated software and reviewed by highly trained observers. Time domain analysis was performed in order to verify the difference in autonomic function in patients with CAN (26,33). Measures included SD of R-R intervals during a 22-h period, SD of the average R-R interval in all 5-min recordings, and mean of the SD of all filtered R-R intervals for all 5-min segments over 22 h.
Statistics.
All analyses were performed with SAS 9.2 (SAS Institute, Cary, NC). The χ2 or Fisher exact test was used for dichotomous variables and the Wilcoxon rank-sum test for continuous variables. Dichotomous variables are listed as percentages. Data of continuous variables are presented as median (range).
A multivariable logistic regression model was created with CAN status as the dependent variable and including sex, age, diabetes duration, HbA1C, total cholesterol, and smoking as variables considered important to coronary artery disease, and the corresponding P values for the independent association of CAN with a given variable are presented in all tables. Univariate linear regression models were created to identify independent predictors of increased calcium score, and these variables were included stepwise in a multiple logistic regression model.
To further compare groups of similar age, we performed a sensitivity analysis on 22 patients in each group. Excluded from this analysis were the four oldest patients with CAN (+CAN) and the eight youngest without CAN (−CAN) to obtain 22 matched pairs.
RESULTS
Patient characteristics.
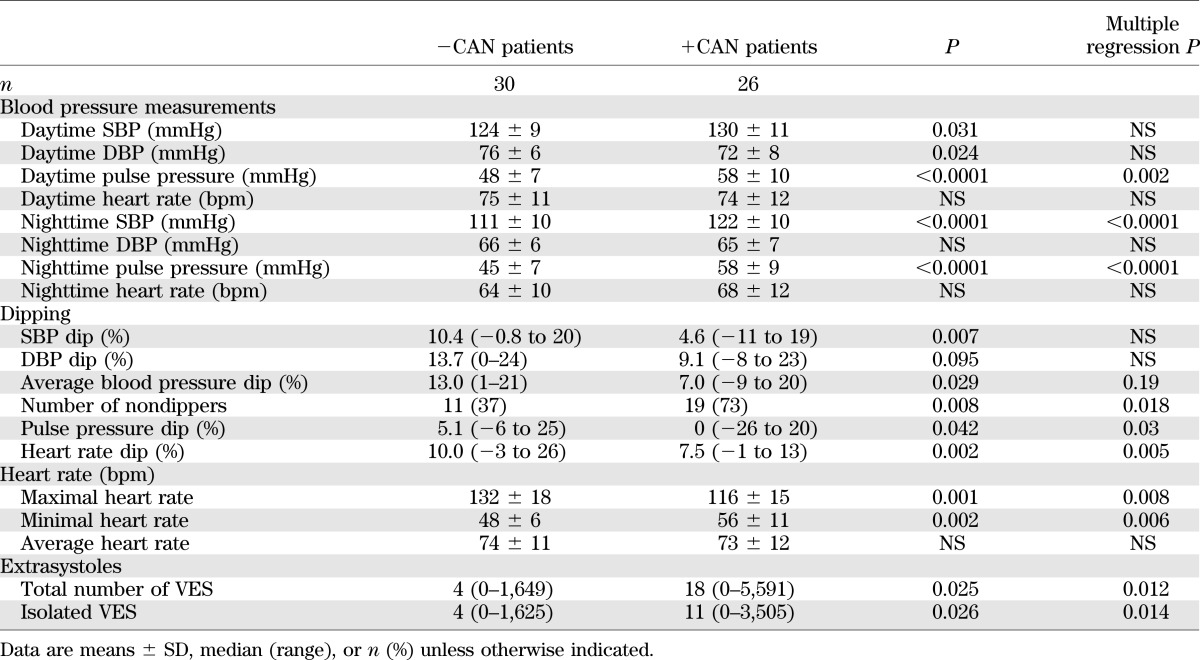
Patients +CAN had a higher mean age and longer diabetes duration compared with patients −CAN (Table 1). The proportions of all other cardiovascular risk factors and recorded diabetes complications were similar in the two groups.
TABLE 1.
