Abstract
Local relapse of tumors after radiation therapy remains a challenge in oncology. To devise rational approaches for preventing this relapse, we have to improve our understanding of how new vessels form in previously irradiated tumors. We propose that tumor regrowth after local irradiation is dependent on blood vessel formation by local endothelial cells without the need for recruitment of endothelial precursor cells from distant nonirradiated tissues or bone marrow. We also suggest that infiltrating myeloid bone marrow–derived cells promote survival of local endothelial cells during the early period after irradiation and angiogenesis during the later stage of tumor regrowth, both via paracrine mechanisms.
Radiation therapy is a widely used method of killing proliferating tumor cells to achieve locoregional control of cancer. Unfortunately, initial tumor response is often followed by relapse. For decades, most radiobiological studies have been focused on the radiosensitivity of cancer cells. However, it is becoming increasingly clear that multiple changes in tumor stroma—including but not limited to hypoxia—may also determine treatment outcome. Moreover, although radiotherapy is a localized treatment, tumor regrowth may be substantially affected by systemic factors.
In particular, ionizing radiation intensifies the recruitment of “distal” stroma, in the form of inflammatory bone marrow–derived cells (BMDCs), to the tumor and its surroundings. As shown recently, these BMDCs of myeloid lineage may facilitate tumor relapse post-radiation (1–4). After a large single dose of local irradiation in preclinical tumor models in mice, two waves of myeloid BMDCs arrive at the treatment site. The first occurs 3–5 days post-radiation (1,4) and is followed by a delayed influx of myeloid BMDCs after about 2 weeks (3). The initial wave is likely mediated by increased expression of “stress-response” molecules in tumors in direct response to radiation (vascular endothelial growth factor, stromal cell–derived factor 1 alpha, endothelial adhesion molecules), and the second is associated with tissue damage leading to hypoxia and activation of hypoxia-responsive genes (hypoxia inducible factor 1 alpha, stromal cell–derived factor 1 alpha, and vascular endothelial growth factor) (3–7). The tumor-protective effect of the recruited myeloid BMDCs has been attributed to their ability to support tumor vasculature after irradiation in a paracrine manner—analogous to that described for radiation-naive tumors (8–10). However, the contributions of specific provascular myeloid BMDC populations, including M2-type macrophages, Tie2+ monocytes and Gr-1+ monocytes, or neutrophils, and the signals that mediate their recruitment and local function are not well characterized.
Moreover, the status of tumor vasculature at certain post-radiation stages remains largely unclear. In general, the tolerance of tumor vessels to irradiation may play a dual role in radiotherapy. A stable vasculature is required to maintain the blood perfusion necessary for cancer cell (re)oxygenation, and therefore radiosensitization, throughout the course of treatment, which is usually fractionated in the clinic. However, functional vessels and viable endothelial cells (ECs) are undesirable after any type of tumor irradiation because they might support the survival and growth of remaining cancer cell clonogens and thus promote the relapse. Despite the intense research on tumor oxygenation during irradiation, little is known about the dynamics of vascular changes and the mechanisms of neovascularization after radiation.
This commentary focuses on revascularization of tumors after non-curative radiotherapy, which is arguably the least understood contributor to tumor regrowth post-radiation. In particular, we address the unanswered question regarding the source of neovessels in relapsing tumors. Many tumor-associated ECs are undoubtedly killed or at least deprived of proliferative capacity by high-dose irradiation. But are those ECs that survive still capable of reestablishing a tumor vasculature during post-radiation recurrence? Or do other players (eg, recruited BMDCs) become more important? To our knowledge, there is no consensus in the literature on this point.
Some researchers proposed that local tumor irradiation at a single dose of 15–20 Gy or higher can “sterilize” sufficient ECs inside and around the tumor to abrogate the growth and sprouting of irradiated vessels (angiogenesis), thereby forcing the tumor to rely on vasculogenesis by infiltrating cells (3,11,12). Indeed, because bone marrow–resident cells or circulating BMDCs receive very little exposure to radiation during tumor treatment, it is reasonable to expect that the recruitment and further incorporation of these cells in tumor vessels would be much more substantial post-radiation vs radiation-naive tumors. If supported by compelling data, this attractive hypothesis would also reconcile an unresolved yet controversial issue in the literature about whether gross tumor response to radiation is associated with vascular damage (13–18). Therefore, the influx of bone marrow–derived vascular precursors to tumors during the regrowth stage post-radiation, when additional cells are necessary for building new vessels, has recently been investigated (3,4). However, neither study could detect any substantial incorporation of BMDCs within the vessels of tumors regrowing post-radiation (3,4). Thus, BMDC-based vasculogenesis is not a major contributor to new vessel formation in irradiated tumors (19). An obvious alternative mechanism is post-radiation angiogenesis, provided local ECs survive and remain functional (14,17).
