Abstract
Serum apolipoprotein (apo)AI and -B have been shown to be associated with diabetic retinopathy, but the underlying mechanisms are unclear. We investigated whether apoAI and apoB levels are associated with measures of systemic and retinal microvascular function in patients with diabetes. We recruited 224 diabetic patients (85 type 1 and 139 type 2) and assessed serum lipids and lipoproteins from fasting blood, skin responses to sodium nitroprusside (endothelium independent) and acetylcholine (ACh) (endothelium dependent) iontophoresis, flicker-light–induced retinal vasodilatation, and retinal vascular tortuosity. After adjustment for age and sex, every SD increase in apoAI level was associated with ACh-induced skin perfusion (mean change 1.27%; P < 0.001 for apoAI) and flicker-light retinal arteriolar vasodilatation (0.33%; P = 0.003) and was associated inversely with arteriolar tortuosity (−2.83 × 10−5; P = 0.044). Each SD increase in apoB was associated with arteriolar tortuosity only (1.75 × 10−5; P = 0.050). These associations, except for apoB, remained in multivariate models. Serum apoAI was associated with increased vasomotor responsiveness to ACh and flickering light and inversely related to retinal vessel tortuosity—a characteristic that has both structural and functional dimensions. These findings provide additional insights into the potential mechanisms of apos in the pathogenesis of diabetic retinopathy and other diabetic microvascular complications.
Diabetic retinopathy and other microvascular complications are major causes of morbidity in patients with diabetes. Recently, we have reported that serum apolipoprotein (apo)AI and apoB levels were strongly associated with the presence and severity of diabetic retinopathy (1), and these associations were more prominent than those of traditional lipids (e.g., total cholesterol). However, the underlying mechanisms in which apos influence diabetic retinopathy remain unclear (1,2).
There is limited evidence that lower apoAI or higher apoB levels are correlated with signs of microvascular dysfunction (3), one of the key events in the pathogenesis of diabetic retinopathy (4). We therefore hypothesize that impaired microvascular function may be an underlying mechanism for the association between apoAI and apoB with diabetic retinopathy.
Systemic and retinal microvascular function can now be assessed noninvasively via dynamic and static vascular assessment. Skin capillary flow during sodium nitroprusside (SNP) and acetylcholine (ACh) iontophoresis reflects endothelium-independent and endothelium-dependent microvascular response, respectively (5,6). It has been suggested that good vasomotor function is associated with sizable vasodilation in response to both ACh and SNP iontophoresis (7), and reduction in Ach- or SNP-induced skin microvascular responses may therefore indicate reduced endothelial- or nonendothelial-related microvascular function, respectively. In addition, reduced retinal vessel response to diffuse luminance flickering and assessment of retinal vascular tortuosity from retinal photographs has been proposed to reflect some degree of functional and structural impairment of retinal microvasculature in people with diabetes (8–11). We previously demonstrated that these measures of microvascular function were associated with diabetic retinopathy in people with diabetes (9,11,12), implicating the role of impaired systemic and retinal microvascular dysfunction in diabetic retinopathy pathogenesis (13).
In this study, we aimed to investigate the association of serum apoAI, apoB, and traditional lipid levels in a cohort of diabetic patients with and without diabetic retinopathy with systemic and retinal microvascular function, assessed using three measures: skin iontophoresis, flicker-light–induced vasodilatation, and retinal vascular imaging.
RESEARCH DESIGN AND METHODS
This was a clinic-based observational study. Details of study population have previously been described (1,9,11,12). We consecutively recruited 224 patients (18–70 years old) with diabetes (85 with type 1 and 139 with type 2) between October 2006 and April 2008 from the eye clinics at the International Diabetes Institute, Melbourne, Australia. We excluded participants who had a history of epilepsy or glaucoma, had undergone previous vitreal surgery, or had a cataract on examination. Type 1 and type 2 diabetes in this study were diagnosed by the treating attending physician and confirmed with participants’ history of age at diagnosis and previous test findings of type 1 diabetes–related markers (e.g., urine ketones). The study followed the tenets of the Declaration of Helsinki and was approved by the institutional ethics committee, with written informed consent from each participant.
Assessment of lipids and apos.
