Abstract
Non-technical summary
Cholecystokinin (CCK) is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein; it also functions to suppress feeding. CCK is also recognized as a neurotransmitter within the central nervous system (CNS). As such, there are receptors for CCK both in the periphery as well as within the CNS. Thus, it is not certain where the principal site of action is for CCK to affect gastric reflexes and feeding behaviour. We show that picomole amounts of systemic CCK are sufficient to modulate gastric reflexes and that these effects are probably mediated via peripheral vagal afferents in the proximal gut. Knowledge of how and where this peptide hormone acts increases our understanding of the regulation of feeding behaviour.
Abstract
Cholecystokinin (CCK) is a potent regulator of visceral functions as a consequence of its actions on vago-vagal reflex circuit elements. This paper addresses three current controversies regarding the role of CCK to control gastric function via vago-vagal reflexes. Specifically: (a) whether CNS vs. peripheral (vagal afferent) receptors are dominant, (b) whether the long (58) vs. short (8) isoform is more potent and (c) whether nutritional status impacts the gain or even the direction of vago-vagal reflexes. Our in vivo recordings of physiologically identified gastric vagal motor neurones (gastric-DMN) involved in the gastric accommodation reflex (GAR) show unequivocally that: (a) receptors in the coeliac–portal circulation are more sensitive in amplifying gastric vagal reflexes; (b) in the periphery, CCK8 is more potent than CCK58; and (c) the nutritional status has a marginal effect on gastric reflex control. While the GAR reflex is more sensitive in the fasted rat, CCK amplifies this sensitivity. Thus, our results are in stark contrast to recent reports which have suggested that vago-vagal reflexes are inverted by the metabolic status of the animal and that this inversion could be mediated by CCK within the CNS.
Introduction
Gastric tone and motility are ultimately controlled by the activity of vagal efferent neurones (Fox & Powley, 1985; Shapiro & Miselis, 1985; Norgren & Smith, 1988; Cajal, 1995)). Vagal efferent effects on the stomach are complex and can be modulated by a variety of incoming information sources. The efferent vagus maintains coordinated excitatory and inhibitory control by acting on cholinergic and non-adrenergic, non-cholinergic components of the gastric myenteric plexus, respectively (Jansson, 1969; Gillis et al. 1989; McCann & Rogers, 1992; Fogel et al. 1996; Krowicki et al. 1997; Rogers et al. 1999, 2003; Curro et al. 2008)). Neurones in the dorsal motor nucleus of the vagus (DMN) are the source of vagal efferent control over the stomach (Shapiro & Miselis, 1985; Rogers et al. 2005)). The majority of gastric-DMN neurones are spontaneously active (Travagli et al. 1991; McCann & Rogers, 1992)) and their activity is powerfully modulated by visceral afferent pathways carried by vagal afferents from the gut (Rogers et al. 2005)). An example of the control of digestion by the vagus is seen in the gastric accommodation reflex, which serves to increase gastric volume in the presence of accumulating ingesta. It also serves as a generator of cyclic retropulsive movements of the stomach to speed the digestion of chyme (Prove & Ehrlein, 1982)). The strength of this reflex is also used as a physiological measure of the relative strength of gastric vagal afferent-mediated satiety signals (Schwartz et al. 1995; Emond et al. 1999)).
The mechanics of the gastric accommodation reflex involve the detection of local distension of the antrum by vagal mechanoreceptors. These vagal afferents, in turn, activate neurones of the nucleus of the solitary tract (NST) through the release of glutamate from their central terminals (Andresen & Yang, 1990; Smith et al. 1998)). The NST neurones activated by gastric vagal afferents inhibit the spontaneously active gastro-excitatory DMN neurones or activate the gastro-inhibitory DMN neurones (McCann & Rogers, 1992; Takahashi & Owyang, 1997)); the physiological result is the immediate withdrawal of excitatory vagal input to the stomach and a rapid gastric relaxation (Rogers et al. 2005)).
Vagal afferents that are responsive to local distension of the gut also possess receptors for the duodenal hormone cholecystokinin (CCK) (Schwartz & Moran, 1994; Peters et al. 2006)). CCK has been shown to activate vagal afferent fibres in the periphery (Corp et al. 1993; Moran et al. 1994)), cell bodies in the nodose ganglion (Peters et al. 2004)), and vagal terminal endings within the NST itself (Rogers & Hermann, 2008)). Not surprisingly, CCK has been shown to cause gastric relaxation through its direct action on vagal afferents (Moran & Schwartz, 1994)) as well as amplify the mechano-sensitivity of these afferents (Schwartz & Moran, 1994)). Thus, if the anticipated effects of CCK to sensitize vagal afferents are physiologically important to the control of the stomach, then these effects should be clearly reflected in the responses of DMN neurones to gastric distension (McCann & Rogers, 1992)). That is, we would predict that gastric-DMN neurones which provide tonic input to the stomach should become more sensitive to the effects of gastric distension under the influence of CCK.
Details regarding the nature of CCK effects on gastrointestinal control have been further complicated by two observations. First, some of our preliminary studies suggested the possibility that the metabolic status (i.e. fed versus fasted) of an animal may impact the sensitivity and, perhaps, the direction of some gastric vago-vagal reflexes. In particular, the receptive relaxation reflex, which is activated by oesophageal distension, is seen to cause a substantial relaxation of the corpus in food-deprived animals. In contrast, there has been the suggestion that fed animals demonstrate an apparent reversal of the reflex (Travagli et al. 2003)). A relatively recent study (Holmes et al. 2009)) suggests this apparent reversal of the receptive relaxation reflex in the fed animal may be attributable to the action of CCK within the vagal reflex circuitry in the dorsal medulla. It is interesting to note that studies by Dockray and colleagues (reviewed by Dockray & Burdyga, 2011)) suggest that the neurochemical phenotype of vagal afferent neurones may depend on the nutritional state of the animal. For example, in fed animals, vagal afferent neurones in the nodose express receptors for peptide YY (Y2) and cocaine- and amphetamine-regulated transcript (CART), while in fasted rats, these neurones express cannabinoid (CB-1) and melanin-concentrating hormone (MCH)-1 receptors. These changes in phenotype expression with different nutritional states are thought to be based on associated changes in circulating levels of CCK, ghrelin and leptin (reviewed by Dockray & Burdyga, 2011)).
Secondly, it appears that the predominant circulating form of the peptide is the longer version, CCK58, and not CCK8 (Reeve et al. 2003)). The in vivo biological effects of these two forms of the peptide may be qualitatively different (Reeve et al. 2004)), though the longer peptide has an approximately 5-fold lower binding affinity for the CCKA type receptor than CCK8 does (Wu et al. 2008)). Nearly all physiological work with CCK on gastric-vagal control has used the eight amino acid peptide; it is not yet known if CCK58 will produce differential effects on vago-vagal reflex circuits.
Therefore, we decided to test the generality of the effects on gastric vago-vagal reflexes of (1) the animal's nutritional state (fed versus fasted) and (2) systemic CCK (both the -8 and -58 isoforms) by recording the spontaneous activity of physiologically identified gastro-excitatory-DMN neurones. In view of the previous results (Travagli et al. 2003; Holmes et al. 2009)), we expected to see that the responsivity of gastric-DMN neurones to graded gastric distensions would be radically altered in the fed state versus the fasted state. Additionally, we anticipated that any such effect on responsivity would be mimicked by the central (versus peripheral) sites of action of CCK.
The current studies indicate that DMN-mediated accommodation reflex activity evoked by gastric distension is modestly depressed in the fed versus fasted state. At physiological doses, CCK acts to potently amplify the DMN-accommodation reflex sensitivity. Additionally, the principal, physiological effect of CCK to amplify gastric vago-vagal reflex sensitivity occurs in the periphery, most likely in intestinal regions served by the coeliac artery (Raybould et al. 1985; Calingasan et al. 1992)). Lastly, CCK8 proved to be more efficacious than CCK58 in amplifying the reflex.
Methods
Ethical approval of animal use
A total of 91 Long–Evans rats (250–500 g) of either sex were obtained from the breeding colony located at Pennington Biomedical Research Centre for these studies. All animals were maintained in a room with a 12 h:12 h light–dark cycle with constant temperature and humidity, and given food and water ad libitum. All experimental protocols were performed according to the guidelines set forth by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committees at the Pennington Biomedical Research Centre.
‘Fed’versus‘fasted’ feeding regime
Rats were randomly assigned to either ‘fed’ or ‘fasted’ groups. In either case, all the animals were maintained on a nutritionally complete liquid diet of Ensure (Abbott Laboratories, Columbus, OH, USA) for a minimum of 24 h before the experiment to facilitate the insertion of the gastric stimulating balloon in the rat (described below). In the rare case where an individual demonstrated a neophobia and did not readily ingest this diet (i.e. minimum of 20 ml intake in 8 h), then this animal was given an additional day of exposure to this diet before being rejected from the study. However, because this liquid diet was so readily accepted by most rats, we frequently needed to refill the bottles a second time during the day to ensure that no animal was food deprived prior to 17.00 h.
