Abstract
Objective
The efficacy of various regimens of initial insulin treatment in poorly controlled type 2 diabetes was compared with regard to diurnal glucose variation.
Design
Randomized controlled trial.
Setting
Insulin therapy initiated on hospital wards, follow-up as outpatients for 12 months.
Subjects
Fifty-two type 2 diabetic patients (HbA1c >7.5%, mean 9.8%) on maximal oral therapy.
Interventions
Insulin only (IO), bedtime insulin with sulphonylurea (glipizide) (IS), or bedtime insulin with metformin (IM).
Main outcome measures
HbA1c and body weight.
Results
HbA1c decreased on average by 1.8, 1.0 and 1.5 percentage points in the IO, IS, and IM groups, respectively (p always <0.025). Body weight increased, most in the IO patients (+6.2 kg), least in the IM patients (+3.4 kg). Analysing all treatment groups combined, a similar HbA1c reduction was observed in patients with overall hyperglycaemia (low fasting plasma glucose/HbA1c ratio) and in patients with fasting hyperglycaemia (high fasting plasma glucose/HbA1c ratio). Within the overall hyperglycaemia group, the IS and IM patients had smaller decreases in HbA1c (−1.5 and −1.3 percentage points, respectively) than the IO patients (−2.7 percentage points). On the other hand, within the fasting hyperglycaemia group HbA1c reductions were −1.2, −0.8 and −1.5 percentage points, in the IO, IS, and IM groups, respectively.
Conclusion
Not all poorly controlled type 2 diabetic patients should automatically be treated with an oral agent and bedtime insulin. Two daily insulin injections is a valid choice, particularly if the patient has overall hyperglycaemia.
Keywords: Family practice, fasting hyperglycaemia, HbA1c, postprandial hyperglycaemia, type 2 diabetes
A widely used regimen when starting insulin treatment in type 2 diabetes has been bedtime insulin in combination with oral hypoglycaemic agents.
When starting insulin, the diurnal glucose profile should be recognized, as it may give useful information in selecting the initial insulin regimen.
Apart from combination therapies, a regimen with two daily insulin injections is also a valid choice, particularly if the patient has overall hyperglycaemia characterized by low fasting glucose in relation to HbA1c.
The prevalence of type 2 diabetes mellitus (T2D) is steadily increasing worldwide [1] and its treatment needs a lot of resources.
Treatment of type 2 diabetes (T2D) is based on dietary and physical activity counselling [2] and antihyperglycaemic medication. Studies on medical treatment of T2D have usually included only a relatively short period of follow-up, although the long-term efficacy of treatment in terms of metabolic control is crucial to avoid future complications [3–5] and to improve quality of life [6].
Normally, insulin is introduced when oral drugs (OHAs) fail. Using insulin in combination with OHAs has become particularly popular [7]. The findings of recent studies have changed treatment regimens so that bedtime insulin is in most cases started when insulin is added to the treatment [5], [7]. The short follow-up period in many studies makes it particularly difficult to evaluate the effect of various insulin treatment regimens on long-term changes in body weight [8].
Patients with T2D vary in the diurnal variation of hyperglycaemia: some are characterized mainly by high postprandial glucose peaks, whereas others have high fasting values and only moderately elevated postprandial values. This aspect has not been taken into account in previous studies on insulin treatment in T2D. Yet a large epidemiological study, DECODE, suggests that high postprandial glucose levels are particularly harmful in terms of macrovascular events [9]. High postprandial glucose is probably also an important risk factor for diabetic microvascular complications [10–12].
C-peptide measurements have been used to assess relative insulin deficiency [13]. It has, however, been suggested that C-peptide has no decisive role for the planning of a T2D patient's therapy, based on the assumption that high C-peptide values during hyperglycaemia reflect severe insulin resistance, and also patients with high HbA1c and insulin resistance should be treated with insulin. Previous studies comparing various insulin treatment regimens in T2D have not taken into account serum C-peptide concentrations [14], [15].