Is there any proof in the literature to support this mechanism? To address this question, we summarize below and in Table 1, the previous preclinical observations, and propose a model reconstructing the dynamics of events in tumor-associated ECs and vasculature in general after a single high dose (approximately 20 Gy) of local gamma- or x-ray irradiation. Such non-curative treatments have been widely used in animal studies and allow a better separation and understanding of post-radiation effects than fractionated irradiation regimens. This analysis is particularly timely because therapies using a single dose or a few large fractions of radiation are being increasingly tested in the clinic (eg, stereotactic body/ablative radiotherapy or radiosurgery) (15,42–44). Although no specific data exist, it has been postulated that such irradiation induces more damage to tumor vasculature than conventional fractionated radiotherapy, which provides a crucial therapeutic benefit (15). The available data are fairly incomplete and inconsistent. Most studies have focused on early vascular effects, and the late post-radiation stages have been less explored. Moreover, the older studies lacked a specific marker for EC staining, and the more recent reports rarely evaluated ECs and vessel structure and function simultaneously. Clearly, there is no experimental consensus on the question of revascularization after radiation, and a logical conceptual framework would help direct future work in this area.
Table 1.
Effects of single-dose local irradiation (12–50 Gy) on endothelial cells (ECs)/vessels and blood perfusion in tumors implanted in rodents, at the four different post-radiation stages
| Tumor type/cell line | Preclinical model | Implantation site* | Radiation dose, Gy† | Measured vascular parameter(s) | Changes in vascular parameters vs control‡ | Reference(s) |
| Stage I: initial tumor response | ||||||
| Walker 256 carcinoma | Rat | Flank, s.c. | 20–30 | Blood flow | ↑ | (20,21) |
| Vascular volume | ↓ | |||||
| KHT sarcoma | Mouse | Flank, i.d. | 20–40 | Blood flow | NS | (22) |
| Neuroblastoma | Mouse | Flank, s.c. | 20 | Vascular volume | ↓ | (23) |
| “Vascular stroma” | ↑ | |||||
| C3H/Bi mammary carcinoma | Mouse | Hind leg, i.m. | 15–45 | Vascular volume | NS | (24,25) |
| Vessel length | ↑↑ | |||||
| Human melanoma E.E. | Mouse | Flank, s.c. | 15 | Vessel length/surface/volume | NS | (26) |
| 36B-10 glioma | Rat | i.c., cerebrum | 20 | Blood flow | ↑↑ | (27) |
| RIF-1 fibrosarcoma | Mouse | s.c. | 20 | Blood flow | ↑↑ | (28) |
| Human LoVo colon adenocarcinoma | Mouse | Flank, s.c. | 12–16 | Vascular volume | ↓↓, then NS | (29) |
| LBDS1 fibrosarcoma | Rat | Fank, s.c. | 20 | Blood flow | NS | (30) |
| B16F1 melanoma and MCA/129 fibrosarcoma | Mouse | Hind limb, s.c. | 15–20 | Apoptosis of ECs | ↓↓ of non-apoptotic ECs | (16,31) |
| C3H mammary carcinoma | Mouse | Foot, s.c. | 20 | Functional vasculature | NS | (32) |
| C38 colon adenocarcinoma | Mouse | Hind leg, s.c. | 20 | Functional vasculature | NS | (33) |
| Human A431 epidermoid carcinoma | Mouse | Hind leg, i.m. | 15 | Vessel density/area | NS | (34) |
| Human NCI-H441 lung adenocarcinoma | Mouse | Hind leg, s.c. | 14 | Vessel density | NS | (35) |
| TRAMP-C1 prostate adenocarcinoma | Mouse | Thigh, i.m. | 25 | Relative EC pixels | ↓ | (36) |