Thus, the ‘fasted’ group was food deprived overnight (approximately 16 h) prior to the acute neurophysiological experiment. In contrast, the ‘fed’ animals had access to fresh Ensure within minutes of the administration of the anaesthesia. Indeed, for the ‘fed’ animals it was necessary to empty the stomach contents prior to instrumentation (described below) for the in vivo experiment. As an additional index of the metabolic state of these two groups of animals, we monitored their relative body weights immediately before anaesthesia compared to their weight on the night before the experiment. After being maintained on the Ensure liquid diet for a minimum of one day, on average, the ‘fasted’ rats lost 15.1 ± 1.3 g (SEM) in body weight during the overnight fast relative to their ‘fed’ counterparts.
Surgical preparation
Rats were deeply anaesthetized with thiobutabarbital (Inactin, Sigma, 150 mg kg−1, intraperitoneal). This long-term anaesthesia is preferred due its minimal interference with autonomic reflexes (Buelke-Sam et al. 1978)). Using aseptic technique, a cannula was secured to the trachea to ensure a clear airway and a catheter was installed into the jugular vein to permit intravenous delivery of either saline or agonists (CCK8 (Sigma, St Louis, MO, USA) or CCK58 (BioSynthesis, Lewisville, TX, USA). In a subset of experiments (cf. Experiment 6; peripheral location of CCK receptors), in place of a jugular catheter, half of the subjects had a cannula installed into the left common carotid artery, the tip of which rested approximately 1 cm proximal to its bifurcation into the internal and external branches (Calingasan et al. 1992)). Lastly, in Experiment 5, an additional catheter was installed in the femoral vein for the peripheral delivery of either saline or the CCK antagonist, lorglumide (Sigma-Aldrich).
A stepping microinfusion pump (Rogers et al. 1982)) was used to deliver intravenous (or intra-arterial) saline or predetermined amounts of either CCK8 or CCK58 (50 μm concentration of either CCK; at a rate of 20 μl min−1). Thus, by turning on the stepping pump infusion lines for specific lengths of time, doses of CCK could be delivered across a range from 30 pmol (2 s infusion) to 1 nmol (60 s infusion). A similar microinfusion pump arrangement was used for the systemic delivery of lorglumide.
A laparotomy was done to expose the stomach and the proximal part of the duodenum. A small incision in the proximal duodenum was made to remove gastric contents via lavage through the pylorus. Once the stomach was empty, a small gastric balloon was inserted through the duodenal incision, past the pylorus, and into the antrum. The gastric balloon was constructed from the small finger of a surgical glove and attached to a piece of Silastic tubing (0.065 inches o.d.) which was exteriorized through the duodenal incision and secured via a purse-string ligature. The abdominal muscle wall and skin were closed with the tubing from the balloon exiting via the incision. The tubing was connected to a pressure transducer (Isotec, Harvard Instruments) to monitor balloon pressure. Instrumented rats were secured in a stereotaxic frame. A midline incision was made in the scalp and the cervical musculature was retracted. The foramen magnum was opened; removal of the dura and arachnoid membranes exposed the caudal portion of the floor of the fourth ventricle. Gastric-related DMN neurones responded to gastric distension volumes of 0.1–1.0 ml air applied to the gastric balloon via a 1 ml syringe.
Extracellular electrophysiological recordings
A single glass micropipette (tip diameter, 1 μm), filled with 2 m NaCl plus 1% pontamine sky blue for iontophoretic marking of recording sites, was used in the identification and recording of neurones in the DMN as described in our earlier studies (McCann & Rogers, 1992; Chen & Rogers, 1997)). Extracellular signals from the micropipette were amplified (5000×; WPI DAM 50 Differential Amplifier) and band-pass filtered (300–3000 Hz; Warner Instruments LPF 202A) before being displayed on an oscilloscope and stored for later analysis on an AM Systems LabChart 7 PC-based waveform analysis system (ADInstruments).
Gastric-DMN neurones are spontaneously active with a basal firing rate between 0.1 and 5 Hz (McCann & Rogers, 1990, 1992; Chen & Rogers, 1997)). Gastric-related DMN neurones were located by their crisp reduction in spontaneous firing that was essentially time-locked to the period of antral balloon distension. Over 95% of physiologically identified gastric-DMN neurones are inhibited by antral distension (i.e. gastro-excitatory DMN neurones that are inhibited by distension), so we are confident that these particular neurones represent the majority of neurones most involved in generating the gastric accommodation reflex (McCann & Rogers, 1990, 1992; Chen & Rogers, 1997; see Fig. 1)).
Figure 1.
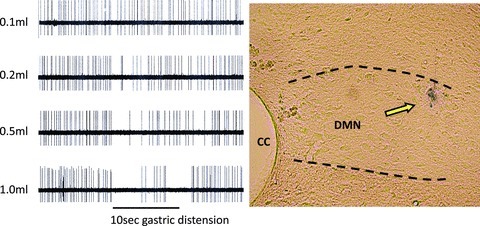
Left panel: raw oscillograph records of the inhibition of spontaneous activity of DMN neurone responding to different volumes of gastric (antral) distension. Over 95% of physiologically identified DMN neurones are inhibited by antral distension (i.e. gastro-excitatory DMN neurones that are inhibited by distension; McCann & Rogers, 1992)). Antral distension volumes of 0.1, 0.2, 0.5 and 1.0 ml in the emptied stomachs of either previously ‘fed’ or ‘fasted’ rats caused a distension-locked reduction in spontaneous activity of the gastric-DMN neurones involved in the gastric accommodation reflex in a distension-volume-dependent manner. Right panel: histological verification of microelectrode recording site. CC, central canal; DMN, dorsal motor nucleus of vagus.
At the end of the recording session, the location of the DMN cell responsive to antral distension could be marked by applying a 2 μA positive direct current for 1 min to the pontamine-filled recording micropipette using a stimulus isolation unit (WPI). The anaesthetized rat was transcardially perfused with PBS followed by 4% paraformaldehyde in PBS. The brainstem was removed and post-fixed overnight in 4% paraformaldehyde, then cryoprotected in 10% sucrose solution. Histological sections (40 μm) were cut on a freezing microtome, mounted onto slides and photographed with a Nikon E800 microscope equipped with a Jenoptic C7 camera. An example of a DMN recording site is shown in Fig. 1.
Experimental procedures
Experiment 1: Stimulation (gastric distension) and response (firing rate) relationships of gastric-DMN neurones in fed versus fasted animals
This subset of studies tested the hypothesis that the ‘fed’ animal demonstrates a suppressed or even an inverted gastric accommodation vago-vagal reflex relative to the reflex elicited in the ‘fasted’ state. Stimulation/response relationships (reflex plots) were generated by injecting and holding volumes of 0.1, 0.2, 0.5 or 1.0 ml of air in the gastric balloon for 10 s. DMN firing rates (FRs) were continuously monitored. The percentage difference between the spontaneous FR of the gastric-DMN neurone 10 s before versus the 10 s during distension was taken as the measure of the strength of the resulting antral distension-DMN reflex. Reflex plots from gastric-DMN neurones of both fed and fasted rats were constructed. The resultant stimulus/response curves generated by fed vs. fasted preparations were analysed for best fit of non-linear regression curves with intercept through zero (Fig. 2)).
Figure 2. Gastric distension inhibits spontaneous DMN activity in a volume-dependent fashion.
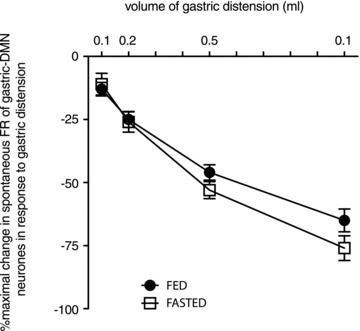
Spontaneous FR activity of DMN neurones is relatively constant. Gastric distension via balloon expansion (0.1–1.0 ml volumes) caused incremental increases in the inhibition of the spontaneous FR of DMN neurones. Basal FR for each individual DMN neurone was considered 100% and then compared to relative amount of FR inhibition caused by each level of distension. Thus, each neurone served as its own control. The resultant stimulus/response curves generated by fed vs. fasted preparations were analysed for best fit of non-linear regression curves with intercept through zero. Fasted animals demonstrated a slight increase in sensitivity to gastric distension at the higher volumes relative to animals that had not been fasted. Non-linear regression analysis of these curves indicate different slopes (‘fed’=−75.6 ± 3.4; ‘fasted’=−87.8 ± 4.1; F1,242= 5.329; P= 0.02).