Fasting (>8 h) blood samples were obtained from each participant during clinical examination to assess blood glucose and HbA1c levels, serum lipid (total, HDL, and LDL cholesterol and triglyceride), and apoAI and apoB levels within 2 weeks of eye examinations. Non-HDL cholesterol was calculated by total cholesterol minus HDL cholesterol level. Details of the blood specimen handling and apoAI and apoB assessments have previously been published (1). Serum apoAI and apoB were assessed using rate immunonephelometry (BN II Nephelometer Dade Behring; Siemens Healthcare Diagnostics, Eschborn, Germany) with kits from the same company at the Department of Medicine, University of Melbourne, (St. Vincent’s Hospital). Intra-assay coefficients of variation for apoAI and apoB were 2.2 and 1.9%, respectively, and interassay coefficients of variation were 5.7 and 2.4%, respectively (1).
Skin microcirculatory function.
Skin microcirculatory function was measured using a laser Doppler flowmetry technique to assess response to iontophoresis of SNP and ACh (6). Iontophoresis delivers vasodilators (SNP and ACh) across the skin using a weak electrical current, with blood flow measured by laser Doppler flowmetry. These assessment procedures have also previously been described (12). All tests were performed on both forearms. Laser Doppler flowmeter (Moor Instruments, Axminster, U.K.) was used to measure the skin blood flux (a function of volume of blood multiplied by velocity). Skin microvascular responses to SNP (endothelial-independent vasodilator) and ACh (endothelial-dependent vasodilator) iontophoresis were recorded. SNP 1% and ACh 1% solutions in distilled water were used for iontophoresis. “Mean baseline flux” was the term for mean flux measured over 120 s preiontophoresis, and “mean response flux” was the term for mean flux over 240 s after the iontophoresis. The responses to SNP or ACh were calculated from the difference between mean response and baseline flux divided by the baseline flux (percentage change relative to baseline measure) for SNP and ACh separately (12).
Flicker-light–induced vasodilatation.
Assessment of flickering-light–dependent vasodilatation was performed in a dark room using the Dynamic Vessel Analyzer (DVA; IMEDOS, Jena, Germany). The detailed examination procedures have previously been published (11,14). Briefly, each participant underwent fundus examination under green light. A selected segment of arteriole and venule was initially measured for 50 s as the baseline, followed by stimulation with flickering light of the same wavelength for 20 s and then a nonflicker period for 80 s. This measurement cycle was repeated twice, with a total duration of 350 s per eye. Retinal arteriolar and venular dilation in response to flickering light was calculated automatically by the DVA built-in software. It was represented as an average increase in the vessel diameter in response to light flicker during the three measurement cycles and was expressed as the percentage increase relative to the baseline diameter size.
Retinal photography and measurement of retinal vascular tortuosity.
Two-field fundus images (disc and macula centered) were taken from each participant before the flicker-light assessment using Canon CF-60UVi equipped with Canon EOS 10D (Canon, Tokyo, Japan). Retinal vessel tortuosity, or curvature of vessel course, was quantitatively measured from digital disc-centered retinal photographs (field 1 of the Early Treatment Diabetic Retinopathy Study [ETDRS] photographic fields), using a semiautomated computer program (Singapore I Vessel Assessment [SIVA], Singapore). Detailed measurement procedures have previously been described (8–10). In brief, a trained grader who was masked to participants’ characteristics applied SIVA to each image and automatically identified the center of the optic disc as well as arterioles and venules within the measurement zone (between 0.5- and 2-disc diameter away from the optic disc). The average of measures from all arterioles and venules was summarized into tortuosity index of arterioles and venules, respectively. Vessel tortuosity index was calculated using the integral of the total squared curvature along the path of the vessel divided by the total length of the vessel arc (15).
Assessment of diabetic retinopathy.
Diabetic retinopathy was graded from two-field fundus photographs at the Centre for Eye Research Australia by graders masked to clinical characteristics (1). The diabetic retinopathy grading was performed following the modified Airlie House Classification system (16), and the presence of diabetic retinopathy was defined as having any retinopathy signs in either the right or the left eye.
Assessment of other risk factors.
All participants underwent a standardized clinical examination and interview using a detailed questionnaire to obtain information including past medical history, current cigarette smoking status, and the use of antihypertensive or lipid-lowering medications or oral hypoglycemic agents. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or current use of antihypertensive medications. Height and weight were measured to determine BMI (1).
Statistical analysis.