| Human A549 lung adenocarcinoma | Mouse | Hind leg, s.c. | 25 | Vessel density | NS | (37) |
| Perfusion | ↑ | |||||
| Human U251 glioblastoma multiforme | Mouse | i.c. | 15 | Relative EC pixels | ↓ | (3) |
| Relative perfusion pixels | NS | |||||
| Human 54A lung tumor | Mouse | Hind limb, s.c. | 21 | Relative EC area | NS | (4) |
| Stage II: Tumor regression phase | ||||||
| Walker 256 carcinoma | Rat | Flank, s.c. | 30 | Vascular volume | ↓↓ | (20) |
| KHT sarcoma | Mouse | Flank, i.d. | 20–40 | Blood flow | ↑ | (22) |
| Neuroblastoma | Mouse | Flank, s.c. | 20 | Vascular volume | ↓↓ | (23) |
| “Vascular stroma” | ↑ | |||||
| C3H/Bi mammary carcinoma | Mouse | Hind leg, i.m. | 30–45 | Vascular volume | ↓ | (24,25) |
| Vessel length | ↑↑↑ | |||||
| Human melanoma E.E. | Mouse | Flank, s.c. | 15–25 | Vessel length/surface/volume | ↓ in microvessels, or NS | (26) |
| LBDS1 fibrosarcoma | Rat | Flank, s.c. | 20 | Blood flow | ↑↑ in tumor center | (30) |
| GL261 glioma | Mouse | Hindlimb, s.c. | 25 | Vessel density | ↓ | (38) |
| TRAMP-C1 prostate adenocarcinoma | Mouse | Thigh, i.m. | 25 | Relative EC pixels | ↓↓ | (36) |
| Human U251 glioblastoma multiforme | Mouse | i.c. | 15 | Relative EC pixels | ↓↓↓ | (3) |
| Relative perfusion pixels | ↓↓ | |||||
| Stage III: Early tumor recurrence | ||||||
| Human melanoma E.E. | Mouse | Flank, s.c. | 15 | Vasculometry | NS | (26) |
| Stage IV: Late tumor recurrence | ||||||
| Walker 256 carcinoma | Rat | Flank, s.c. | 30 | Vascular volume | ↓ (partial recovery) | (20) |
| C3H/Bi mammary carcinoma | Mouse | Hind leg, i.m. | 15–45 | Vascular volume | NS | (24,25) |
| Vessel length | NS | |||||
| Human melanoma E.E. | Mouse | Flank, s.c. | 15 | Vessel length/surface/volume | ↑ in microvessels | (26) |
| LBDS1 fibrosarcoma | Rat | Flank, s.c. | 20 | Blood flow | NS | (30) |
| K1735 melanoma | Mouse | Hind limb, s.c. | 12 | Vessel density | NS, then ↓ | (39) |
| Human 54A lung tumor | Mouse | Hind limb, s.c. | 20 | Perfused vessel density | ↓ | (40) |
| Human NCI-H441 lung adenocarcinoma | Mouse | Hind limb, s.c. | 14 | Vessel density | ↓ | (35) |
| TRAMP-C1 prostate adenocarcinoma | Mouse | Thigh, i.m. | 25 | Relative EC pixels | ↓↓ | (36) |
| NR-S1 squamous cell carcinoma | Mouse | Hind leg, s.c. | 50 | Vessel density | ↑↑ | (41) |
| Human U251 glioblastoma multiforme | Mouse | i.c. | 15 | Relative EC pixels | ↓↓ (partial recovery) | (3) |
| Relative perfusion pixels | NS | |||||
| MCa8 mammary carcinoma | Mouse | Hind limb, s.c. | 20 | Vessel density | NS | (4) |
i.c. = intracranially; i.d. = intradermally; i.m. = intramuscularly; s.c = subcutaneously.
This table summarizes the effects of treatment seen after single doses of radiation within the indicated range (data from experiments using doses outside this range or dose fractionation from these studies are not included). For simplicity, doses in Roentgens (R) in older studies are translated here in Gy (100 R is approximately equal to 1 Gy).
↓ indicates a decrease and ↑ indicates an increase in measured vascular parameters; the number of arrows is a reflection of the strength of the effect. NS indicates a non–statistically significant effect.
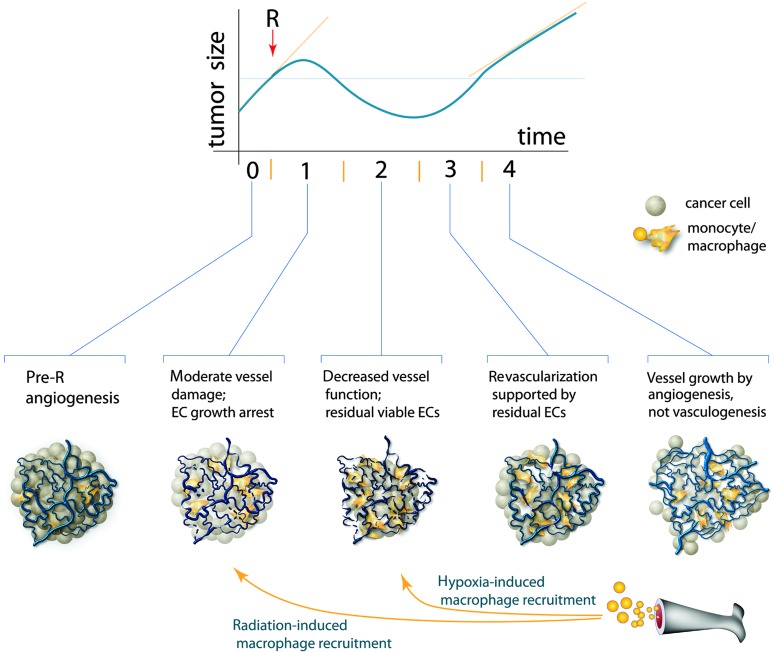
We propose such a framework, in which the tumor vascular response to irradiation occurs in four stages, which approximately corresponds to the phases of tumor size changes (Figure 1).