Experiment 2: Effect of CCK8 or CCK58 on gastric distension reflex activity of gastric-DMN neurones in fed or fasted animals
We examined whether systemic CCK8 or CCK58 could modulate the gastric distension reflex of inhibition of identified gastric-DMN neurone activity in either fasted or fed rats. Previous studies (Cooper et al. 2008)) have shown that 1 nmol amounts of systemically administered CCK8 or CCK58 are sufficient to generate cFOS activation in the dorsal vagal complex. Therefore, a 1 nmol dose of either saline or CCK8 or 58 was given i.v. to both fed and fasted rats that were prepared as described above. Based on the results of the studies in part 1, we chose to use the 0.5 ml gastric distension volume as a standard gastric distension challenge for these groups. (To verify that repetitive 0.5 ml distensions of the stomach did not show adaptation in the change of gastric-DMN firing rate, we subjected one of our ‘fed’ preparations to 10 cycles of distension/release, spaced at 1 min intervals and observed a consistent (average 44.1 ± 2.0% (SEM)) inhibition of firing rate under control conditions.)
Systemic administration of 1 nmol of either CCK8 or 58 had a pronounced acute effect to completely suppress spontaneous gastric-DMN neurone activity. Recovery from this CCK plus gastric distension-induced suppression of gastric-DMN firing rate often took several minutes to recover, thus making it difficult to be certain that the same unit could be held for repetitive trials. Therefore, the reflex plots before (i.e. ‘basal’ condition) and after 1 nmol CCK (either CCK8 or CCK58) could not be paired to the same individual gastric-DMN unit. Rather, within each individual animal, the response to 0.5 ml of gastric distension was recorded from multiple individual gastric-DMN units under ‘basal’ (saline) conditions. After 1 nmol i.v. CCK (either CCK8 or CCK58), different individual gastric-DMN neurones in the same animal were tested for their response to 0.5 ml gastric distension (Fig. 3)).
Figure 3. Effect of systemic CCK8 or CCK58 (1nmol, i.v.) on the sensitivity of DMN neurones to gastric distension.
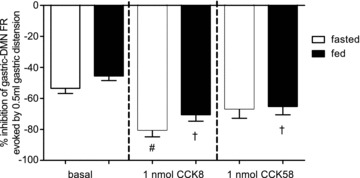
As also seen in Figs 1 and 2, under basal conditions, the spontaneous FR of gastro-excitatory-DMN neurones was reduced approximately 50% in response to 0.5 ml antral distension, regardless of the metabolic status of the animal. In this subset of experiments, fed or fasted animals received intravenous (i.v.) delivery of 1 nmol of either CCK8 or CCK58. Two-way analysis of variance revealed a main effect of the 1 nmol CCK treatment (F2,139= 14.48; P < 0.0001), but no main effect of the metabolic state nor was there an interaction between these two sources of variance. Bonferroni post tests between basal and each of the two different CCK subtypes indicate that CCK8 amplifies the inhibition of gastric-DMN neurones to 0.5 ml antral distension in either the fed or fasted state (#P < 0.0001); in contrast CCK58 significantly amplifies this inhibition only in the fed state (†P < 0.05) relative to the basal responses.
These data were subjected to a ‘2’ (fed vs. fasted) by ‘3’ (saline, CCK8, or CCK58) two-way analysis of variance to determine if there were interactions between metabolic state and different drug treatments. Bonferroni post tests were made between basal and each of the two different CCK subtypes; values of P < 0.05 were considered statistically significant differences.
Experiment 3: Low dose (i.v.) CCK8 and CCK58 effects on spontaneous activity of gastric-DMN neurones
This subset of studies was designed to determine: (a) the lower limits of systemic doses of either form of CCK to inhibit the spontaneous activity of identified gastric-DMN neurones and (b) to see if the effects of CCK, at these lower doses, to inhibit gastric-DMN reflex responses are affected by the metabolic status of the animals. All surgical and experimental preparations were the same as described above. The stepping microinfusion pump was used to deliver intravenous saline or either CCK8 or CCK58 (50 μm concentration of either CCK; at a rate of 20 μl min−1; pump for 6 or 10 s = 2.0 or 3.3 μl; doses = 100 or 160 pmol). These doses approximate levels that have been shown to activate vagal afferents (Schwartz & Moran, 1994; Rogers & Hermann, 2008)) and cause satiety via i.v. injection (Cox, 1998)). These doses of CCK are similar to amounts released into the circulation following a meal (Raybould et al. 1985; Reidelberger et al. 1989, 1994; Calingasan et al. 1992; Chowdhury & Rayford, 2001)).
Gastric-DMN neurones were pre-identified as before (see Fig. 1)) by their inhibition of spontaneous FR in response to 0.5 ml gastric distension. The spontaneous FR of identified gastric-DMN neurones was then monitored in response to randomized i.v. doses of CCK8 or 58 (100 or 160 pmol) or 3.3 μl saline. Unlike the prolonged inhibition of spontaneous activity seen with the high doses (1 nmol) of CCK8 or 58, these 100 or 160 pmol quantities resulted in quick onset and transient inhibitions of the spontaneous FR of gastric-DMN neurones (see example in Fig. 4)). The percentage maximal change in spontaneous FR of gastric-DMN neurones occurring within 2 min following these i.v. injections in either fed or fasted animals is shown in Fig. 5. These data were subjected to a ‘2’ (fed vs. fasted) by ‘5’ (doses of saline or CCK) two-way analysis of variance to determine if there were interactions between metabolic state and different drug treatments on the spontaneous FR of identified gastric-DMN neurones; Bonferroni post tests compared changes in FR responses to CCK doses to those elicited by i.v. saline. P values less than 0.05 were considered statistically significant.
Figure 4. Raw oscillographic record of a gastric-DMN neurone firing rate (FR) in response to antral distension before and after CCK8 i.v.
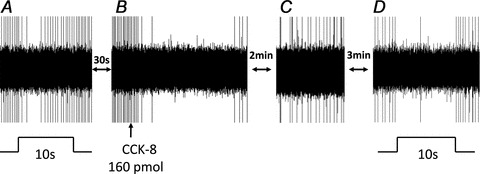
A, basal spontaneous FR of DMN neurone is transiently reduced during the 10 s antral distension (0.5 ml volume). B, after basal FR recovered, CCK8 (160 pmol in 3.3 μl total volume) was injected i.v. and evoked a pronounced suppression of spontaneous DMN activity. C, within 2–3 min after CCK8 i.v., spontaneous FR returns to a steady level. D, after intravenous CCK8, suppression of spontaneous FR of same gastric-DMN neurone evoked by 10 s antral distension for 10 s is amplified relative to the response elicited prior to CCK8 exposure.
Figure 5. Percentage maximal change in spontaneous FR of identified gastric-DMN neurones occurring within 2 min following 100 or 160 pmol i.v. injections of CCK8 or 58 (or 3.3 μl i.v. saline) in either fed or fasted animals.
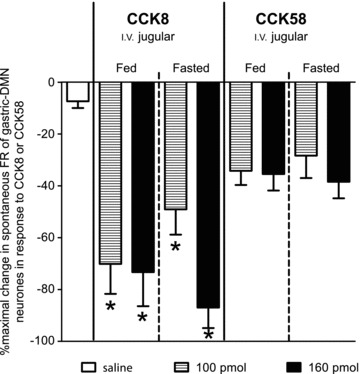
Comparable numbers of identified gastric-DMN neurones were sensitive to either CCK8 (29 neurones sensitive/32 neurones identified) or CCK58 (26 neurones sensitive/28 neurones identified). Two-way analysis of variance (metabolic state versus doses of CCK) revealed that there was no interaction between metabolic state of the animal or CCK doses. There was a main effect of CCK on spontaneous DMN firing rate (F4,63= 19.61; P < 0.0001) that is exclusively attributable to CCK8. Note, there was no main effect of metabolic state (F4,63= 0.13; P= 0.72).
Experiment 4: Effect of systemic CCK8 (160 pmol) on gastric distension reflex activity of gastric-DMN neurones (paired observations)
The goal of this section was to determine if the amplification of the reflex sensitivity of gastric-DMN neurones observed at 1 nmol of CCK8 would also occur at the more physiological dose of 160 pmol. All surgical and experimental preparations (e.g. fed vs. fasted) were as described above; again, 0.5 ml gastric distension volume was the standard of comparison between these groups. Given that the spontaneous activity of the gastric-DMN neurones recovered after attenuation from the systemic administration of 160 pmol CCK8, it was possible to record the firing rate of the same DMN neurone before and after injection of CCK8. Evaluation of the gastric distension reflex was repeated after a minimum of 5 min after the injection of CCK8, to ensure that the gastric-DMN neurone had fully recovered its spontaneous firing activity. Therefore, the reflex responses of each gastric-DMN unit before and after systemic CCK8 could be directly compared (Fig. 6)). These data were subjected to a ‘2’ (fed vs. fasted) by ‘2’ (before vs. after CCK8) two-way analysis of variance to determine if there were interactions between metabolic state and CCK8 on the gastric-distension-induced change in gastric-DMN firing rate. Bonferroni post tests compared changes in FR responses to gastric distension before vs. after systemic CCK8 within the fed or fasted groups; P values less than 0.05 were considered statistically significant.