Clinical characteristics and laboratory results
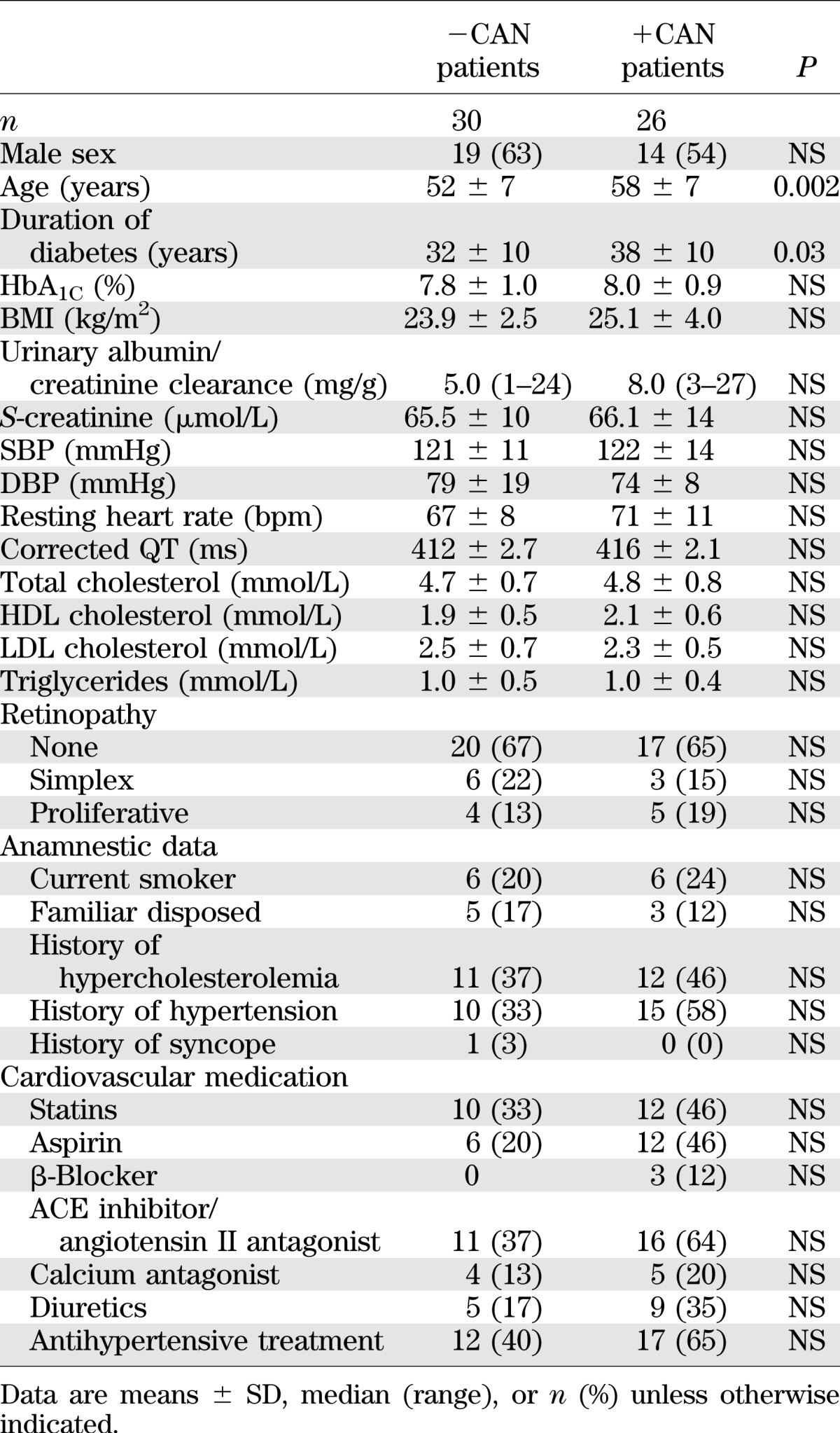
Whereas +CAN tended to be associated with higher levels of high-sensitivity C-reactive protein than −CAN (median 1.1 mg/L [range 0.2–13] vs. 0.7 mg/L [0.4–14.4], respectively, P = 0.06), levels of NTproBNP and cystatin C were similar in +CAN and −CAN patients (10.3 μmol/L [6.1–28.9] vs. 18.6 μmol/L [7.8–56.5], P = 0.18, and 0.64 mg/L [0.5–0.72] vs. 0.67 mg/L [0.45–1.2], P = 0.16, respectively). Peripheral vibration threshold was significantly higher in patients +CAN compared with that in patients −CAN (23 V [8–50] vs. 17 V [6–35], P < 0.01).
MSCT.
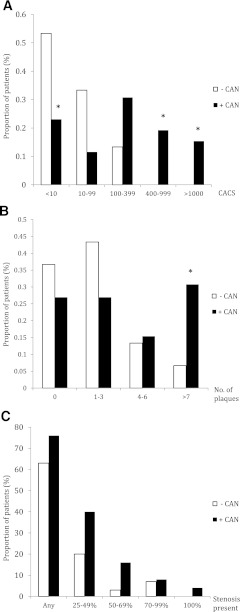
The mean CACS was significantly higher in +CAN than in −CAN patients (Table 2). Categories of increasing CACS according to CAN status are illustrated in Fig. 1A. The proportions of patients having a CACS ≥400 were significantly higher in patients +CAN than in those −CAN. While nine patients +CAN were found in categories of CACS >400, the highest CACS in −CAN was 312. Furthermore, a CACS <10 was less common in patients +CAN than in patients −CAN.
TABLE 2.