Figure 1.
Proposed model of tumor growth and corresponding changes in endothelial cell/vessel status after single-dose local irradiation (R). After irradiation (approximately 20 Gy), tumors normally continue to enlarge for a few days, then shrink (depicted) or become stable in size (not shown), and then they start to regrow. The time scale varies for different tumors, but the whole span is typically a few weeks. We have divided the post-radiation events into four different stages (I–IV), including two waves of the vessel-rescuing recruitment of myelomonocytes. Note that angiogenesis is required in stage IV only. EC = endothelial cell.
Stage I: Initial Tumor Response
One group of researchers proposed that massive tumor EC apoptosis (within a few hours after irradiation) is the major determinant of therapeutic outcome (16,31), but this effect has not been independently reproduced. In contrast, multiple other reports summarized in Table 1 have shown absent or modest changes in vessel structure and function during the first few days after irradiation. In addition, EC proliferation, which is associated with angiogenesis in growing tumors, is likely arrested by radiation in a p53-dependent manner (45). As shown both in preclinical experiments (34) and in samples from rectal carcinoma patients [after five daily fractions of 5 Gy each] (46), this growth arrest may be preferential in endothelial vs tumor cells and potentially enhances the DNA repair and survival of the irradiated ECs. Therefore, the modest post-radiation decrease of vascular density may be simply explained by the lack of new vessel formation in a tumor mass that continues to increase in volume for a few days post-radiation (36), rather than by pruning of existing vessels. However, the impact of vessel “stasis” vs pruning post-radiation remains unknown. Nevertheless, as the majority of tumor vessels remain perfused with blood, they provide a conduit for the rapid recruitment of myeloid BMDCs post-radiation (1,4). These cells become an extra source of pro-survival factors for ECs, which could become more critical as the irradiated tumor cells gradually disappear.
Stage II: Tumor Regression Phase
The next stage of vascular changes post-radiation, before overt tumor recurrence, is less understood (Table 1). During this phase, tumor size either decreases (Figure 1) or remains stable, depending on tumor radiosensitivity. The death and rapid elimination of cancer cells may even result in an increased ratio of vessels to cancer cells in regressing tumors (23–26). Once again, the moderate drop of vessel/EC density in tumors not shrunk after radiation treatment (36,38) may actually indicate a minor change in absolute numbers of ECs per tumor. However, it remains unknown what fractions of irradiated tumor-associated ECs have been killed and lysed, and how many of the surviving ECs become permanently cell cycle–arrested (likely because of cell senescence) or retain proliferation potential at this stage. In addition, the mere presence of residual ECs does not guarantee integrity of the vasculature or sufficient blood perfusion. The progressive decrease of perfusion may lead to hypoxia and, in turn, to a second wave of myeloid BMDC recruitment (3).
Stage III: Early Tumor Recurrence
As shown in Table 1, there is virtually no evidence concerning the status of the ECs and tumor vasculature at this stage post-radiation. However, there are two sources of indirect information. First, the existing data on residual ECs and vessels during stage II post-radiation, and second, the intriguing observations that the regrowth of certain radiosensitive tumors may be accelerated post-radiation (47,48). It is therefore conceivable that ECs and vessel fragments that survived stages I and II may be sufficient to quickly reestablish a functional vasculature to support rapid tumor regrowth. It remains unclear if EC proliferation is necessary at this stage. Together with residual tumor and host-derived cells, the myeloid BMDCs recruited previously likely play a supporting role, by supplying growth factors necessary for the reformation and maintenance of functional vasculature.