Figure 6. Responses of identified gastric-DMN neurones to modest antral distension (0.5 ml) before and after systemic administration of CCK8 (160 pmol) in either fed or fasted animals were made as paired observations.
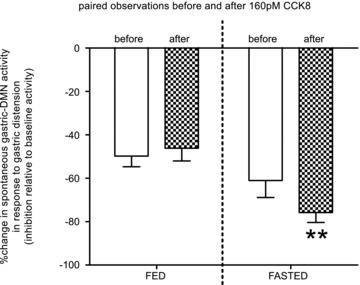
Repeated measures two-way analysis of variance revealed that the matching effect of these paired observations was extremely significant (F17,17= 9.82; P < 0.0001) and there was a significant interaction between metabolic state and the presence of CCK8 (F1,17= 12.42; P= 0.0026); the effect of CCK to amplify the inhibition of gastric-DMN FR in response to modest gastric distension was more evident in the fasted animals. Given that the interaction is statistically significant, the P values of the CCK main effect (F1,17= 4.54; P= 0.048) and the metabolic main effect (F1,17= 6.23; P= 0.023) are more difficult to interpret. However, it can be readily appreciated that in the fasted animal, exogenous CCK (given systemically at approximate physiological doses) amplifies the inhibition of gastric-DMN firing rate in response to antral distension. That is, at physiological doses, exogenous, systemic CCK does not modulate the ‘fasted’ gastric accommodation reflex to resemble the reflex elicited in the ‘fed’ state.
Experiment 5: Central versus peripheral site of action of CCK8 effects on spontaneous activity of gastric-DMN neurones
All surgical and experimental preparations were the same as described above with the exception that these experiments were done only in fasted rats (i.e. the group which demonstrated the maximum effects of systemic CCK inhibition of spontaneous gastric-DMN activity). This subset of experiments was designed to determine if the effects of systemic CCK8 (160 pmol) on the spontaneous activity of pre-identified gastric-DMN neurones is the result of central or peripheral sites of action. The CCKA antagonist, lorglumide (Sigma) was administered either via the jugular catheter (10 mg kg−1 in 0.3 ml volume (Burdyga et al. 2008; Sartor & Verberne, 2010)), or was placed (2 μl volume containing 30 nmol) directly onto a small piece of filter paper (∼2 mm2) apposed onto the floor of the fourth ventricle (Viard et al. 2007; Holmes et al. 2009)).
The application of 30 nmol of lorglumide (formula weight ∼500 g mol−1) directly onto the floor of the fourth ventricle (4V) translates into a 3–30 mm concentration when one accounts for an estimated volume of 10–100 μl of CSF at that site during the experiment. Note that this dosage and concentration have been shown to be sufficient to suppress the effects of large concentrations of CCK applied centrally (Viard et al. 2007; Holmes et al. 2009)). In contrast, the peripheral dose (10 mg kg−1) used by others (Burdyga et al. 2008; Sartor & Verberne, 2010)) amounts to 3.25 mg in a 325 g rat. However, this dose is placed into the peripheral circulation with a volume of approximately 25–30 ml. Thus, the actual circulating peripheral concentration is in the order of 6.5 μm. Therefore, when taking the body fluid dilution factors into account, the peripheral concentration is approximately 1/400 to 1/4000 of the concentration of the centrally applied lorglumide.
Gastric-DMN neurones were pre-identified by their inhibition of spontaneous activity in response to 0.5 ml gastric distension. The spontaneous FR of identified gastric-DMN neurones was monitored continuously. A demonstration of the change in spontaneous activity in response to systemic CCK8 (160 pmol) was established for each animal. Subsequently (approximately 10 min after the first exposure to low dose, systemic CCK8), lorglumide was administered (either peripherally (i.v.) or centrally (4V)) 10 min before the next exposure to systemic CCK8. The percentage maximal change in spontaneous FR of gastric-DMN neurones in response to CCK8 during either route of lorglumide antagonism is shown in Fig. 7A. Examples of central or peripheral antagonism of CCK changes in gastric-DMN activity over time are shown in Fig. 7B and C. A one-way analysis of variance of percentage maximal changes in gastric-DMN FR in response to systemic CCK was done. Bonferroni post tests were carried out comparing the CCK-induced changes in gastric-DMN FR for either route of administration of lorglumide against the CCK alone control. P values less than 0.05 were considered statistically significant.
Figure 7. Peripheral versus central antagonism of the effects of systemic CCK8 on DMN spontaneous activity.
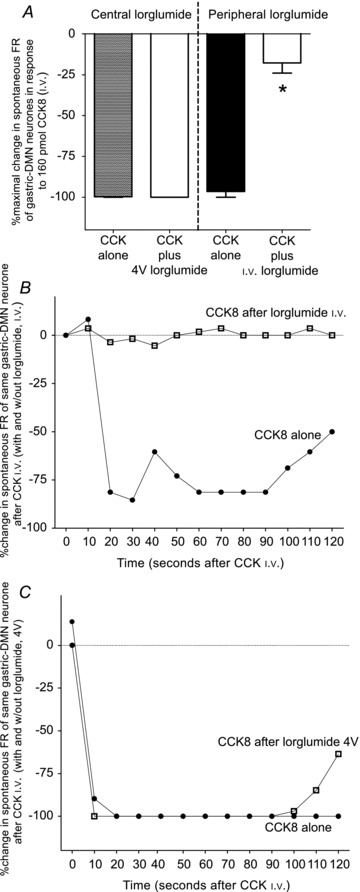
A, bar graph represents averaged maximal effects of CCK8 to inhibit spontaneous DMN firing rate with and without lorglumide administered either centrally or peripherally. Application of lorglumide to the 4V floor has no effect on the attenuation of the spontaneous activity of the gastric-DMN neurones evoked by systemic CCK8 in fasted rats. In contrast, i.v.-administered lorglumide reduced the attenuating effect of CCK8 on the spontaneous activity of the gastric-DMN neurones by approximately 80% (F3,16= 112; P < 0.0001). B, plot of the spontaneous activity of an identified gastric-DMN neurone exposed to CCK8 with and without i.v.-administered lorglumide. Note that the CCK8 effect is completely blocked after i.v. lorglumide. C, similar plot of the spontaneous activity of an identified gastric-DMN neurone exposed to CCK8 with and without 4V-administered lorglumide. Spontaneous activity of this gastric-DMN neurone was not affected following 4V application of lorglumide.
Experiment 6: Verification of coeliac/mesenteric site of action for CCK
Previous studies on CCK action to regulate feeding (Kraly, 1984; Edwards et al. 1986)) and the activity of reflex control circuitry in the brainstem (Holmes et al. 2009)) have used relatively high doses or concentrations of the hormone. However, careful physiological and behavioural studies (Raybould et al. 1985; Calingasan et al. 1992)) have shown that physiological doses (i.e. 30–70 pmol) of CCK applied intra-arterially at the juncture of the coeliac artery activate vagal afferents and suppress feeding. Thus, the delivery of CCK to this coeliac/mesenteric circulation should preferentially activate vagal afferents in the proximal gut, the presumed locus of physiological action for the peptide (Raybould et al. 1985; Calingasan et al. 1992)). Experimental and surgical preparations were as described above. Only fasted animals were used in this subgroup of experiments. The groups differed only in terms of the placement of the catheter (i.e. jugular (i.v.) versus near-coeliac (i.a.)). As described above, a stepping microinfusion pump was used to deliver saline, 30 pmol, or 60 pmol of CCK8 via either circulatory route.
Gastric-DMN neurones were pre-identified as before (see Fig. 1)) by their reduction of spontaneous FR in response to 0.5 ml gastric distension. The spontaneous FR of identified gastric-DMN neurones was then monitored in response to randomized doses of 30 or 60 pmol of CCK8 or 1.4 μl saline administered either systemically (i.v.) or specifically to the coeliac/mesenteric circulation (i.a.). The percentage maximal change in spontaneous FR of gastric-DMN neurones occurring within 2 min following these injections is shown in Fig. 8.
Figure 8. Percentage maximal change in spontaneous FR of identified gastric-DMN neurones occurring within 2 min following 0, 30, or 60 pmol injections of CCK8 into either the systemic (jugular (i.v.)) or coeliac/mesenteric (near coeliac (i.a.)) circulation of fasted animals.