Multislice computed tomography calcium scoring
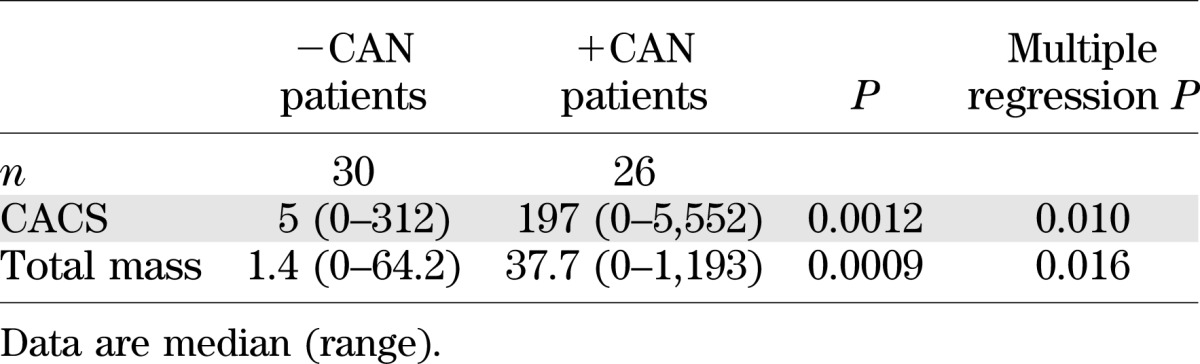
FIG. 1.
MSCT findings. A: CACS in +CAN and −CAN patients. B: Number of coronary plaques per patient according to CAN status. C: Prevalence of coronary stenosis of increasing severity according to CAN status. *P < 0.05.
With use of the CACS of each individual patient to find the corresponding percentile in a background population according to age and sex, the median percentile was significantly higher in patients +CAN compared with that in patients −CAN (median 92 [range 0–99] vs. 39 [0–98], respectively, P = 0.0205). Similarly, the proportion of patients with a CACS above the 95th percentile according to age and sex was significantly higher in patients +CAN compared with −CAN (13 [72%] vs. 5 [17%], P = 0.0077).
Coronary computed tomography angiography revealed focal coronary plaques in 38 (69%) patients. Obstructive lesions were found in 9 (16%) and occlusions in 1 (2%) patient.
Patients +CAN had a higher median number of plaque lesions than −CAN patients (three vs. one, respectively, P = 0.039) and a higher proportion of patients with more than seven plaque lesions (Fig. 1B) in univariate analysis. However, the prevalence of obstructive stenosis was not significantly higher (Fig. 1C).
Absence of elevated CACS did not exclude obstructive stenosis, as three patients had coronary atherosclerosis with a CACS of zero. Two of these patients had minimal plaques (<25%), but one patient (+CAN) had a 70–99% noncalcified stenosis.
Echocardiography.
All patients had a normal left ventricular ejection fraction. CAN status had no significant impact on left ventricular systolic function as measured by conventional echocardiography, but TDI measures of longitudinal systolic function (s′) were significantly lower in +CAN than in −CAN (Table 3).
TABLE 3.
Echocardiography findings
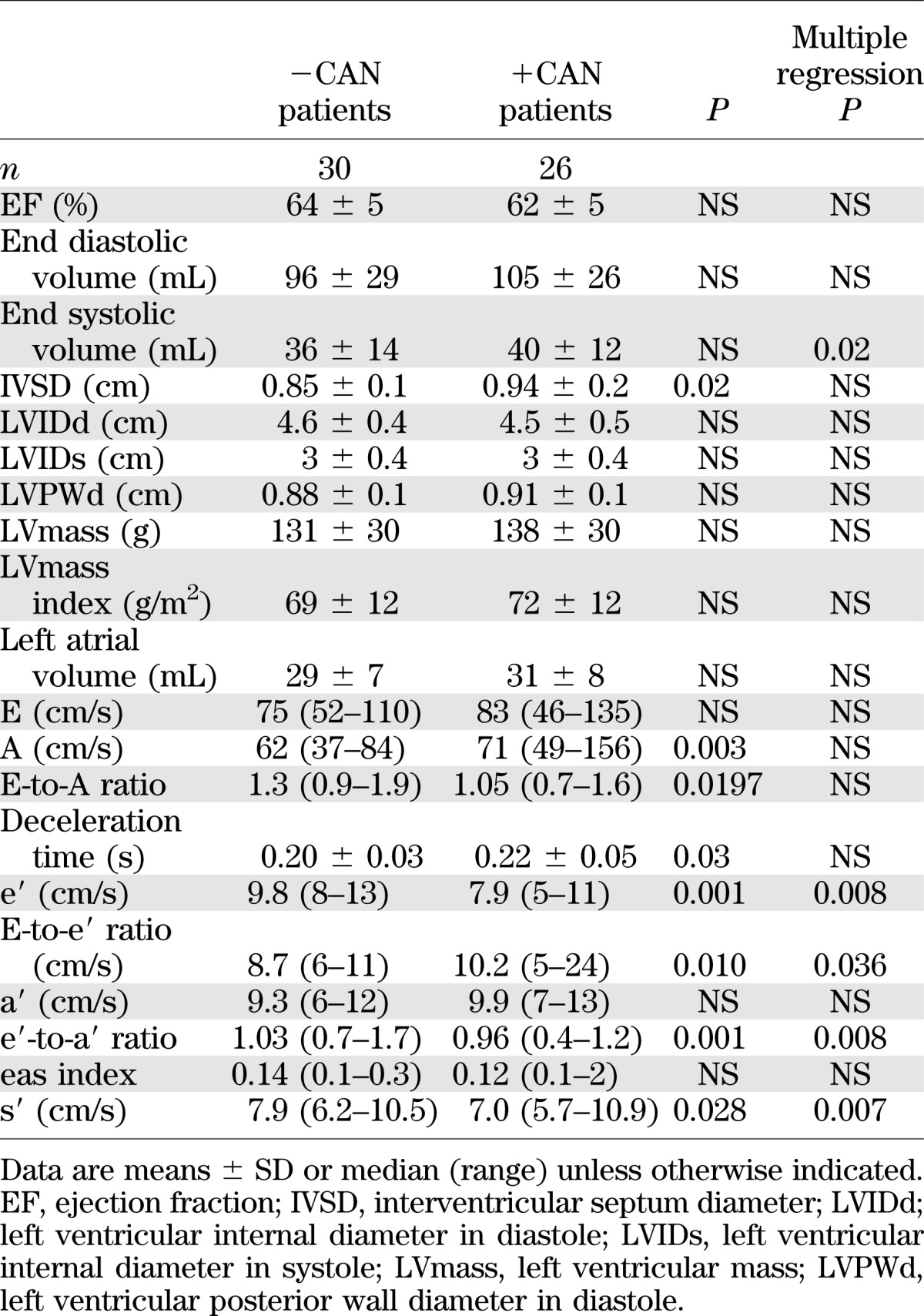
TDI measures of left ventricular diastolic function indicated higher filling pressures (E-to-e′ ratio) and impaired diastolic performance according to both e′ and e′-to-a′ ratio in +CAN compared with −CAN, whereas the eas index was not significantly different in the two groups (Table 3).
Ambulatory blood pressure measurements.
Patients +CAN had higher SBP and lower DBP but mean arterial blood pressure similar to that in −CAN patients. Accordingly, the pulse pressure was significantly higher in patients +CAN compared with that in −CAN patients (Table 4).
TABLE 4.
Twenty-four-hour blood pressure and Holter measurements
The nocturnal drop in blood pressure was lower in +CAN patients compared with that in −CAN patients with respect to both SBP and DBP. The number of patients with abnormal dipping was significantly higher in +CAN patients compared with −CAN patients.
Ambulatory electrocardiography.