The aim of this study was to compare initial insulin treatments, either with insulin alone or with combination therapy (insulin + glipizide or insulin + metformin), in T2D patients whose HbA1c was above 7.5% on OHA treatment, using HbA1c and weight gain as long-term outcome measures. The study focused on the following two questions: first, does the outcome differ between patients with predominantly fasting hyperglycaemia (high fasting glucose/HbA1c ratio) and patients with overall hyperglycaemia (low fasting glucose/HbA1c ratio), and second, does the efficacy of insulin treatment depend on the degree of endogenous insulin secretion.
Material and methods
Patients
The study was carried out in the years 1994–1998. Inclusion criteria were: type 2 diabetes for more than 5 years, age 40–75 years, body mass index (BMI) <35 kg/m2, HbA1c >7.5% and fasting serum/plasma glucose above 8.0 mmol/l. To avoid inclusion of T1D patients and clearly insulin deficient T2D patients, a postprandial C-peptide value (simultaneous serum glucose >7 mmol/l) exceeding 0.6 nmol/l was required.
Patients with severe cardiac insufficiency, serum creatinine >150 µmol/l, or alanine aminotransferase >80 IU/l were excluded.
Study design
The patients were first divided into two groups according to the type of hyperglycaemia: those with fasting hyperglycaemia (the ratio of fasting serum glucose and glycosylated haemoglobin equal to or exceeding 1.3) and those with overall hyperglycaemia (the ratio below 1.3).
Each patient was then randomized into one of five treatment groups: (1) insulin only (NPH twice daily), (2) bedtime NPH insulin + glipizide, (3) bedtime NPH insulin + metformin, (4) bedtime Lente insulin + glipizide, and (5) bedtime Lente insulin + metformin.
Patients treated with insulin only received either NPH or Lente insulin twice daily, i.e. in the morning and at bedtime. The dose of glipizide was 10 mg in the morning, and the dose of metformin 2.5 g/day or the highest dose tolerated by the patient. There was no statistical difference with regard to the change in HbA1c between the groups treated with NPH or Lente insulin and therefore these groups were combined in the final analysis.
The final study groups were:
insulin only (injections in the morning and at bedtime);
insulin + glipizide;
insulin + metformin.
The first phase of the study was carried out as an inpatient, as was the general practice at that time. Self-monitoring of plasma glucose (four times daily) was recommended.
The follow-up consisted of outpatient clinical visits at 2 weeks, 4 weeks, 2 months, 3 months, 6 months, 9 months, and 12 months (6 hospital outpatient clinics and 3 health centres). On each visit, the patient's weight and fasting serum/plasma glucose were recorded, and the results of home blood glucose monitoring were reviewed. The insulin dose was then adjusted. HbA1c was determined at 3 months, 6 months, 9 months, and 12 months (the final visit). Plasma cholesterol, HDL cholesterol, triglycerides, and urinary microalbumin were recorded at baseline and at 12 months (see Table I).
Table I.
Body weight, glucose control, insulin dose, and serum lipid values in 52 type 2 diabetics before and during 12 months of insulin treatment.
| Baseline | 3 months | 6 months | 9 months | 12 months | Change1 | CI | |
| Body weight (kg) | 84.2 | 85.3 | 86.5 | 86.0 | 89.1 | 4.4 | 3.17,5.59 |
| BMI (kg/m2) | 28.5 | 28.9 | 29.3 | 29.3 | 30.1 | 1.5 | 1.09,1.89 |
| FPG (mmol/l) | 12.5 | 9.2 | 9.1 | 8.8 | 8.7 | −3.8 | −5.03,−2.54 |
| HbA1c (percentage points) | 9.9 | 8.8 | 8.8 | 8.7 | 8.5 | −1.4 | −1.77,−0.98 |
| Daily insulin dose (IU) | 25.1 | 32.5 | 37.1 | 37.9 | 42.0 | 16.8 | 10.87,22.70 |
| Serum cholesterol (mmol/l) | 5.5 | 5.6 | 0.1 | −0.13,0.34 | |||
| Serum HDL-cholesterol (mmol/l) | 1.0 | 1.2 | 0.1 | 0.05,0.20 | |||
| Serum triglycerides (mmol/l) | 2.3 | 2.1 | −0.3 | −0.53,0.01 | |||
| Serum LDL-cholesterol (mmol/l) | 3.5 | 3.6 | 0.1 | −0.16,0.30 |
BMI = body mass index; FPG = fasting plasma glucose; HbA1c = glycosylated haemoglobin. Values are mean±95% CI. 1Baseline vs. 12 months.