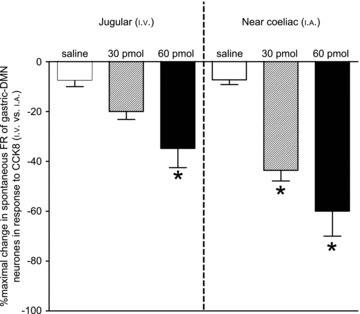
Two-way analysis of variance revealed that the route of administration was very significant (F1,36= 9.23; P= 0.0044) as was the dose of CCK8 (F2,36= 19.04; P < 0.0001). There was no interaction between these sources of variance. *P < 0.05 Bonferroni multiple comparisons to saline.
These data were subjected to a ‘2’ (route) by ‘3’ (dose) two-way analysis of variance to determine if there were interactions between route of administration (i.e. i.v.versusi.a.) and different drug doses (i.e. 0, 30, or 60 pmol CCK8). Bonferroni post tests were made between saline control and each of the two different circulation routes; values of P < 0.05 were considered statistically significant differences.
Results
Experiment 1: Graded antral distensions and gastric-DMN activity in ‘fed’versus‘fasted’ rats
Antral distension volumes of 0.1, 0.2, 0.5 and 1.0 ml in the emptied stomachs of either previously ‘fed’ or ‘fasted’ rats caused a distension-locked reduction in the spontaneous activity of the gastric-DMN neurones involved in the gastric accommodation reflex. These reductions in gastric-DMN activity were significant and clearly distension-volume dependent (Fig. 1)). The results were qualitatively similar between ‘fed’versus‘fasted’ rats. Stimulus/response curves generated for these metabolic states (Fig. 2)) indicate that fasted rats were somewhat more sensitive to the higher amplitude distension signals compared with fed rats. Non-linear regression analysis of these curves indicate different slopes (‘fed’=−75.6 ± 3.4; ‘fasted’=−87.8 ± 4.1; F1,242= 5.329; P= 0.02) with the ‘fasted’ group demonstrating a modestly higher sensitivity to distension (93 individual gastric-DMN neurones; 22 animals).
Experiment 2: Effect of systemic (intravenous; i.v.) CCK8 or CCK58 (1 nmol) on the sensitivity of DMN neurones to gastric distension
Our first set of experiments demonstrated that the spontaneous firing rate of gastro-excitatory-DMN neurones was reduced approximately 50% in response to 0.5 ml antral distension, regardless of the metabolic status of the animal (Figs 1 and 2)). Therefore, this level of gastric distension (0.5 ml) was then chosen as our constant stimulus for the subsequent studies on gastric-DMN reflex sensitivity.
Although Holmes et al. reported a reversal of the vago-vagal-mediated oesophageal-gastric reflex after application of 15 nmol CCK8 onto the floor of the fourth ventricle (Holmes et al. 2009)), previous studies by Cooper et al. had shown that 1 nmol quantities of peripherally administered CCK8 and CCK58 activate both the myenteric plexus and the dorsal vagal complex (Cooper et al. 2008)). Therefore, the present subset of experiments used i.v. (jugular) delivery of 1 nmol of either CCK8 or CCK58. In either case, 1 nmol CCK (i.v.) produced an immediate, complete, and long duration (several minutes) inhibition of gastric-DMN spontaneous activity. Thus, the ability to ‘hold’ an individual gastric-DMN unit was difficult as it was not clear if the unit had been lost or was still inhibited. Therefore, it was necessary to evaluate the effects of 0.5 ml distension in individual gastric-DMN units prior to CCK i.v. injections (‘basal’) to be compared to the effects of 0.5 ml distension on individual gastric-DMN units within one hour after the 1 nmol CCK injection (i.e. each animal was able to provide data for both ‘basal’ and ‘CCK’ conditions; Fig. 3)).
Two-way analysis of variance to determine if there were interactions between metabolic state and different drug treatments revealed that although there was a main effect of the 1 nmol CCK i.v. treatment (F2,139= 14.48; P < 0.0001), there was no main effect of the metabolic state nor was there an interaction between these two sources of variance. Bonferroni post tests between basal and each of the two different CCK subtypes indicate that CCK8 amplifies the inhibition of gastric-DMN neurones to 0.5 ml antral distension in either the fed or fasted state (P < 0.0001); in contrast CCK58 significantly amplifies this inhibition only in the fed state (P < 0.05) relative to the basal responses (146 individual gastric-DMN neurones; 18 animals).
Experiment 3: Low dose (i.v.) CCK8 and CCK58 effects on spontaneous activity of identified gastric-DMN neurones
The previous experiments showed that peripheral administration of either CCK8 or CCK58 (1 nmol; i.v.) accentuated the inhibitory reflex effect of gastric distension on the spontaneous activity of gastric-DMN neurones. Qualitatively, there was little difference in the relative effects of CCK 8 versus 58 on gastric-DMN reflex sensitivity. However, post-prandial circulating levels of CCK8 have been reported to be approximately 80 pg ml−1 (Reidelberger et al. 1989, 1994; Chowdhury & Rayford, 2001)). If we assume a total blood volume of 25–30 ml in an adult rat, a 1 nmol dose of CCK (i.e. 1.1 μg) translates into approximately 44,000 pg ml−1; more than 500-fold above physiological levels. Therefore, perhaps this dose (of either form of CCK) produced a ‘ceiling’ effect, thus obstructing subtle effects due to isoform differences or the fed/fasted state of the animals. More recently, systemic administration of 100–1000 pmol kg−1 CCK8 has been used to evoke changes in gastric and vagal nerve activity (Okano-Matsumoto et al. 2011)). As a consequence, this next subset of experiments was designed to determine the effects of more physiological doses (100 and 160 pmol) of these two isoforms of CCK on the spontaneous activity of identified gastric-DMN neurones.
Identified gastric-DMN neurones were equally likely to be sensitive to either CCK8 or CCK58 at these doses (CCK8: of the 32 identified gastric-DMN neurones, 29 were sensitive to CCK8. For comparison, CCK58: of the 28 identified gastric-DMN neurones, 26 were sensitive to CCK58). See example of raw oscilloscope recordings in Figure 4.
Two-way analysis of variance (metabolic state versus doses of CCK) revealed that there was no interaction between metabolic state of the animal or CCK doses. There was a main effect of CCK on spontaneous gastric-DMN firing rate (F4,63= 19.61; P < 0.0001) and this was, statistically, exclusively attributable to CCK8 (Fig. 5)). That is, at these lower i.v. doses, CCK8 was considerably more potent than CCK58 in reducing spontaneous gastric-DMN activity (Bonferroni multiple comparisons post tests). In these experiments, there was no main effect of metabolic state (F4,63= 0.13; P= 0.72; 72 individual gastric-DMN neurones; 23 animals).
Experiment 4: Effect of systemic CCK8 (160 pmol) on gastric distension reflex activity of gastric-DMN neurones (paired observations)
These studies were designed to determine the response of identified gastric-DMN neurones to modest antral distension (0.5 ml) before and after systemic administration of CCK8 (160 pmol) in either fed or fasted animals, i.e. paired observations (Fig. 6)). In these studies, repeated measures two-way analysis of variance revealed that the matching effect of these paired observations was extremely significant (F17,17= 9.82; P < 0.0001). Also, there was a significant interaction between metabolic state and the presence of CCK8 (F1,17= 12.42; P= 0.0026); the effect of CCK to amplify the inhibition of gastric-DMN FR in response to modest gastric distension was more evident in the fasted animals (Fig. 6)). Given that this interaction is statistically significant, the P values of the CCK main effect (F1,17= 4.54; P= 0.048) and the metabolic main effect (F1,17= 6.23; P= 0.023) are more difficult to interpret. However, it can be readily appreciated that in the fasted animal, exogenous CCK (given systemically i.v. at approximate physiological doses) amplifies the inhibition of gastric-DMN firing rate in response to antral distension. That is, at physiological doses, exogenous, systemic CCK does not modulate the ‘fasted’ gastric accommodation reflex to resemble the reflex elicited in the ‘fed’ state (n= 19 gastric-DMN units (paired observations); 8 animals).
Experiment 5: Central versus peripheral site of action of CCK8 effects on spontaneous activity of gastric-DMN neurones
It is known that distension of the antrum activates mechanoreceptors in the wall of the stomach that ultimately lead, through the vago-vagal circuit, to the inhibition of the gastric-DMN neurones (McCann & Rogers, 1992; Rogers et al. 2005)). In the previous sections, we demonstrated that systemic CCK8 can also lead to attenuation of the responsiveness of gastric-DMN neurones.
These CNS effects may be limited to CCK8 acting directly on the same vagal afferents that are activated by the antrum (Moran & Schwartz, 1994; Baptista et al. 2005; Rogers et al. 2005)); some of the effects of CCK8 may also be attributable to the direct activation of CCKA receptors in the dorsal vagal complex (Holmes et al. 2009)).