Stage IV: Late Tumor Recurrence
After a recurring tumor reaches a size close to that at the onset of irradiation, its growth rate typically decreases. This well-known phenomenon is referred to as the “tumor bed effect” and is attributed to defects in neovascularization (49). However, no single vascular parameter from previous reports in various tumors seems to unequivocally indicate this deficiency (Table 1). At the same time, it is clear that new vessel formation at this stage becomes necessary for further tumor growth. Because BMDCs have not been detected in the vessels of relapsed tumors (3,4), we propose that the dominant mechanism at this stage is angiogenesis (ie, proliferation and sprouting of ECs that have survived irradiation). Although EC proliferation may even increase during this phase (39), it is conceivable that angiogenesis may be less efficient than that in radiation-naive tumors because of the post-mitotic death of some of the irradiated ECs (14). Another local source of angiogenesis at this stage could be ECs recruited from preexisting vessels from nonirradiated adjacent tissue (47, 50), but the impact of this mechanism has not been thoroughly investigated. Irrespective of the source of ECs for angiogenesis, certain myeloid BMDCs (eg, Tie2+ monocytes) may support vessel formation in a paracrine manner in this phase (3,4).
Perspectives
This theoretical model of vascular dynamics in irradiated tumors could and should be validated using modern technologies. Moreover, additional aspects also merit further investigation. For example, other stromal cells might influence tumor neovascularization post-radiation (eg, pericytes, tumor-associated fibroblasts, or lymphocytes), but their function in irradiated tumors remains poorly understood. In addition, tumor vasculature is fairly heterogeneous, with distinct patterns and growth rates between peripheral and central areas (51–54). Therefore, one could expect a substantial zonal variability of tumor vascular responses to radiation. For example, if EC proliferation occurs preferentially at the periphery of tumors, the vessels in these areas might be more vulnerable to radiation. However, the post-radiation recurrence of tumors often occurs at a tumor edge (55,56). Acute hypoxia related to intermittent perfusion (57) or the increased pericyte coverage of these vessels (58) may help explain this paradox. Also, particle irradiations, which have now entered a broader clinical use, might have peculiar effects on tumor vessels (59,60). Finally, almost all experiments on vascular response to radiation published to date have been performed using tumors implanted ectopically (subcutaneously or intramuscularly) in rodents. However, increasing evidence suggests that a valid organ-specific tumor microenvironment is essential for appropriately modeling tumor growth and angiogenesis in mice (61,62). Thus, all the mechanisms discussed above need to be studied in orthotopically implanted or autochthonous (spontaneous and carcinogen-induced) tumors in animals and whenever possible, in patients.
Several recent discoveries in tumor vascular biology may also influence the multistage model of post-radiation neovascularization proposed here. For example, it has been recently demonstrated that a fraction of ECs in growing tumors may be derived via trans-differentiation from cancer cells (63) or from cancer stem-like cells (eg, in glioblastomas) (64–67). As stem-like cells may be more radioresistant than other neoplastic cells (68,69), it is conceivable that cancer stem cell–derived ECs are more resistant to radiation. In this larger context, the term “vasculogenesis” might be invoked to describe neovascularization in irradiated tumors from tumor stem cells. Vasculogenesis may also occur from endothelial precursor cells of non-bone marrow origin that are recruited to tumors from nonirradiated adjacent tissues and/or circulation (70,71). It is also conceivable that tumor neovascularization via other local mechanisms—intussusception or vascular co-option—could differentially occur post-radiation. However, the evidence for any of these phenomena post-radiation in tumors is currently lacking.
In summary, there is an urgent need to better understand the cellular mechanisms of neovascularization in irradiated tumors. Experimental models should be built to be more relevant to human tumor biology and treatment, and clinical studies should be designed to answer these questions. Only when the source of ECs and the dynamics of post-radiation vessel formation are better understood, will we be able to identify the molecular pathways coordinating the process to eventually target this critical component of relapse.
Funding
US National Institutes of Health (P01-CA080124, R01-CA115767, R01-CA085140, R01-CA126642, and T32-CA073479 to RKJ; R01-CA159258 to DGD); Federal Share/National Cancer Institute Proton Beam Program Income grants to RKJ, DGD, and LLM; Department of Defense Award (W81XWH-10 – 1-0016 to RKJ); American Cancer Society grant (120733-RSG-11-073-01-TBG to DGD); and a National Foundation for Cancer Research grant (to RKJ).
Footnotes
The funders had no role in the writing of the commentary or the decision to submit the commentary for publication. Dr. R. K. Jain is a consultant for Noxxon Pharma, AG; has received grants and has pending contracts with Roche and MedImmune; and is a member of the board and holds stock for Xtuit Pharmaceuticals. There is no significant financial or other competing interest to the commentary.