Analyses were performed using Intercooled STATA version 10.1 for Windows (StataCorp, College Station, TX). Clinical characteristics of participants with and without diabetic retinopathy were compared using χ2 test for proportions and t test or Mann-Whitney U test for means. We analyzed serum apos and traditional lipids as independent variables and skin iontophoresis, flicker-light–induced vasodilatation, and tortuosity index as dependent variables. Responses to iontophoresis of SNP and ACh of right and left arm were used in the analyses. Similarly, flicker-light–induced vasodilatation and tortuosity index of right and left eyes were also used. Both arteriolar and venular tortuosity indexes were positively skewed; therefore, log transformation was applied. We used t test for clustered data for the analyses of skin iontophoresis, flicker-light–induced vasodilatation, and retinal vessel tortuosity to account for within-cluster correlation between right and left sides. Independent variables were assessed categorically (in quartiles) to explore any potential threshold values and continuously (per SD increase). General linear model with additional cluster command was used to estimate the mean difference of dependent variables, and every SD increase in serum apos and lipids was used to account for the correlation between right and left side of the arms or eyes. We controlled for age and sex in the analyses (model 1) and further for diabetes duration, HbA1c, SBP, use of insulin, oral diabetes and lipid-lowering medications, and presence of diabetic retinopathy (except for analysis stratified by diabetic retinopathy presence) (model 2). Covariables were selected for model 2 to include established diabetic retinopathy risk factors (duration of diabetes, HbA1c, and SBP) and diabetes and lipid-related medications that may be associated with either microvasculatural changes or apo levels. Presence of diabetic retinopathy was also chosen as a covariable in model 2 to ensure that these associations were independent of diabetic retinopathy status. Further analyses were performed to stratify according to the absence and presence of diabetic retinopathy or type 1 or type 2 diabetes.