Therefore, lorglumide, an antagonist at the CCKA receptor, was used to determine the site of action of systemic CCK8 on the vago-vagal circuit involved in the gastric accommodation reflex. Previous studies have shown that 5–10 mg kg−1 systemic lorglumide will block peripheral, but not central, effects of CCK (Sartor & Verberne, 2010)). Conversely, application of 30–40 nmol of lorglumide into the 4V will block central, but not peripheral effects of CCK (Viard et al. 2007)). We employed both routes of lorglumide administration to evaluate the probable site of action of systemic CCK to effect a reduction in spontaneous gastric-DMN activity.
Figure 7 represents the data of peripheral versus central antagonism of the effects of systemic CCK8 on gastric-DMN spontaneous activity. The bar graph (Fig. 7A)) represents averaged maximal effects of CCK8 to inhibit spontaneous gastric-DMN firing rate with and without lorglumide administered either centrally (4V) or peripherally (i.v.). Clearly, application of lorglumide to the 4V floor has no effect on the attenuation of the spontaneous activity of the gastric-DMN neurones evoked by systemic CCK8. In contrast, i.v.-administered lorglumide reduced the attenuating effect of CCK8 on the spontaneous activity of the gastric-DMN neurones by approximately 80% (F3,16= 112; P < 0.0001). Lorglumide (i.v. or 4V) had no effect, by itself, on gastric-DMN firing in these fasted animals (data not shown) (n= 8 gastric-DMN neurones; paired observations; 7 animals).
Examples of individual, paired observations (i.e. on the same gastric-DMN neuron) are shown in Fig. 7B and C. Figure 7B is a plot of the spontaneous activity of an identified gastric-DMN neurone exposed to CCK8 with and without peripherally (i.v.) administered lorglumide. Note that the CCK8 effect is completely blocked after i.v. lorglumide. Figure 7C shows a similar plot of the spontaneous activity of an identified gastric-DMN neurone exposed to CCK8 with and without centrally (4V) administered lorglumide. Here, in contrast, the spontaneous activity of this gastric-DMN neurone was not affected following 4V application of lorglumide (Fig. 7C)).
Experiment 6: Verification of coeliac/mesenteric site of action for CCK
These studies evaluated the response of identified gastric-DMN neurones to either systemic (jugular, i.v.) or coeliac/mesenteric (coeliac, i.a.) administration of CCK8 (0, 30, or 60 pmol) in fasted animals (Fig. 8)). Two-way analysis of variance (i.e. route vs. dose) revealed that the route of administration was very significant (F1,36= 9.23; P= 0.0044) as was the dose of CCK8 (F2,36= 19.04; P < 0.0001). These data show that administration of 30 pmol of CCK8 via the near-coeliac i.a. route, statistically, significantly suppresses gastric-DMN spontaneous activity compared with the i.v. route. Thus, these data verify that the principal, physiological effect of CCK to amplify gastric vago-vagal reflex sensitivity occurs in the periphery, most likely in intestinal regions served by the coeliac artery (Raybould et al. 1985; Calingasan et al. 1992)).
Discussion
DMN neurones are powerful regulators of gastric tone and motility. This control over gastric function is exerted through DMN synaptic inputs onto enteric cholinergic neurones (Schemann & Grundy, 1992; Rogers et al. 2005)) that, in turn, regulate the excitability of gastric smooth muscle. DMN neurones integrate visceral afferent inputs (via the NST), descending CNS afferent activity, and circulating ‘afferent’ factors such as gut hormones and cytokines in order to regulate gastric function (Rogers et al. 2005)). DMN activity is, therefore, a very sensitive overall measure of second-by-second CNS control over the stomach.
It has been suggested (Travagli et al. 2003; Dockray & Burdyga, 2011)) that the metabolic state of the animal (i.e. ‘fed’versus‘fasted’) could have a significant impact on the sensitivity of vago-vagal reflexes responsible for controlling the gastric phase of digestion as well as the impact of CCK to modulate these vago-vagal gastric control reflexes. Based on immunohistochemical (IHC) studies, Dockray and Burdyga have proposed that the feeding status of the animal may be reflected in the density/expression of receptors (e.g. Y2R, CB1, MCH1R) on vagal afferents (Dockray & Burdyga, 2011)). They suggest that, in turn, this changing receptor population may place a bias on the responsiveness of the vago-vagal reflex circuits that control the stomach as well as on components involved in visceral afferent satiety mechanisms. Others have proposed that CCK itself may be responsible for radical shifts in not only the magnitude but also the direction of gastric vagal reflexes (Holmes et al. 2009)).
We examined the responsiveness of physiologically identified gastric-DMN neurones to graded gastric antral distension under both fed and fasted conditions. In the basal condition (i.e. no exogenous CCK), we noted a small but significant reduction in the sensitivity of DMN neurones to antral distension in the fed versus fasted rats (Fig. 2)). Also, fasted rats tended to be slightly more sensitive than fed rats to the inhibitory effects of CCK8 (Fig. 3)). However, it was abundantly clear that ‘high’ doses (1 nmol) of systemic CCK8 produced a significant increase in reflex sensitivity regardless of feeding status (Fig. 3)). These results suggest that CCK has a dominant effect to increase the sensitivity of vagal reflex controls over gastric motility and tone. Several metabolic parameters associated with feeding status are available to modulate reflex sensitivity by controlling the expression of receptors on vagal afferents that, in turn, regulate afferent sensitivity to mechanical or chemical stimuli (Dockray & Burdyga, 2011)). However, these effects were, for the most part, subtle compared with that exerted by CCK in these studies.
The review by Dockray and Burdyga (Dockray & Burdyga, 2011)) strongly suggests that metabolic status or exposure to CCK alters the expression of several presynaptic vagal receptors which could shift the sensitivity of vagal afferents to primary visceral stimuli such as gastric distension and leptin (Schwartz et al. 1993)). For example, their IHC studies show that fasting elevates, while feeding suppresses, the expression of CB1-receptors in nodose ganglion cell bodies (Dockray & Burdyga, 2011)). Predictions based on these IHC results would conclude that the fasted animal (i.e. elevated CB1 receptor expression) would demonstrate a reduced responsiveness of vagal afferents to distension or CCK due to the activation of vagal presynaptic CB1 receptors by endogenous ligands (i.e. anandamide) (Derbenev et al. 2004)). However, the results obtained in the present in vivo study did not bear out these predictions. Indeed, neurophysiological recordings from identified gastric vago-vagal neurones in the DMN showed the converse: fasted rats demonstrated a slightly higher sensitivity to CCK and gastric distension than recently fed animals. Ironically, the effects of gastric distension in the fasted rat were further amplified by exogenous CCK.
While the plasticity of neurochemical phenotype of vagal afferent neurones documented by Dockray and Burdyga is no doubt correct, our neurophysiological results suggest that the interplay between the numerous modulators released in fed versus fasted animals may alter vagal afferent reflex sensitivity or the sensitivity of the second order NST neurones (and, ultimately, the DMN efferent neurones) in unpredictable ways. Alternatively, in the intact animal, vago-vagal reflex sensitivity may be drastically altered by the action of descending CNS inputs differentially active in fed versus fasted states. One such example would include thyrotropin-releasing hormone (TRH) which is released onto NST and DMN neurones at the onset of feeding (Martinez et al. 2001, 2002). TRH powerfully desensitizes vago-vagal reflexes by simultaneously inhibiting NST neurones involved in gastric reflex control while activating gastroexcitatory DMN neurones (McCann et al. 1989; Rogers et al. 2005)).
Although fasted animals showed a slightly greater gastric accommodation reflex (i.e. higher sensitivity to gastric distension) and response to CCK than their ‘fed’ counterparts, we did not observe a reversal of this reflex in the fed animal nor did systemic CCK evoke such a reversal of a vago-vagal reflex as has been previously reported (Holmes et al. 2009)). These differing observations may reflect differences in the organization of the gastric accommodation reflex (studied here) versus the oesophageal gastric reflex as well as methodological differences (Holmes et al. 2009)). The present study utilized intravenous doses of CCK that at least approximated amounts released into the portal circulation after meals and fitted within the demonstrated CCK dose versus activation curves observed for vagal afferent fibres and terminals both in vivo and in vitro (Schwartz et al. 1995; Rogers & Hermann, 2008; Okano-Matsumoto et al. 2011)). The study by Holmes et al. used intra-brainstem injection concentrations of CCK in the millimolar range (dorsal vagal complex: 60 nl of 7.5 mm CCK = 450 pmol dose; 4V: 2 μl of 7.5 mm CCK = 15 nmol dose; Holmes et al. 2009)). Such high CCK concentrations could have unusual effects on the function of vago-vagal circuitry.