References
- 1.Li F, Sonveaux P, Rabbani ZN, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. . 2007;26(1):63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. . 2008;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. . 2010;120(3):694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozin SV, Kamoun W, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor re-growth after local irradiation. Cancer Res. . 2010;70(14):5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. . 1999;59(14):3374–3378. [PubMed] [Google Scholar]
- 6.Hallahan DE, Chen AY, Teng M, Cmelak AJ. Drug-radiation interactions in tumor blood vessels. Oncology (Williston Park). . 1999;13(10) suppl 5:71–77. [PubMed] [Google Scholar]
- 7.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. . 2011;17(8):2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. . 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 9.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. . 2008;8(8):618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 10.Loges S, Schmidt T, Carmeliet P. “Antimyeloangiogenic” therapy for cancer by inhibiting PlGF. Clin Cancer Res. . 2009;15(11):3648–3653. doi: 10.1158/1078-0432.CCR-08-2276. [DOI] [PubMed] [Google Scholar]
- 11.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. . 2011;11(4):239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 12.Liu SK, Bham SA, Fokas E, et al. Delta-like ligand 4-notch blockade and tumor radiation response. J Natl Cancer Inst. . 2011;103(23):1778–1798. doi: 10.1093/jnci/djr419. [DOI] [PubMed] [Google Scholar]
- 13.Budach W, Taghian A, Freeman J, Gioioso D, Suit HD. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst. . 1993;85(12):988–993. doi: 10.1093/jnci/85.12.988. [DOI] [PubMed] [Google Scholar]
- 14.Denekamp J. Limited role of vasculature-mediated injury in tumor response to radiotherapy. J Natl Cancer Inst. . 1993;85(12):935–937. doi: 10.1093/jnci/85.12.935. [DOI] [PubMed] [Google Scholar]
- 15.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. . 2005;8(2):89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. . 2003;300(5622):1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield JP, Cobb WS, Lyden D. Resisting arrest: a switch from angiogenesis to vasculogenesis in recurrent malignant gliomas. J Clin Invest. . 2010;120(3):663–667. doi: 10.1172/JCI42345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa K, Boucher Y, Kashiwagi S, Fukumura D, Chen D, Gerweck LE. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. . 2007;67(9):4016–4021. doi: 10.1158/0008-5472.CAN-06-4498. [DOI] [PubMed] [Google Scholar]
- 19.Kozin SV, Duda DG, Munn LL, Jain RK. Is vasculogenesis crucial for the regrowth of irradiated tumours? Nat Rev Cancer. . 2011;11(7):532. doi: 10.1038/nrc2007-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song CW, Levitt SH. Vascular changes in Walker 256 carcinoma of rats following x irradiation. Radiology. . 1971;100(2):397–407. doi: 10.1148/100.2.397. [DOI] [PubMed] [Google Scholar]
- 21.Song CW, Payne JT, Levitt SH. Vascularity and blood flow in x-irradiated Walker carcinoma 256 of rats. Radiology. . 1972;104(3):693–697. doi: 10.1148/104.3.693. [DOI] [PubMed] [Google Scholar]
- 22.Kallman RF, DeNardo GL, Stasch MJ. Blood flow in irradiated mouse sarcoma as determined by the clearance of xenon-133. Cancer Res. . 1972;32(3):483–490. [PubMed] [Google Scholar]
- 23.Song CW, Sung JH, Clement JJ, Levitt SH. Vascular changes in neuroblastoma of mice following x-irradiation. Cancer Res. . 1974;34(9):2344–2350. [PubMed] [Google Scholar]
- 24.Hilmas DE, Gillette EL. Morphometric analyses of the microvasculature of tumors during growth and after x-irradiation. Cancer. . 1974;33(1):103–110. doi: 10.1002/1097-0142(197401)33:1<103::aid-cncr2820330116>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Hilmas DE, Gillette EL. Microvasculature of C3H/Bi mouse mammary tumors after x-irradiation. Radiat Res. . 1975;61(1):128–143. [PubMed] [Google Scholar]
- 26.Solesvik OV, Rofstad EK, Brustad T. Vascular changes in a human malignant melanoma xenograft following single-dose irradiation. Radiat Res. . 1984;98(1):115–128. [PubMed] [Google Scholar]
- 27.Spence AM, Graham MM, Abbott GL, Muzi M, Lewellen TK. Blood flow changes following 137Cs irradiation in a rat glioma model. Radiat Res. . 1988;115(3):586–594. [PubMed] [Google Scholar]