RESULTS
Of 224 patients with diabetes, the median (interquartile range [IQR]) age was 59 years (51–66), 59.4% were male, median duration of diabetes was 15 years (9–21), 38% (n = 85) had type 1 diabetes, and 64.3% (144) had diabetic retinopathy. Compared with patients without diabetic retinopathy, those with diabetic retinopathy had lower apoAI and higher apoB and apoB/AI levels, reduced retinal vascular responses to flickering light, reduced skin capillary response to iontophoresis of SNP and ACh, and more tortuous retinal arterioles (Supplementary Table 1) (1).
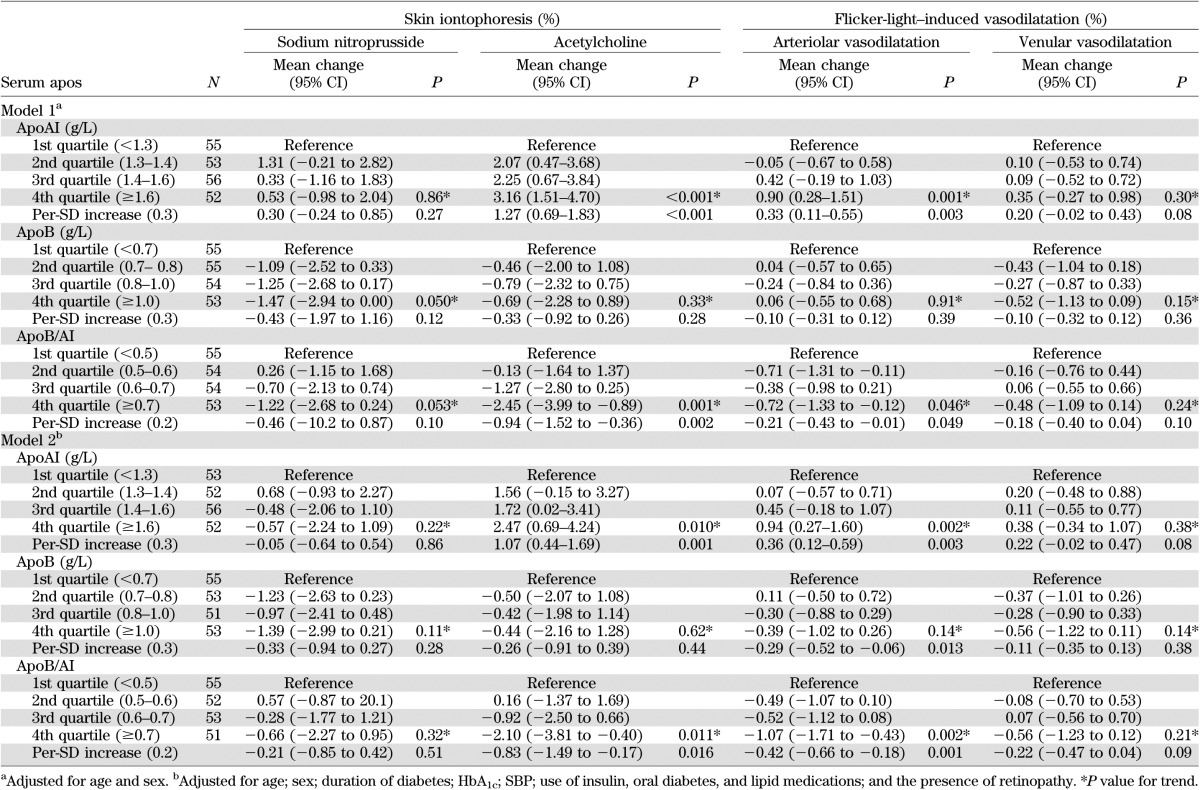
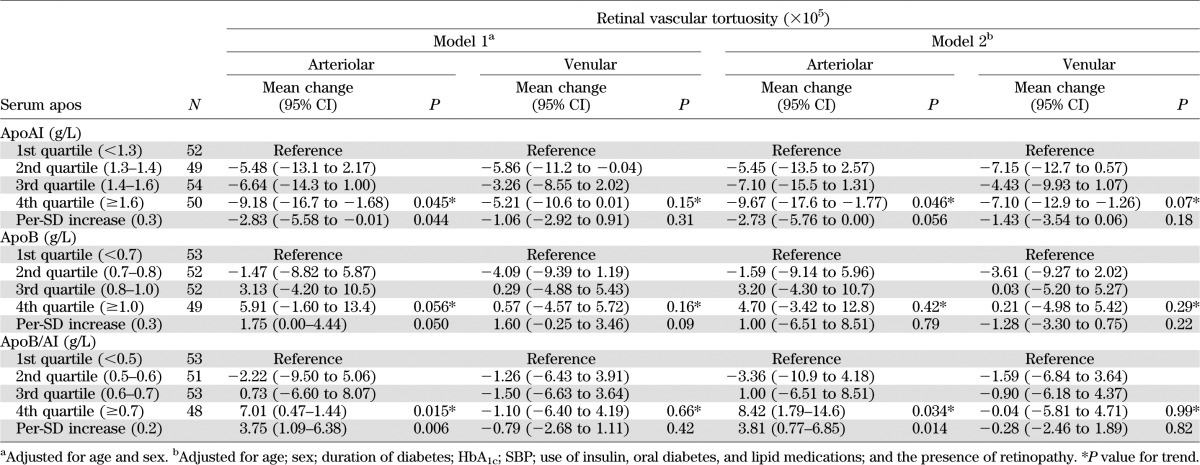
Tables 1 and 2 show the associations of serum apo levels with skin capillary responses to iontophoresis of SNP and ACh, flicker-light–induced retinal vasodilatation (Table 1), and retinal vascular tortuosity index (Table 2). In model 1, every SD increase in apoAI level was positively associated with ACh-induced skin perfusion (mean change 1.27%; P < 0.001) and flicker-light–induced arteriolar vasodilatation (mean change 0.33%; P = 0.003) (Table 1) but was inversely associated with retinal arteriolar tortuosity index (mean change −2.83 × 10−5; P = 0.044) (Table 2). ApoB was only significantly associated with increased arteriolar tortuosity (mean change of tortuosity index 1.75 × 10−5 per-SD increase in apoB; P = 0.050) (Table 2). ApoB/AI was inversely associated with ACh-induced skin perfusion (mean change −0.94% per-SD increase in apoB/AI; P = 0.002) and arteriolar vasodilatation (mean change −0.21%; P = 0.049) but was positively associated with arteriolar tortuosity index (mean change 3.75 × 10−5; P = 0.006) (Table 2). In model 2, these associations remained statistically significant or stronger, except for the association of apoAI with arteriolar tortuosity index, which became marginally nonsignificant (P = 0.056), and the association of apoB with arteriolar tortuosity index, which showed no association (Tables 1 and 2). No association was found for SNP-induced skin perfusion, venular dilatation, and venular tortuosity.
TABLE 1.
Associations between serum apos and skin iontophoresis and flicker-light–induced vasodilatation
TABLE 2.