Biochemical and statistical methods
Fasting plasma glucose (FPG) and plasma lipids were determined using standard laboratory methods. HbA1c was assessed using HPLC (reference range 4.2–6.0%). C-peptide (meal-stimulated) was determined by means of the Double Antibody C-Peptide method (RIA, Diagnostic Products Corp., LA, CA, USA).
Statistical analyses were performed using the SSPS program, version 14 (t-test, paired t-test, and regression analysis, when appropriate).
Ethical and other aspects
The study was approved by the joint Ethical Committee of the University of Turku and Turku University Central Hospital. Oral consent was requested and recorded at baseline. The study was an investigator-initiated study with the ordinary clinical resources.
Results
Baseline data
A total of 52 patients were eligible and randomized for treatment at 9 study centres, of which 6 were hospitals (45 patients) and 3 were community health centres (7 patients). There were 35 men and 17 women (mean age 62 years). The mean initial BMI was 28.5 kg/m2 (Table I). The mean initial fasting plasma glucose was 12.5 mmol/l (range 6.8–20.2) and initial HbA1c 9.9% (range 7.6–13.3).
Postprandial glucose was not analyzed because of its greater variability depending on the carbohydrate content of the meal and the exact time of measurement.
The majority of patients (30/52) had fasting hyperglycaemia (ratio FPG/HbA1c ≥ 1.3; group A) while approximately 40% (22/52) had overall hyperglycaemia (ratio FPG/HbA1c < 1.3; group B). The limit of the FPG/HbA1c ratio was set at 7.8 mmol/l/6%, with 7.8 being the diagnostic limit of plasma glucose for the diagnosis of diabetes and 6% being the upper reference limit of HbA1c.
Metabolic control
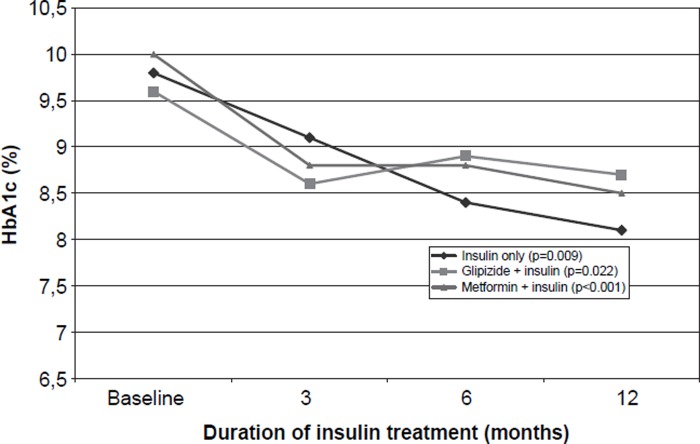
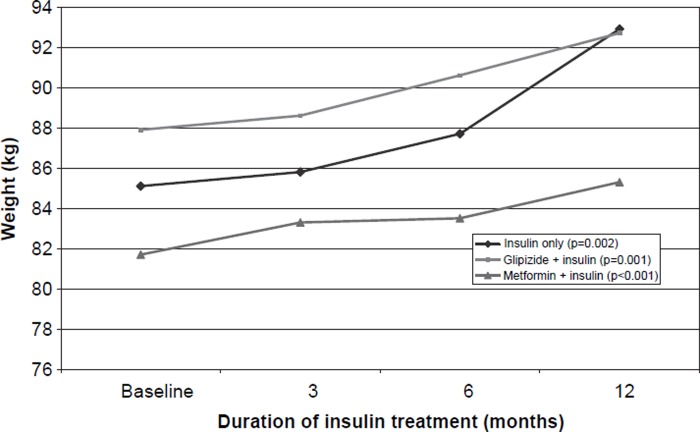
As a result of the insulin therapy, the mean HbA1c decreased by 1.4 percentage points during the 12 months of treatment (Figure 1). The mean BMI increased from the initial 28.5 kg/m2 to 30.1 kg/m2, reflecting a mean weight gain of almost 5 kg (Table I, Figure 2). All these changes were statistically significant. HDL cholesterol increased slightly; otherwise there were no changes in the lipid values.