- 28.Tozer GM, Bhujwalla ZM, Griffiths JR, Maxwell RJ. Phosphorus-31 magnetic resonance spectroscopy and blood perfusion of the RIF-1 tumor following X-irradiation. Int J Radiat Oncol Biol Phys. . 1989;16(1):155–164. doi: 10.1016/0360-3016(89)90023-0. [DOI] [PubMed] [Google Scholar]
- 29.Kalofonos H, Rowlinson G, Epenetos AA. Enhancement of monoclonal antibody uptake in human colon tumor xenografts following irradiation. Cancer Res. . 1990;50(1):159–163. [PubMed] [Google Scholar]
- 30.Tozer GM, Myers R, Cunningham VJ. Radiation-induced modification of blood flow distribution in a rat fibrosarcoma. Int J Radiat Biol. . 1991;60(1-2):327–334. doi: 10.1080/09553009114552081. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Barros M, Thin TH, Maj J, et al. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. . 2010;70(20):8179–8186. doi: 10.1158/0008-5472.CAN-10-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horsman MR, Nielsen T, Ostergaard L, Overgaard J. Radiation administered as a large single dose or in a fractionated schedule: role of the tumour vasculature as a target for influencing response. Acta Oncol. . 2006;45(7):876–880. doi: 10.1080/02841860600900068. [DOI] [PubMed] [Google Scholar]
- 33.Ljungkvist AS, Bussink J, Kaanders JH, Wiedenmann NE, Vlasman R, van der Kogel AJ. Dynamics of hypoxia, proliferation and apoptosis after irradiation in a murine tumor model. Radiat Res. . 2006;165(3):326–336. doi: 10.1667/rr3515.1. [DOI] [PubMed] [Google Scholar]
- 34.Itasaka S, Komaki R, Herbst RS, et al. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. Int J Radiat Oncol Biol Phys. . 2007;67(3):870–878. doi: 10.1016/j.ijrobp.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng L, Ou G, Itasaka S, et al. TS-1 enhances the effect of radiotherapy by suppressing radiation-induced hypoxia-inducible factor-1 activation and inducing endothelial cell apoptosis. Cancer Sci. . 2008;99(11):2327–2335. doi: 10.1111/j.1349-7006.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen FH, Chiang CS, Wang CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res. . 2009;15(5):1721–1729. doi: 10.1158/1078-0432.CCR-08-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fokas E, Hanze J, Kamlah F, et al. Irradiation-dependent effects on tumor perfusion and endogenous and exogenous hypoxia markers in an A549 xenograft model. Int J Radiat Oncol Biol Phys. . 2010;77(5):1500–1508. doi: 10.1016/j.ijrobp.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 38.Newcomb EW, Lukyanov Y, Alonso-Basanta M, et al. Antiangiogenic effects of noscapine enhance radioresponse for GL261 tumors. Int J Radiat Oncol Biol Phys. . 2008;71(5):1477–1484. doi: 10.1016/j.ijrobp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai JH, Makonnen S, Feldman M, Sehgal CM, Maity A, Lee WM. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol Ther. . 2005;4(12):1395–1400. doi: 10.4161/cbt.4.12.2331. [DOI] [PubMed] [Google Scholar]
- 40.Kozin SV, Winkler F, Garkavtsev I, Hicklin DJ, Jain RK, Boucher Y. Human tumor xenografts recurring after radiotherapy are more sensitive to anti-vascular endothelial growth factor receptor-2 treatment than treatment-naive tumors. Cancer Res. . 2007;67(11):5076–5082. doi: 10.1158/0008-5472.CAN-06-3664. [DOI] [PubMed] [Google Scholar]
- 41.Nojiri K, Iwakawa M, Ichikawa Y, et al. The proangiogenic factor ephrin-A1 is up-regulated in radioresistant murine tumor by irradiation. Exp Biol Med (Maywood). . 2009;234(1):112–122. doi: 10.3181/0806-RM-189. [DOI] [PubMed] [Google Scholar]
- 42.Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys. . 2008;71(2):324–325. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. . 2010;7(1):44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 44.Tipton K, Launders JH, Inamdar R, Miyamoto C, Schoelles K. Stereotactic body radiation therapy: scope of the literature. Ann Intern Med. . 2011;154(11):737–745. doi: 10.7326/0003-4819-154-11-201106070-00343. [DOI] [PubMed] [Google Scholar]
- 45.Burdelya LG, Komarova EA, Hill JE, et al. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. . 2006;66(19):9356–9361. doi: 10.1158/0008-5472.CAN-06-1223. [DOI] [PubMed] [Google Scholar]
- 46.Baeten CI, Castermans K, Lammering G, et al. Effects of radiotherapy and chemotherapy on angiogenesis and leukocyte infiltration in rectal cancer. Int J Radiat Oncol Biol Phys. . 2006;66(4):1219–1227. doi: 10.1016/j.ijrobp.2006.07.1362. [DOI] [PubMed] [Google Scholar]
- 47.Wurschmidt F, Beck-Bornholdt HP, Vogler H. Radiobiology of the rhabdomyosarcoma R1H of the rat: influence of the size of irradiation field on tumor response, tumor bed effect, and neovascularization kinetics. Int J Radiat Oncol Biol Phys. . 1990;18(4):879–882. doi: 10.1016/0360-3016(90)90411-c. [DOI] [PubMed] [Google Scholar]