Associations between serum apos and retinal vascular tortuosity
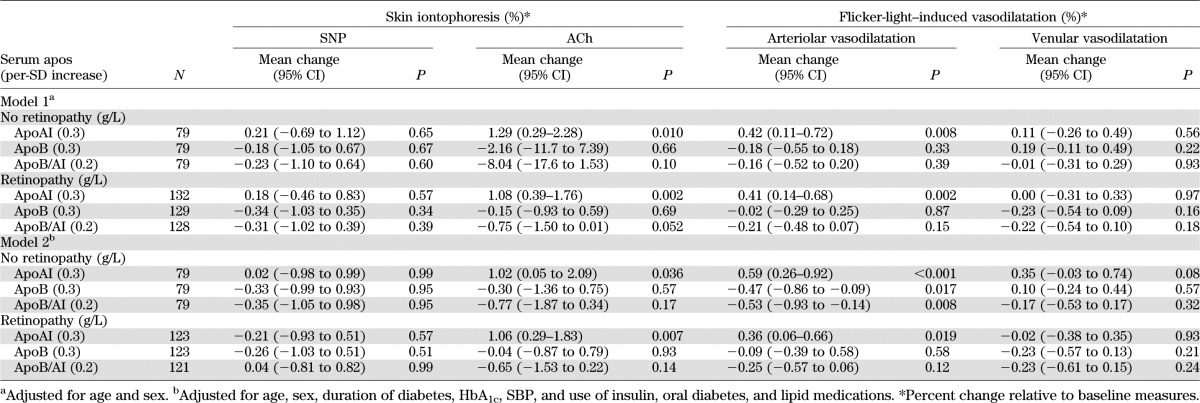
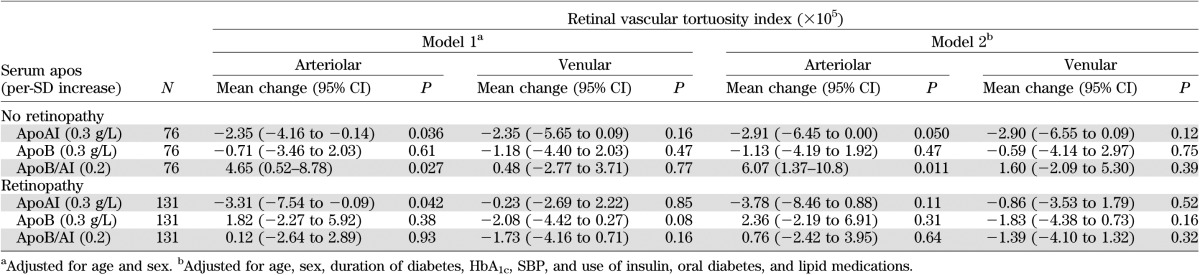
The associations of serum apos with SNP- and ACh-induced skin microvascular responses, flicker-light–induced retinal vasodilatation, and retinal vascular tortuosity in stratified subgroups by the presence of diabetic retinopathy are shown in Tables 3 and 4. Increased apoAI level, but not apoB and apoB/AI levels, was significantly associated with increased ACh-induced skin perfusion and flicker-light–stimulated arteriolar vasodilatation in both the nondiabetic retinopathy and diabetic retinopathy groups and in both models 1 and 2 (all P < 0.05) (Table 3). Increased apoAI and apoB/AI levels were significantly associated with decreased and increased arteriolar tortuosity, respectively, in patients without diabetic retinopathy only (Table 4).
TABLE 3.
Associations between serum apos and skin iontophoresis and flicker-light–induced vasodilatation stratified by retinopathy presence
TABLE 4.
Associations between serum apos and retinal vascular tortuosity index stratified by retinopathy presence
In the analyses of traditional lipids, only HDL cholesterol was associated with response to iontophoresis of SNP (mean change 0.68%; P = 0.049 per-SD increase in HDL cholesterol) and ACh (mean change 1.27%; P < 0.001 per-SD increase in HDL cholesterol). No association was found for other traditional lipids (Supplementary Tables 2 and 3).
DISCUSSION
In this study, we have demonstrated that serum apoAI and apoB/AI levels were associated with three measures of vascular function: 1) ACh-induced skin microvascular responses, 2) flicker-light–induced retinal arteriolar vasodilatation, and 3) retinal arteriolar tortuosity. These associations, particularly of apoAI, remained significant in patients without diabetic retinopathy. By contrast, we demonstrated limited association of traditional lipid levels with ACh-induced skin microvascular responses, except for HDL cholesterol, which contains apoA1. Our findings suggest that an adverse profile of serum apos, but not traditional lipids, is a marker of systemic and retinal microvascular dysfunction, possibly endothelium related, and may play a role in diabetic retinopathy—as previously observed (1).
We recently demonstrated that apoAI and apoB levels are strongly associated with diabetic retinopathy and therefore may be better biomarkers of diabetic retinopathy than traditional lipids (1). Yet, the mechanisms underlying these associations remain elusive. ApoAI and apoB have been increasingly recognized for their important role in cardiovascular disease risk (17,18), and these mechanisms have mainly been studied in relation to large vessels. Previous studies have shown that higher level of apoAI may improve the vascular endothelial function in coronary arteries (19), while apoB has opposite effects (20,21). In keeping with this, animal studies also demonstrated an improvement in endothelium-dependent vascular responses among rats being treated with an apoAI mimetic (22) and accelerated vascular endothelial dysfunction in pigs fed an apoB-rich diet (20). Nevertheless, these apos have not been given much attention in small-vessel diseases. There is limited evidence that apoAI may improve endothelium-mediated response of small peripheral arteries (3).