Interpretation of the CCK effect to regulate visceral afferent responsiveness could be confused by the fact that several isoforms of this gut hormone may be found in the circulation. Indeed, the 58 amino acid form of CCK may be the principal version of the circulating hormone while the sulfated eight amino acid form has been used almost exclusively in physiological and behavioural studies (Reeve et al. 2003)). Our results with higher doses (1 nmol i.v.) of CCK8 versus CCK58 indicate that both forms produce comparable increases in sensitivity of the accommodation reflex as measured by responses of DMN neurones. However, at lower doses (100–160 pmol; i.v.) of CCK8 or CCK58, it was clear that the eight amino acid form was the more potent. This difference is probably due to the reduced affinity that CCK58 has for the CCK receptor compared with CCK8 (Wu et al. 2008)). At higher doses, the difference in potency between the two isoforms would not be apparent as the receptors would probably be saturated in either case.
There is wide acceptance of the concept that CCK released from the duodenum regulates autonomic and behavioural functions through action on vagal afferents (Moran & Schwartz, 1994)). The site of action within the vagal afferent trunk is a subject for debate. Vagal afferent fibres in the abdomen (Moran & Schwartz, 1994)), the cell bodies of origin of the afferent vagus (Peters et al. 2004, 2006) and terminal endings of the vagus within the brainstem (Rogers & Hermann, 2008)) are all sensitive to CCK at concentrations from 10 to 1000 pm. While there is evidence that CCK can evoke changes in feeding behaviour through direct action within the CNS (Reidelberger et al. 1994)), arithmetic considerations argue strongly for peripherally released CCK action on vagal afferents within the coeliac–portal circulation (Calingasan et al. 1992; Berthoud & Patterson, 1996; Cox & Randich, 1997; Okano-Matsumoto et al. 2011)). The general plasma concentration of CCK stimulated by nutrients or HCl applied to the lumen of the duodenum is in the range of 5–10 pm, a concentration below the minimum sensitivity of central vagal afferents for CCK (Rogers & Hermann, 2008)). However, the CCK concentrations in the portal circulation are nearly ten times higher and well within the demonstrated concentration versus activation curves for vagal afferents (Cantor & Rehfeld, 1989; Reidelberger et al. 1989)). So, CCK released by the gut as a function of normal digestion only reaches concentrations effective for activation of vagal afferents in the proximal gut itself. Our present results add support to this view in that gastric-DMN neuronal activity could be reliably suppressed by very small (30 pmol) doses of CCK applied to the near-coeliac arterial circulation (as in Calingasan et al. 1992)) but not the general intravenous circulation. These results are reinforced by our demonstration that 4V application of lorglumide (at doses previously shown to block CNS action of high doses of CCK) had no effect on the ability of systemic CCK to inhibit gastric-DMN neurones. Conversely, i.v. administration of lorglumide (at doses previously shown to be effective in blocking systemic effects of CCK to invoke satiety) completely blocked i.v. CCK inhibition of DMN activity.
This is not to say that there are no physiologically relevant intra-CNS CCK receptor populations. CCK is an abundant transmitter peptide in numerous CNS pathways (Kiss et al. 1984; King & Bishop, 1990)). Synaptic concentrations of the peptide could be expected to be high, relative to the circulation.
Summary
We find that neurones in the DMN are extremely sensitive reporters of changes in gastric volume. Most gastric-DMN neurones demonstrate a noticeable reduction of spontaneous firing rate in response to antral distensions as small as 0.1 ml. This study shows that the nutritional status of the animal plays a modest role in the regulation of this reflex; that is, fasted rats show a small but significant increase in reflex sensitivity over their recently fed counterparts.
Additionally, we observed that intravenously delivered CCK produces significant increases in the sensitivity of gastric-DMN neurones to vago-vagal reflex responses, especially at pharmacological doses. High doses (1 nmol, i.v.) of either CCK8 or 58 comparably sensitize gastric-DMN neurones to the effects of antral distension. Such high doses apparently override the more subtle effects of metabolic status (i.e. fed versus fasted) to alter reflex sensitivity. However, regardless of whether we used the high dose (1 nmol; refer to Fig. 3)) or the more physiologically relevant dose (160 pmol; refer to Fig. 5)) of CCK intravenously, the gastric accommodation reflex of the fasted animal did not decrease in magnitude so as to resemble the reflex elicited in the ‘fed’ animals nor did it reverse in direction (i.e. an excitation as opposed to an inhibition in spontaneous firing rate of gastric-DMN neurones). Perhaps unpredictable effects of high CNS doses of CCK could be responsible for the reversal of gastric vago-vagal reflexes described previously (Holmes et al. 2009)).
Systemic administration of exogenous CCK at doses comparable to those released after meals still produce a sensitization of reflex effects, though these effects are modulated by the animals’ metabolic status. Note that physiological doses of CCK sensitize the gastric accommodation reflex in fasted but not fed animals. While the specific basis for this effect is not clear, hormones released during periods of nutrient excess (e.g. PP, PYY, insulin, leptin) versus surfeit (e.g. ghrelin, cortisol, glucagon) may have a significant effect on presynaptic vagal afferent receptor populations that regulate the first order visceral afferent synapse in the NST (Dockray & Burdyga, 2011)). Alternatively, the activity of CNS pathways involved in eliciting the cephalic phase changes in digestive functions through actions at the NST or DMN could bias vago-vagal reflex responses to distension or CCK.
Finally, while it is clear that the vagal afferent fibre is sensitive to CCK literally from the periphery (Schwartz & Moran, 1994)) to cell body of origin (Peters et al. 2004, 2006) and to synaptic terminal (Rogers & Hermann, 2008)), it is highly likely, on arithmetic grounds alone, that the main effect of physiological release of CCK to affect vagal afferents is mediated in the periphery. CCK concentrations typically found in the general circulation after meals are less than the minimal sensitivity of vagal afferent processes within the CNS (Rogers & Hermann, 2008)). However, Calingasan and colleagues (Calingasan et al. 1992)) found that the delivery of physiologically relevant quantities of CCK to the coeliac–portal circulation of the proximal gut produced significant reductions in feeding behaviour that were not seen following general i.v. delivery of the same doses. The present work shows that the same is true for vago-vagal reflex sensitivity to CCK. That is, delivery of very small quantities of CCK directly to the proximal gut via a near-coeliac i.a. route inhibits DMN neurones responsible for maintaining gastric tone while the same doses of CCK applied i.v. were less effective. These results were supported by findings that i.v. lorglumide completely suppressed DMN neurone inhibition by CCK, while the application of much higher concentrations of lorglumide to the 4V had no effect to block the peripheral effects of CCK delivery.
Taken together, the results of this study support the view that the amplification of gastric vago-vagal reflexes by CCK is potent, linear, dose dependent and mediated through vagal afferent receptors located in the proximal gut.
Acknowledgments
This work was supported by the National Institutes of Health Grants DK56373 and NS55866. The authors thank Dr Paula Geiselman for advice on statistical analysis.
Glossary
Abbreviations
- CCK
cholecystokinin
- DMN
dorsal motor nucleus of the vagus
- FR
firing rate
- IHC
immunohistochemical
- NST
nucleus of the solitary tract
- 4V
fourth ventricle
Author contributions
All the experiments were carried out at the Laboratory of Autonomic Neurosciences, Pennington Biomedical Research Centre. All three authors contributed to (1) the conception and design of the experiments, (2) collection, analysis and interpretation of data, and (3) drafting the article or revising it critically for important intellectual content. All authors approved the final version of the manuscript.