Mean 24-h heart rate was independent of CAN status, but the maximal increase and decrease in heart rate were lower in +CAN compared with −CAN patients (Table 4).
The total number of ventricular extrasystoles (VES) was significantly higher in +CAN compared with −CAN patients. The majority of the VES were isolated, and the overall prevalence of consecutive VES was low and without difference between the groups. The number of patients having >30 VES/h, which has been defined as frequent (32), were not significantly higher in +CAN than in the −CAN patients.
In the time domain analysis of HRV patients +CAN compared with −CAN had significantly lower SD of R-R intervals during a 22-h period (median 99 [range 47–185] vs. 152 [99–208], P = 0.0005) as well as SD of the average R-R interval in all 5-min recordings and mean of the SD of all filtered R-R intervals for all 5-min segments over 22 h (data not shown).
Statistical analysis.
Variables independently associated with increased CACS from univariate analysis were DBP, pulse pressure, and measures of diastolic function (E-to-e′ ratio, interventricular septum diameter [IVSD], E, A, and left atrial volume). When all significant variables were included stepwise in a multiple logistic regression model, CAN remained an independent predictor of CACS (P = 0.0009) together with age (P = 0.0136), diabetes duration (P = 0.0010), and daytime DBP (P = 0.0269). Including BMI, pulse pressure, and/or urine albumin excretion in the model did not change the significance of any of the presented results.
Sensitivity analysis.
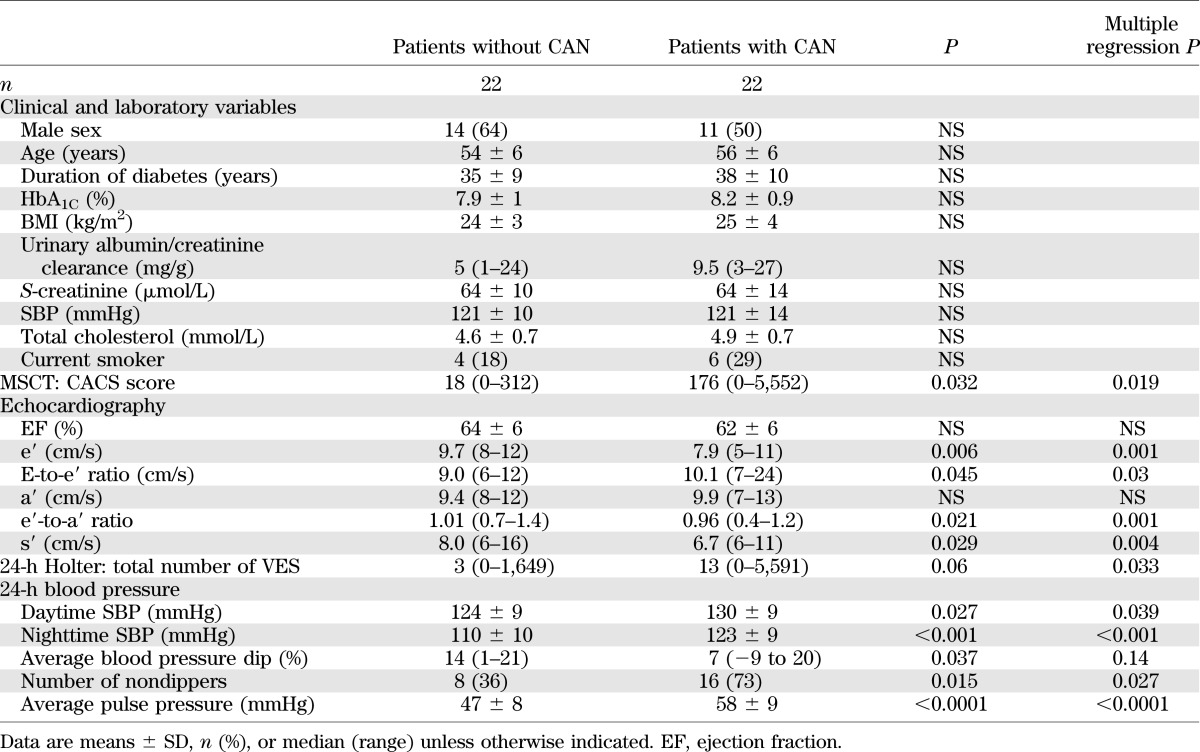
A sensitivity analysis of 22 matched patient pairs (+CAN and −CAN) is presented in Table 5. In this analysis, there was no significant difference in age, sex, diabetes duration, HbA1C, or other cardiovascular risk factors between +CAN and −CAN patients. In this subset of patients, results regarding CACS, echocardiography, ambulatory blood pressure, and Holter monitoring did not differ from those of the entire study population (Table 5).
TABLE 5.
Findings of analysis of 22 patients with and without CAN of comparable mean age and diabetes duration
Coexistence of CAN and markers of increased cardiovascular risk.
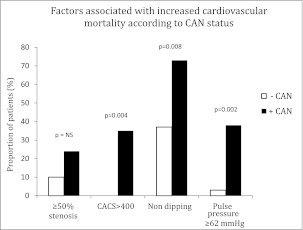
A higher proportion of patients +CAN had markers associated with increased cardiovascular mortality, including a CACS ≥400 (hazard ratio 2.99–3.24 for mortality [15]), abnormal dipping (2.16 for mortality [21]), a pulse pressure ≥62 mmHg (1.8 for mortality [34]), and a trend toward a higher proportion of patients with coronary artery stenosis ≥50% (41 for a composite end point of death, nonfatal myocardial infarction, and revascularization [35]) (Fig. 2).
FIG. 2.
The proportions of patients with different markers associated with increased cardiovascular risk according to CAN status.