Our findings provide first-line evidence showing that apoAI and apoB/AI may affect systemic and retinal microvascular function, consistently shown by responses to iontophoresis of Ach, flicker-light–induced arteriolar vasodilatation, and arteriolar tortuosity. ACh-induced skin microvascular responses, in contrast to SNP, are specifically endothelium dependent (5,6). During the iontophoresis, ACh may stimulate nitric oxide (NO) production, while SNP is an NO donor to vascular smooth muscle cells (5,6). Since NO is a mediator of endothelium-derived relaxing factor (23,24), NO production during ACh iontophoresis provokes endothelium-dependent vasodilatation in skin microcirculation, which has been proposed to reflect systemic microcirculation (6). Retinal arteriolar vasodilatation in response to flicker-light stimulation has been demonstrated to signify retinal vascular capacity to adapt to increased blood supply to active neurons in stimulated neuroretina (25,26). Additionally, retinal arteriolar tortuosity may indicate incapability of blood vessels to adapt to intravascular pressure and increased shear stress (27–29). The failure to perform such adaptive changes can be due to the loss of vascular flexibility as a result of vascular cell damage and can thus appear as vessel undulation (9,27,29).
Mechanisms explaining the beneficial associations of apoAI and deteriorating associations of apoB/AI with microvascular function seen in this study may be similar to findings in larger vessels (2,20,30). ApoAI is the structural protein of usually vasoprotective HDL, and apoB is the main component of VLDL, IDL, and LDL (31). ApoAI can promote vasoprotective mechanisms via its ability to promote reverse cholesterol transport from peripheral tissue to the liver (32) and to inhibit LDL from oxidation, which may induce smooth muscle cell cytotoxicity and vascular endothelial dysfunction (30,32)—its antiplatelet and anti-inflammatory effects (30). By contrast, apoB is responsible for delivering lipids and cholesterol from the intestine and the liver to peripheral tissue and at the same time is a reflection of the total number of atherogenic particles, which if oxidized, are toxic to arterial walls (18,21). Therefore, apoB/AI levels may reflect both damaging and protective lipoprotein pathways (21,31). The magnitude of the apo-associated changes in vascular measures seemed to be very small. Nevertheless, microvasculature has a major influence on systemic hemodynamics (33), and such small changes have been shown to be significantly associated with diabetic retinopathy in our previous studies (9,11,12); thus, they are likely to be clinically meaningful.
In our analysis stratified by diabetic retinopathy, apoAI remained significantly associated with response to iontoporesis of ACh and flicker-light–induced vasodilatation. However, its association with arteriolar tortuosity was attenuated after controlling for other diabetes risk factors (e.g., diabetes duration, HbA1c, SBP) and medication. This may be due to relatively small numbers with insufficient power to detect modest associations—or the association between apoAI and arteriolar tortuosity is not independent of other factors. The latter is consistent with our previous hypothesis that tortuous vessels may indicate early vascular damage as a result of adverse systemic exposures in diabetes (e.g., high HbA1c) (8,9), in addition to an adverse apos profile. Nevertheless, we demonstrated significant associations even in the absence of diabetic retinopathy, which support our primary hypothesis that the associations between apos and microvascular changes are independent of diabetic microvascular complications and could play a role in the pathways to diabetic retinopathy.
Our findings, if confirmed in future studies, have strong clinical implications. These results suggest new insights into underlying mechanisms for the influence of apoAI and apoB on diabetic retinopathy and other diabetic microvascular complications, which possibly affect microvascular endothelial dysfunction. In addition, our findings may also provide clues to the possible mechanisms by which fenofibrate, which improves the apo profile (34), slowed progression of diabetic retinopathy in recent type 2 diabetes trials (34,35). Further research to investigate the effect of apos modifying treatment in the development/progression of diabetic retinopathy is therefore the key in this area.