References
- Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol. 2005;94:2763–2771. doi: 10.1152/jn.00351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996;156:123–131. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- Buelke-Sam J, Holson JF, Bazare JJ, Young JF. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci. 1978;28:157–162. [PubMed] [Google Scholar]
- Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci. 2008;28:11583–11592. doi: 10.1523/JNEUROSCI.2493-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SRy. Histology of the Nervous System. Vol. 1. New York: Oxford University Press; 1995. [Google Scholar]
- Calingasan N, Ritter S, Ritter R, Brenner L. Low-dose near-celiac arterial cholecystokinin suppresses food intake in rats. Am J Physiol Regul Integr Comp Physiol. 1992;263:R572–R577. doi: 10.1152/ajpregu.1992.263.3.R572. [DOI] [PubMed] [Google Scholar]
- Cantor P, Rehfeld JF. Cholecystokinin in pig plasma: release of components devoid of a bioactive COOH-terminus. Am J Physiol Gastrointest Liver Physiol. 1989;256:G53–G61. doi: 10.1152/ajpgi.1989.256.1.G53. [DOI] [PubMed] [Google Scholar]
- Chen CH, Rogers RC. Peptide YY and the Y2 agonist PYY-(13-36) inhibit neurons of the dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol. 1997;273:R213–R218. doi: 10.1152/ajpregu.1997.273.1.R213. [DOI] [PubMed] [Google Scholar]
- Chowdhury P, Rayford PL. Effect of food restriction on plasma cholecystokinin levels and exocrine pancreatic function in rats. Ann Clin Lab Sci. 2001;31:376–382. [PubMed] [Google Scholar]
- Cooper MS, Reeve JR, Jr, Raboin SJ, Abdalla MO, Green GM, Sayegh AI. Cholecystokinin-58 and cholecystokinin-8 produce similar but not identical activations of myenteric plexus and dorsal vagal complex. Regul Pept. 2008;148:88–94. doi: 10.1016/j.regpep.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Corp ES, McQuade J, Moran TH, Smith GP. Characterization of type A and type B CCK receptor binding sites in rat vagus nerve. Brain Res. 1993;623:161–166. doi: 10.1016/0006-8993(93)90024-h. [DOI] [PubMed] [Google Scholar]
- Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1390–R1396. doi: 10.1152/ajpregu.1998.274.5.R1390. [DOI] [PubMed] [Google Scholar]
- Cox JE, Randich A. CCK-8 activates hepatic vagal C-fiber afferents. Brain Res. 1997;776:189–194. doi: 10.1016/s0006-8993(97)01036-6. [DOI] [PubMed] [Google Scholar]
- Curro D, Ipavec V, Preziosi P. Neurotransmitters of the non-adrenergic non-cholinergic relaxation of proximal stomach. Eur Rev Med Pharmacol Sci. 2008;12(Suppl. 1):53–62. [PubMed] [Google Scholar]
- Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201:313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- Edwards GL, Ladenheim EE, Ritter RC. Dorsomedial hindbrain participation in cholecystokinin-induced satiety. Am J Physiol Regul Integr Comp Physiol. 1986;251:R971–R977. doi: 10.1152/ajpregu.1986.251.5.R971. [DOI] [PubMed] [Google Scholar]
- Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1545–R1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- Fogel R, Zhang X, Renehan WE. Relationships between the morphology and function of gastric and intestinal distention-sensitive neurons in the dorsal motor nucleus of the vagus. J Comp Neurol. 1996;364:78–91. doi: 10.1002/(SICI)1096-9861(19960101)364:1<78::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985;341:269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- Gillis RA, Quest JA, Pagani FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. In: Wood JD, editor. Handbook of Physiology, The Gastrointestinal System, Motility and Circulation. Bethesda, MD, USA: American Physiological Society; 1989. pp. 621–683. [Google Scholar]
- Holmes GM, Tong M, Travagli RA. Effects of brain stem cholecystokinin-8s on gastric tone and esophageal-gastric reflex. Am J Physiol Gastrointest Liver Physiol. 2009;296:G621–631. doi: 10.1152/ajpgi.90567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson G. Vago-vagal reflex relaxation of the stomach in the cat. Acta Physiol Scand. 1969;75:245–252. doi: 10.1111/j.1748-1716.1969.tb04376.x. [DOI] [PubMed] [Google Scholar]
- King JS, Bishop GA. Distribution and brainstem origin of cholecystokinin-like immunoreactivity in the opossum cerebellum. J Comp Neurol. 1990;298:373–384. doi: 10.1002/cne.902980309. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Williams TH, Palkovits M. Distribution and projections of cholecystokinin-immunoreactive neurons in the hypothalamic paraventricular nucleus of rat. J Comp Neurol. 1984;227:173–181. doi: 10.1002/cne.902270204. [DOI] [PubMed] [Google Scholar]
- Kraly FS. Vagotomy does not alter cholecystokinin's inhibition of sham feeding. Am J Physiol Regul Integr Comp Physiol. 1984;246:R829–R831. doi: 10.1152/ajpregu.1984.246.5.R829. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Hermann GE, Rogers RC. Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J Auton Nerv Syst. 1989;26:107–112. doi: 10.1016/0165-1838(89)90158-6. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol. 1992;453:401–411. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Barrachina MD, Ohning G, Tache Y. Cephalic phase of acid secretion involves activation of medullary TRH receptor subtype 1 in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1310–G1319. doi: 10.1152/ajpgi.00222.2002. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Tache Y. Central TRH receptor 1 antisense blocks cold-induced gastric emptying but not brain c-Fos induction. Peptides. 2001;22:81–90. doi: 10.1016/s0196-9781(00)00359-4. [DOI] [PubMed] [Google Scholar]
- Moran TH, Kornbluh R, Moore K, Schwartz GJ. Cholecystokinin inhibits gastric emptying and contracts the pyloric sphincter in rats by interacting with low affinity CCK receptor sites. Regul Pept. 1994;52:165–172. doi: 10.1016/0167-0115(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Moran TH, Schwartz GJ. Neurobiology of cholecystokinin. Crit Rev Neurobiol. 1994;9:1–28. [PubMed] [Google Scholar]
- Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 1988;273:207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- Okano-Matsumoto S, McRoberts JA, Tache Y, Adelson DW. Electrophysiological evidence for distinct vagal pathways mediating CCK-evoked motor effects in the proximal versus distal stomach. J Physiol. 2011;589:371–393. doi: 10.1113/jphysiol.2010.196832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Karpiel AB, Ritter RC, Simasko SM. Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology. 2004;145:3652–3657. doi: 10.1210/en.2004-0221. [DOI] [PubMed] [Google Scholar]
- Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1544–R1549. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- Prove J, Ehrlein HJ. Motor function of gastric antrum and pylorus for evacuation of low and high viscosity meals in dogs. Gut. 1982;23:150–156. doi: 10.1136/gut.23.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Gayton RJ, Dockray GJ. CNS effects of circulating CCK8: involvement of brainstem neurones responding to gastric distension. Brain Res. 1985;342:187–190. doi: 10.1016/0006-8993(85)91373-3. [DOI] [PubMed] [Google Scholar]
- Reeve JR, Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol. 2003;285:G255–G265. doi: 10.1152/ajpgi.00523.2002. [DOI] [PubMed] [Google Scholar]
- Reeve JR, Jr, Wu SV, Keire DA, Faull K, Chew P, Solomon TE, Green GM, Coskun T. Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. Am J Physiol Gastrointest Liver Physiol. 2004;286:G395–G402. doi: 10.1152/ajpgi.00020.2003. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Kalogeris TJ, Solomon TE. Plasma CCK levels after food intake and infusion of CCK analogues that inhibit feeding in dogs. Am J Physiol Regul Integr Comp Physiol. 1989;256:R1148–R1154. doi: 10.1152/ajpregu.1989.256.5.R1148. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Varga G, Liehr RM, Castellanos DA, Rosenquist GL, Wong HC, Walsh JH. Cholecystokinin suppresses food intake by a nonendocrine mechanism in rats. Am J Physiol Regul Integr Comp Physiol. 1994;267:R901–R908. doi: 10.1152/ajpregu.1994.267.4.R901. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides. 2008;29:1716–1725. doi: 10.1016/j.peptides.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE, Ebly EM. An inexpensive multichannel infusion/withdrawal pump system. Brain Res Bull. 1982;8:543–546. doi: 10.1016/0361-9230(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE, Travagli RA. Brainstem control of gastric function. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 4th edn. Elsevier Academic Press; 2005. pp. 851–875. [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor DM, Verberne AJ. Gastric leptin: a novel role in cardiovascular regulation. Am J Physiol Heart Circ Physiol. 2010;298:H406–H414. doi: 10.1152/ajpheart.00997.2009. [DOI] [PubMed] [Google Scholar]
- Schemann M, Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol Gastrointest Liver Physiol. 1992;263:G709–G718. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am J Physiol Regul Integr Comp Physiol. 1993;265:R872–R876. doi: 10.1152/ajpregu.1993.265.4.R872. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH. CCK elicits and modulates vagal afferent activity arising from gastric and duodenal sites. Ann N Y Acad Sci. 1994;713:121–128. doi: 10.1111/j.1749-6632.1994.tb44058.x. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Tougas G, Moran TH. Integration of vagal afferent responses to duodenal loads and exogenous CCK in rats. Peptides. 1995;16:707–711. doi: 10.1016/0196-9781(95)00033-g. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Musings on the wanderer: what's new in our understanding of vago-vagal reflexes? III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G180–G187. doi: 10.1152/ajpgi.00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard E, Zheng Z, Wan S, Travagli RA. Vagally mediated, nonparacrine effects of cholecystokinin-8s on rat pancreatic exocrine secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G493–G500. doi: 10.1152/ajpgi.00118.2007. [DOI] [PubMed] [Google Scholar]
- Wu SV, Harikumar KG, Burgess RJ, Reeve JR, Jr, Miller LJ. Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G641–G647. doi: 10.1152/ajpgi.90390.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