DISCUSSION
The current study demonstrates an association between the presence of CAN and several distinct signs of subclinical cardiovascular disease in type 1 diabetic patients with normal urinary albumin excretion rate. Patients with CAN were characterized by increased coronary calcium deposit, impaired left ventricular function, increased arterial pulse pressure, a higher prevalence of nondipping, and increased ventricular ectopia compared with patients without CAN.
Long-term type 1 diabetes carries an excess cardiovascular mortality (36), and nephropathy is a major contributing factor (37,38). Thus, even in asymptomatic patients a greater atherosclerotic plaque burden was demonstrated by magnetic resonance imaging in diabetic patients with albuminuria compared with patients with normal albumin excretion rate (39).
CAN has also been associated with high cardiovascular mortality (2). However, since nephropathy and CAN generally develop in parallel in type 1 diabetic patients, the relative contribution of CAN to cardiovascular disease has been difficult to identify (5). In an attempt to overcome this important confounder, the present CAN population was identified in a subset of type 1 diabetic patients characterized by long duration of diabetes yet with absence of nephropathy (i.e., normal albumin excretion rate).
CACS is a powerful predictor of clinical coronary artery disease (17). An association between CACS and reduced cardiac autonomic function has recently been reported in both type 1 (40–42) and type 2 (43) diabetes. Colhoun et al. (40) observed an association between CACS and CAN independent of age and triglycerides but not independent of BMI and SBP. By contrast, Rodrigues et al. (42) found an association between CAN and progression of CACS, independent of all confounders tested, including inflammatory markers. In the current study, we extended the evaluation of the coronary arteries with a combination of CACS and computed tomography angiography and included measurements of several other potential confounders, including left ventricular function and 24-h blood pressure.
CACS was markedly higher in patients +CAN, with a median CACS of 196 compared with a median CACS of 5 in patients −CAN. With use of MESA data as reference (29,30), 72% of patients +CAN had CACS similar to that in subjects in the upper 95th percentile according to age and sex, whereas this was only the case for 17% of patients −CAN. Likewise, the estimated arterial age for a person with CACS of 5 is 52 years (95% CI 48–56 years) (30,44), in agreement with the median age of patients –CAN. By contrast, a CACS of 196 corresponds to an estimated arterial age of 77 years (75–80), which is almost 20 years more than the actual median age of patients +CAN.