Strengths of our study include comprehensive assessments of markers of microvascular-specific endothelial dysfunction, measurements of both apos and traditional lipids, and photographic assessment of diabetic retinopathy using standardized grading protocols. Despite the relatively small sample size, our study sample was typical of clinical diabetic populations with diabetic retinopathy and comparable with the Multi-Ethnic Study of Atherosclerosis (MESA) (36), which showed strong associations with diabetes duration and HbA1c level (1), reassuring the generalizability of our findings to other diabetic populations. Limitations should also be noted. First, the cross-sectional nature of this study provides no temporal interpretation of reported associations. Second, we were unable to perform analysis stratified by diabetes type or diabetic retinopathy severity owing to the relatively small number of study sample. Third, a reduction in ACh-induced skin microvascular responses might also be influenced by the number and function of cutaneous ACh receptors. Therefore, cautious interpretation is needed, since we have no measures of cutaneous ACh receptors (6,37). Nevertheless, there has been no previously documented evidence showing that apoAI and ACh receptors are correlated. Our results demonstrated a dose-response relationship between apoAI and ACh-induced skin microvascular responses. Therefore, it is likely that apoAI has influence on peripheral microvascular function, regardless of the presence or absence of alteration in the number and function of skin ACh receptors. As the number and function of skin ACh receptors are influenced by inflammation status (38), we have also controlled for factors (e.g., duration of diabetes, HbA1c) that potentially excite inflammatory processes and, additionally, an inflammatory marker (C-reactive protein level) that we measured in this sample (data not shown). However, the observed association remained significant.
Fourth, emerging evidence has also suggested that the use of fenofibrate may have profound effects on microvascular function (39). In this study, we did not obtain detailed enough information regarding the use of specific lipid medications (e.g., duration of the medications, dosage, frequency, and compliance of the patients) to be able to specifically assess the effect of fenofibrate on our outcome measures. Nonetheless, there was only one participant treated with fenofibrate in this study. Therefore, the possibility of confounding effects by lipid medications should have been very minimal. Finally, we did not have data on serum apos and skin iontophoresis from people without diabetes; therefore, we were unable to perform these analyses compared with nondiabetic subjects. Our findings are thus only applicable to subjects with diabetes and cannot be generalized to subjects without diabetes.
In summary, we report novel associations of serum apos with markers of systemic and retinal microvascular dysfunction, possibly endothelium related, in patients with diabetes without and with diabetic retinopathy. Our findings may elucidate the role of apos in diabetic retinopathy and support previous evidence that serum apos are stronger biomarkers for diabetic retinopathy, and possibly other diabetic microvascular complications, than traditional lipids.
ACKNOWLEDGMENTS
The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian Government. This study was supported by a Diabetes Australia Research Trust grant (to J.J.W., T.Y.W., J.S., and T.T.N.) and by the National Health and Medical Research Council Centre for Clinical Research Excellence #529923–Translational Clinical Research in Major Eye Diseases.
No potential conflicts of interest relevant to this article were reported.
M.B.S. contributed to data collection, researched data analysis, and wrote the manuscript. T.Y.W. obtained funding for the study, provided supervision, contributed to discussions, and critically reviewed and edited the manuscript. T.T.N. obtained funding, contributed to study design and data collection, researched data interpretation, and contributed to discussion. R.K. contributed to discussion and reviewed and revised the manuscript. A.J.J. contributed to interpretation of the study findings and discussion and reviewed and edited the manuscript. J.S. obtained funding and reviewed and edited the manuscript. C.R. researched data analysis and interpretation. J.J.W. obtained funding, provided supervision, contributed to discussion, and critically reviewed and edited the manuscript. M.B.S. and J.J.W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Connie Karschimkus and Dr. Andrzej Januszewski (Department of Medicine, University of Melbourne, Melbourne, Australia) for technical assistance related to apoAI and -B assays.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1272/-/DC1.