- 48.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. . 2005;5(7):516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 49.Milas L, Milas M. Tumor bed phenomenon: effect on tumor progression and therapy. In: Rubin DB, editor. The Radiation Biology of the Vascular Endothelium. Boca Raton, FL: CRC Press LLC; 1998. pp. 209–229. [Google Scholar]
- 50.Sonveaux P, Brouet A, Havaux X, et al. Irradiation-induced angiogenesis through the up-regulation of the nitric oxide pathway: implications for tumor radiotherapy. Cancer Res. . 2003;63(5):1012–1019. [PubMed] [Google Scholar]
- 51.Endrich B, Reinhold HS, Gross JF, Intaglietta M. Tissue perfusion inhomogeneity during early tumor growth in rats. J Natl Cancer Inst. . 1979;62(2):387–395. [PubMed] [Google Scholar]
- 52.Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. . 2003;162(1):183–193. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giatromanolaki A, Sivridis E, Koukourakis MI. Tumour angiogenesis: vascular growth and survival. Apmis. . 2004;112(7–8):431–440. doi: 10.1111/j.1600-0463.2004.apm11207-0804.x. [DOI] [PubMed] [Google Scholar]
- 54.Tilki D, Kilic N, Sevinc S, Zywietz F, Stief CG, Ergun S. Zone-specific remodeling of tumor blood vessels affects tumor growth. Cancer. . 2007;110(10):2347–2362. doi: 10.1002/cncr.23024. [DOI] [PubMed] [Google Scholar]
- 55.Eddy HA. Tumor vascular responses following irradiation. Microvasc Res. . 1980;20(2):195–211. doi: 10.1016/0026-2862(80)90007-2. [DOI] [PubMed] [Google Scholar]
- 56.Yamaura H, Matsuzawa T. Tumor regrowth after irradiation; an experimental approach. Int J Radiat Biol Relat Stud Phys Chem Med. . 1979;35(3):201–219. doi: 10.1080/09553007914550241. [DOI] [PubMed] [Google Scholar]
- 57.Dewhirst MW. Intermittent hypoxia furthers the rationale for hypoxia-inducible factor-1 targeting. Cancer Res. . 2007;67(3):854–855. doi: 10.1158/0008-5472.CAN-06-4744. [DOI] [PubMed] [Google Scholar]
- 58.Hlushchuk R, Riesterer O, Baum O, et al. Tumor recovery by angiogenic switch from sprouting to intussusceptive angiogenesis after treatment with PTK787/ZK222584 or ionizing radiation. Am J Pathol. . 2008;173(4):1173–1185. doi: 10.2353/ajpath.2008.071131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi Y, Teshima T, Kawaguchi N, et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. . 2003;63(14):4253–4257. [PubMed] [Google Scholar]
- 60.Grabham P, Hu B, Sharma P, Geard C. Effects of ionizing radiation on three-dimensional human vessel models: differential effects according to radiation quality and cellular development. Radiat Res. . 2011;175(1):21–28. doi: 10.1667/RR2289.1. [DOI] [PubMed] [Google Scholar]
- 61.Fidler IJ. Angiogenic heterogeneity: regulation of neoplastic angiogenesis by the organ microenvironment. J Natl Cancer Inst. . 2001;93(14):1040–1041. doi: 10.1093/jnci/93.14.1040. [DOI] [PubMed] [Google Scholar]
- 62.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. . 2007;170(3):793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sajithlal GB, McGuire TF, Lu J, Beer-Stolz D, Prochownik EV. Endothelial-like cells derived directly from human tumor xenografts. Int J Cancer. . 2010;127(10):2268–2278. doi: 10.1002/ijc.25251. [DOI] [PubMed] [Google Scholar]
- 64.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. . 2010;468(7325):824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 65.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Feature article: transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. . 2011;108(11):4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. . 2010;468(7325):829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 67.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. . 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. . 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 69.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. . 2008;8(7):545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 70.Ergun S, Hohn HP, Kilic N, Singer BB, Tilki D. Endothelial and hematopoietic progenitor cells (EPCs and HPCs): hand in hand fate determining partners for cancer cells. Stem Cell Rev. . 2008;4(3):169–177. doi: 10.1007/s12015-008-9028-y. [DOI] [PubMed] [Google Scholar]
- 71.Aicher A, Rentsch M, Sasaki K, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. . 2007;100(4):581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]