Despite a higher CACS among +CAN patients, computed tomography angiography did not reveal significant differences in the prevalence of obstructive stenoses. It could be speculated whether the increased calcification without increased luminal coronary plaque burden is due to a different localization of the calcified area relative to the intima and media. Surgical sympathectomy leads to arterial media calcification, a condition frequently found in the lower extremities in patients with diabetic neuropathy (45), and it has been suggested that arterial media calcification could be the result of autonomic denervation (40,46). Sympathetic denervation may cause dedifferentiation of vascular smooth muscle cells, and these alterations are associated with extracellular matrix production and migration to the intima—changes that are seen in atherosclerosis (40,47). Another suggested mechanism of how CAN could result in increased calcification is through loss of neuropeptides, involving biochemical pathways similar to those observed in studies on calcification processes in bone metabolism (46).
Data from the current study could be interpreted as supportive of the concept of increased media sclerosis. However, the relationship between CACS and luminal stenoses is known to be only modest (48), and a nonsignificant difference in the prevalence of stenoses does not necessarily imply that the calcification is differently located in the arterial wall. Furthermore, the existence of media sclerosis has been questioned (49).
Regardless of the location of the calcification, the difference in CACS could be caused by other confounding factors. Many cardiovascular parameters are interrelated, and associations could merely reflect that these conditions share common risk factors.
The presence of CAN was associated with a decreased systolic and diastolic function of the left ventricle. We found a decrease in s′ of approximately 1 cm/s in patients +CAN compared with patients −CAN. A similar decrease in s′ in a study of the background population corresponded to a hazard ratio of 1.35 for 5-year mortality (19), suggesting that the observed left ventricular dysfunction might have prognostic importance. Several echocardiographic measures of diastolic function were univariately associated with CACS. Blood pressure variables are other factors associated with CAN, CACS, and left ventricular function. However, CAN remained independently associated with CACS, even when these variables were included in multivariable models.
An association between CAN and nondipping has been attributed to impaired vagal activity during nighttime in patients with CAN (50). In the current study, nondipping was more prevalent in patients with CAN, but it was not an isolated phenomenon in these patients, since 37% of patients without CAN were also nondippers. Likewise, a recent study did not find CAN to be the main causal factor for nondipping in type 1 diabetes (51). Nonetheless, blood pressure levels at night were significantly higher in +CAN compared with patients −CAN, suggesting a relationship between CAN and nocturnal blood pressure regulation.
CAN was also found to be associated with increased pulse pressure. In addition to being associated with increased cardiovascular risk, arterial pulse pressure is considered an indirect marker of arterial stiffness (22). With use of other methods, arterial stiffness has been shown to contribute to left ventricular diastolic dysfunction (52) and has been suggested as a link between CAN and cardiovascular disease (53).
We did not observe a significant difference in mean corrected QT or in the prevalence of QTc prolongation. Very few studies have reported on ventricular ectopia associated with CAN. We found no signs of pathological arrhythmias, but CAN was associated with an increased number of isolated premature ventricular beats.
Though interrelationships are difficult to exclude, CAN remained independently associated with CACS even when differences in cardiovascular parameters were adjusted for in multivariable analysis and sensitivity analysis was applied, and CAN was found to be associated with markers of increased cardiovascular risk (Fig. 2).
This study had limitations. First, owing to stringent inclusion criteria and since autonomic dysfunction is a rare isolated complication in long-term diabetes (10), it was only possible to match patients for age, sex, and diabetes duration in a limited number of patients, and several of the variables measured are age dependent. However, both multivariable logistic regression models and sensitivity analysis of smaller groups of patients with no differences in demographics and cardiovascular risk factors were performed. In these analyses, all reported findings remained similar and significant. Second, investigations were only carried out on diabetic patients; reference values on the different measurements from a nondiabetic control group were therefore not available. Third, we did not have information on HRV on all patients in our registry. Many patients had HRV measured in previous studies, but some of the patients had HRV measured owing to clinical suspicion of CAN. We cannot exclude having captured a particular subgroup of patients being free of long-term diabetes complications not similar to the most common phenotype of diabetic patients, and our findings can only to a limited extent be extrapolated to the entire diabetic population.
In the analysis of ambulatory blood pressure measurements, a fixed method was used and not diary time. This could potentially have biased the results if differences in the sleep patterns between the two groups exist.
In the comparison between CACS and individual reference values from the MESA study, diabetes duration was a parameter that could not be accounted for. The longer diabetes duration in +CAN patients must be remembered when evaluating these data.
Confounding effects from differences in antihypertensive treatment and use of diuretics cannot be excluded, even though the differences were nonsignificant in demographics.
In conclusion, CAN was associated with several distinct signs of subclinical cardiovascular disease in type 1 diabetic patients with normoalbuminuria. These included increased coronary calcium deposit, subtle impairment of left ventricle systolic and diastolic function, increased arterial pulse pressure, a higher prevalence of nondipping, and marginally increased ventricular ectopia, and CAN was independently associated with CACS even with adjustment for confounding factors.
ACKNOWLEDGMENTS
This study was supported by a grant from Arvid Nilssons Foundation. The MSCT Cardiac Research Unit, Rigshospitalet, is supported by a grant from the A.P. Møller og Hustru Chastine McKinney Møllers Fond til almene Formaal.