REFERENCES
- 1.Sasongko MB, Wong TY, Nguyen TT, et al. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care 2011;34:474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chait A, Montes VN. Apolipoproteins and diabetic retinopathy. Diabetes Care 2011;34:529–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferré R, Aragonès G, Plana N, et al. High-density lipoprotein cholesterol and apolipoprotein A1 levels strongly influence the reactivity of small peripheral arteries. Atherosclerosis 2011;216:115–119 [DOI] [PubMed] [Google Scholar]
- 4.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23:1496–1508 [DOI] [PubMed] [Google Scholar]
- 5.Debbabi H, Bonnin P, Ducluzeau PH, Lefthériotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens 2010;23:541–546 [DOI] [PubMed] [Google Scholar]
- 6.Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med 2008;18:109–116 [DOI] [PubMed] [Google Scholar]
- 7.Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol 1996;496:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasongko MB, Wang JJ, Donaghue KC, et al. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care 2010;33:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasongko MB, Wong TY, Nguyen TT, Cheung CY, Shaw JE, Wang JJ. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia 2011;54:2409–2416 [DOI] [PubMed] [Google Scholar]
- 10.Sasongko MB, Wong TY, Donaghue KC, et al. Retinal arteriolar tortuosity is associated with retinopathy and early kidney dysfunction in type 1 diabetes. Am J Ophthalmol 2012;153:176.e1–183.e1 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TT, Kawasaki R, Wang JJ, et al. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care 2009;32:2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TT, Shaw JE, Robinson C, et al. Diabetic retinopathy is related to both endothelium-dependent and -independent responses of skin microvascular flow. Diabetes Care 2011;34:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hecke MV, Dekker JM, Nijpels G, et al. Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia 2005;48:1300–1306 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TT, Kawasaki R, Kreis AJ, et al. Correlation of light-flicker-induced retinal vasodilation and retinal vascular caliber measurements in diabetes. Invest Ophthalmol Vis Sci 2009;50:5609–5613 [DOI] [PubMed] [Google Scholar]
- 15.Hart WE, Goldbaum M, Côté B, Kube P, Nelson MR. Measurement and classification of retinal vascular tortuosity. Int J Med Inform 1999;53:239–252 [DOI] [PubMed] [Google Scholar]
- 16.Diabetic retinopathy study. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the Diabetic Retinopathy. Invest Ophthalmol Vis Sci 1981;21:210–226 [PubMed] [Google Scholar]
- 17.Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQueen MJ, Hawken S, Wang X, et al. INTERHEART study investigators Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 2008;372:224–233 [DOI] [PubMed] [Google Scholar]
- 19.Zeiher AM, Schächlinger V, Hohnloser SH, Saurbier B, Just H. Coronary atherosclerotic wall thickening and vascular reactivity in humans. Elevated high-density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation 1994;89:2525–2532 [DOI] [PubMed] [Google Scholar]
- 20.Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol 1999;19:2981–2992 [DOI] [PubMed] [Google Scholar]
- 21.Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med 2006;259:437–446 [DOI] [PubMed] [Google Scholar]
- 22.Peterson SJ, Husney D, Kruger AL, et al. Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J Pharmacol Exp Ther 2007;322:514–520 [DOI] [PubMed] [Google Scholar]
- 23.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 1987;84:9265–9269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987;327:524–526 [DOI] [PubMed] [Google Scholar]
- 25.Dorner GT, Garhofer G, Kiss B, et al. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol 2003;285:H631–H636 [DOI] [PubMed] [Google Scholar]
- 26.Kondo M, Wang L, Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta Ophthalmol Scand 1997;75:232–235 [DOI] [PubMed] [Google Scholar]
- 27.Goodfellow J, Ramsey MW, Luddington LA, et al. Endothelium and inelastic arteries: an early marker of vascular dysfunction in non-insulin dependent diabetes. BMJ 1996;312:744–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol 2005;25:957–962 [DOI] [PubMed] [Google Scholar]
- 29.Kylstra JA, Wierzbicki T, Wolbarsht ML, Landers MB, 3rd, Stefansson E. The relationship between retinal vessel tortuosity, diameter, and transmural pressure. Graefes Arch Clin Exp Ophthalmol 1986;224:477–480 [DOI] [PubMed] [Google Scholar]
- 30.Shah PK, Kaul S, Nilsson J, Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part II. Circulation 2001;104:2498–2502 [DOI] [PubMed] [Google Scholar]
- 31.Davidson MH. Apolipoprotein measurements: is more widespread use clinically indicated? Clin Cardiol 2009;32:482–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill SA, McQueen MJ. Reverse cholesterol transport—a review of the process and its clinical implications. Clin Biochem 1997;30:517–525 [DOI] [PubMed] [Google Scholar]
- 33.Johnson PC. Overview of the microcirculation. In Handbook of Physiology: Microcirculation. Tuma RF, Duran WN, Ley K, Eds. Amsterdam, Elsevier, 2008, p. 6–20 [Google Scholar]
- 34.Chew EY, Ambrosius WT, Davis MD, et al. ACCORD Study Group. ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keech AC, Mitchell P, Summanen PA, et al. FIELD study investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370:1687–1697 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen TT, Alibrahim E, Islam FM, et al. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: the multi-ethnic study of atherosclerosis. Diabetes Care 2009;32:1704–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 2006;27:503–508 [DOI] [PubMed] [Google Scholar]
- 38.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 2006;86:1309–1379 [DOI] [PubMed] [Google Scholar]
- 39.Hiukka A, Maranghi M, Matikainen N, Taskinen MR. PPARalpha: an emerging therapeutic target in diabetic microvascular damage. Nat Rev Endocrinol 2010;6:454–463 [DOI] [PubMed] [Google Scholar]