U.M.M. was supported by a scholarship from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
U.M.M. researched data and wrote the manuscript. T.J. included patients, contributed to discussion, and reviewed the manuscript. L.K. researched data and reviewed the manuscript. H.K. reviewed the manuscript. A.S.M. screened patients. U.D. and P.R. reviewed the manuscript. J.H. contributed to discussion and reviewed the manuscript. K.F.K. researched data and reviewed the manuscript. U.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the European Society of Cardiology Congress 2011, Paris, France, 27–31 August 2011, and at the 47th European Association for the Study of Diabetes Annual Meeting, Lisbon, Portugal, 12–16 September 2011.
The authors thank Tina Bock-Pedersen, MSCT Cardiac Research Unit, Rigshospitalet, for excellent technical assistance.
REFERENCES
- 1.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–397 [DOI] [PubMed] [Google Scholar]
- 2.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003;26:1895–1901 [DOI] [PubMed] [Google Scholar]
- 3.Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev 1994;10:339–383 [DOI] [PubMed] [Google Scholar]
- 4.Orchard TJ, LLoyd CE, Maser RE, Kuller LH. Why does diabetic autonomic neuropathy predict IDDM mortality? An analysis from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Res Clin Pract 1996;34(Suppl.):S165–S171 [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003;26:1553–1579 [DOI] [PubMed] [Google Scholar]
- 6.Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med 1993;10:820–824 [DOI] [PubMed] [Google Scholar]
- 7.Wirta O, Pasternack A, Mustonen J, Laippala P. Renal and cardiovascular predictors of 9-year total and sudden cardiac mortality in non-insulin-dependent diabetic subjects. Nephrol Dial Transplant 1997;12:2612–2617 [DOI] [PubMed] [Google Scholar]
- 8.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2006;29:334–339 [DOI] [PubMed] [Google Scholar]
- 9.Lykke JA, Tarnow L, Parving HH, Hilsted J. A combined abnormality in heart rate variation and QT corrected interval is a strong predictor of cardiovascular death in type 1 diabetes. Scand J Clin Lab Invest 2008;68:654–659 [DOI] [PubMed] [Google Scholar]
- 10.Bellavere F, Ferri M, Guarini L, et al. Prolonged QT period in diabetic autonomic neuropathy: a possible role in sudden cardiac death? Br Heart J 1988;59:379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossing P, Breum L, Major-Pedersen A, et al. Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet Med 2001;18:199–205 [DOI] [PubMed] [Google Scholar]
- 12.Suarez GA, Clark VM, Norell JE, et al. Sudden cardiac death in diabetes mellitus: risk factors in the Rochester diabetic neuropathy study. J Neurol Neurosurg Psychiatry 2005;76:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Airaksinen KE. Silent coronary artery disease in diabetes—a feature of autonomic neuropathy or accelerated atherosclerosis? Diabetologia 2001;44:259–266 [DOI] [PubMed] [Google Scholar]
- 14.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–2336 [DOI] [PubMed] [Google Scholar]
- 15.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003;228:826–833 [DOI] [PubMed] [Google Scholar]
- 16.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807–814 [DOI] [PubMed] [Google Scholar]
- 17.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43:1663–1669 [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Yip GW, Wang AY, et al. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol 2003;41:820–826 [DOI] [PubMed] [Google Scholar]
- 19.Mogelvang R, Sogaard P, Pedersen SA, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009;119:2679–2685 [DOI] [PubMed] [Google Scholar]
- 20.Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med 2000;17:360–364 [DOI] [PubMed] [Google Scholar]
- 21.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002;20:2183–2189 [DOI] [PubMed] [Google Scholar]
- 22.Rönnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop PH, Finnish Diabetic Nephropathy (FinnDiane) Study Group Altered age-related blood pressure pattern in type 1 diabetes. Circulation 2004;110:1076–1082 [DOI] [PubMed] [Google Scholar]
- 23.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med 1977;31:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesfaye S, Boulton AJ, Dyck PJ, et al. Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardi L, Spallone V, Stevens M, et al. on behalf of the Toronto Consensus Panel on Diabetic Neuropathy* Investigation methods for cardiac autonomic function in human research studies. Diabetes Metab Res Rev. 21 June 2011 [Epub head of print]21695761 [Google Scholar]
- 26.Spallone V, Ziegler D, Freeman R, et al. on behalf of the Toronto Consensus Panel on Diabetic Neuropathy* Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 22 June 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Greenland P, Bonow RO, Brundage BH, et al. American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Society of Atherosclerosis Imaging and Prevention. Society of Cardiovascular Computed Tomography ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2007;49:378–402 [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Cerqueira M, Hodgson JM, et al. American College of Cardiology Foundation Appropriate Use Criteria Task Force. Society of Cardiovascular Computed Tomography. American College of Radiology. American Heart Association. American Society of Echocardiography. American Society of Nuclear Cardiology. North American Society for Cardiovascular Imaging. Society for Cardiovascular Angiography and Interventions. Society for Cardiovascular Magnetic Resonance ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr 2010;4:407–.e1–e33. [DOI] [PubMed] [Google Scholar]
- 29.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113:30–37 [DOI] [PubMed] [Google Scholar]
- 30.CAC score reference values [article online], 2012. Seattle, WA, Mesa Coordinating Center. Available from http://www.mesa-nhlbi.org/CACReference.aspx 2011. Accessed 12 November 2012
- 31.Raff GL, Abidov A, Achenbach S, et al. Society of Cardiovascular Computed Tomography SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr 2009;3:122–136 [DOI] [PubMed] [Google Scholar]
- 32.The Scientific Committee Consensus document on non-invasive ambulatory blood pressure monitoring. J Hypertens Suppl 1990;8:S135–S140 [PubMed] [Google Scholar]
- 33.May O, Arildsen H. Long-term predictive power of heart rate variability on all-cause mortality in the diabetic population. Acta Diabetol 2011;48:55–59 [DOI] [PubMed] [Google Scholar]
- 34.Schram MT, Kostense PJ, Van Dijk RA, et al. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002;20:1743–1751 [DOI] [PubMed] [Google Scholar]
- 35.Abdulla J, Asferg C, Kofoed KF. Prognostic value of absence or presence of coronary artery disease determined by 64-slice computed tomography coronary angiography a systematic review and meta-analysis. Int J Cardiovasc Imaging 2011;27:413–420 [DOI] [PubMed] [Google Scholar]
- 36.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
- 37.Retnakaran R, Zinman B. Type 1 diabetes, hyperglycaemia, and the heart. Lancet 2008;371:1790–1799 [DOI] [PubMed] [Google Scholar]
- 38.Libby P, Nathan DM, Abraham K, et al. National Heart, Lung, and Blood Institute. National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 2005;111:3489–3493 [DOI] [PubMed] [Google Scholar]
- 39.Kim WY, Astrup AS, Stuber M, et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation 2007;115:228–235 [DOI] [PubMed] [Google Scholar]
- 40.Colhoun HM, Francis DP, Rubens MB, Underwood SR, Fuller JH. The association of heart-rate variability with cardiovascular risk factors and coronary artery calcification: a study in type 1 diabetic patients and the general population. Diabetes Care 2001;24:1108–1114 [DOI] [PubMed] [Google Scholar]
- 41.Thilo C, Standl E, Knez A, et al. Coronary calcification in long-term type 1 diabetic patients—a study with multi slice spiral computed tomography. Exp Clin Endocrinol Diabetes 2004;112:561–565 [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues TC, Ehrlich J, Hunter CM, Kinney GL, Rewers M, Snell-Bergeon JK. Reduced heart rate variability predicts progression of coronary artery calcification in adults with type 1 diabetes and controls without diabetes. Diabetes Technol Ther 2010;12:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon SS, Choi YK, Seo HA, et al. Relationship between cardiovascular autonomic neuropathy and coronary artery calcification in patients with type 2 diabetes. Endocr J 2010;57:445–454 [DOI] [PubMed] [Google Scholar]
- 44.McClelland RL, Nasir K, Budoff M, Blumenthal RS, Kronmal RA. Arterial age as a function of coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2009;103:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goebel FD, Füessl HS. Mönckeberg’s sclerosis after sympathetic denervation in diabetic and non-diabetic subjects. Diabetologia 1983;24:347–350 [DOI] [PubMed] [Google Scholar]
- 46.Jeffcoate WJ, Rasmussen LM, Hofbauer LC, Game FL. Medial arterial calcification in diabetes and its relationship to neuropathy. Diabetologia 2009;52:2478–2488 [DOI] [PubMed] [Google Scholar]
- 47.Kacem K, Bonvento G, Seylaz J. Effect of sympathectomy on the phenotype of smooth muscle cells of middle cerebral and ear arteries of hyperlipidaemic rabbits. Histochem J 1997;29:279–286 [DOI] [PubMed] [Google Scholar]
- 48.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998;31:126–133 [DOI] [PubMed] [Google Scholar]
- 49.Micheletti RG, Fishbein GA, Currier JS, Fishbein MC. Mönckeberg sclerosis revisited: a clarification of the histologic definition of Mönckeberg sclerosis. Arch Pathol Lab Med 2008;132:43–47 [DOI] [PubMed] [Google Scholar]
- 50.Bernardi L, Ricordi L, Lazzari P, et al. Impaired circadian modulation of sympathovagal activity in diabetes. A possible explanation for altered temporal onset of cardiovascular disease. Circulation 1992;86:1443–1452 [DOI] [PubMed] [Google Scholar]
- 51.Cabezas-Cerrato J, Hermida RC, Cabezas-Agricola JM, Ayala DE. Cardiac autonomic neuropathy, estimated cardiovascular risk, and circadian blood pressure pattern in diabetes mellitus. Chronobiol Int 2009;26:942–957 [DOI] [PubMed] [Google Scholar]
- 52.Abhayaratna WP, Barnes ME, O’Rourke MF, et al. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol 2006;98:1387–1392 [DOI] [PubMed] [Google Scholar]
- 53.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 2010;33:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]