Figure 1.
HbA1c in various insulin treatment groups of type 2 diabetic patients before and during insulin treatment; p-values for the difference between the baseline and 12-month values.
Figure 2.
Body weight (kg) in various insulin treatment groups of type 2 diabetic patients before and during insulin treatment; p-values for the difference between the baseline and 12-month values.
The HbA1c values diminished markedly in patients receiving insulin only and in patients treated with insulin and metformin, whereas insulin combined with glipizide seemed not to be equally effective (Table II). The weight increased most when two daily insulin doses were used, and the weight gain tended to be smaller in patients using insulin and metformin. Patients receiving glipizide or metformin had a final insulin dose that was approximately 50% lower than the dose of those who were treated with insulin only.
Table II.
Glucose control, body weight and insulin dose in the three treatment groups of type 2 diabetic patients before and during 12 months of insulin treatment.
| Fasting plasma glucose mmol/l |
HbA1c (%) |
Body weight (kg) |
Insulin dose (IU) |
|||||||||||||||||||||
| Treatment | n | Baseline | 3 months | 6 months | 12 months | Change* | CI | Baseline | 3 months | 6 months | 12 months | Change* | CI | Baseline | 3 months | 6 months | 12 months | Change* | CI | Baseline | 3 months | 6 months | 12 months | Min–max |
| Insulin only | 11 | 13.3 | 10.5 | 8.5 | 10.2 | −4.0 | −8.1,0.1 | 9.8 | 9.1 | 8.4 | 8.1 | −1.8 | −3.1,−0.6 | 85.1 | 85.8 | 87.7 | 92.9 | 6.3 | 2.9,9.7 | 42.7 | 56.0 | 63.5 | 71,1 | 30–160 |
| Glipizide + insulin | 15 | 12.3 | 9.5 | 10.0 | 9.4 | −3.1 | −5.8,−0.3 | 9.6 | 8.6 | 8.9 | 8.7 | −1.0 | −1.8,−0.2 | 87.9 | 88.6 | 90.6 | 92.7 | 4.7 | 2.4,7.0 | 18.3 | 25.9 | 31.3 | 37,9 | 18–82 |
| Metformin + insulin | 26 | 12.3 | 8.4 | 8.2 | 7.6 | −4.2 | −5.7,−2.7 | 10.0 | 8.8 | 8.8 | 8.5 | −1.5 | −1.9,−1.0 | 81.7 | 83.3 | 83.5 | 85.3 | 3.4 | 1.7,5.1 | 21.1 | 26.2 | 29.3 | 33,1 | 10–103 |
Impact of the type of hyperglycaemia
We found a smaller decrease in the fasting plasma glucose in Group B than in Group A (Table III). Fasting glucose tended to decrease most in patients taking metformin and insulin, independent of the type of hyperglycaemia. HbA1c decreased similarly in Groups A and B, when all treatment groups were analyzed together (Table III). Among patients with overall hyperglycaemia, the decline in HbA1c tended to be greatest in the patients taking insulin only (−2.7 percentage points). Among fasting hyperglycaemia patients, the decrease in HbA1c tended to be smallest in patients on sulphonylurea and insulin.
Table III.
Glucose control, body weight, C-peptide and insulin dose according to type of hyperglycaemia in the three insulin treatment groups of type 2 diabetic patients at baseline and 12 month follow-up visit.
| Fasting plasma glucose (mmol/l) |
HbA1c (%) |
Body weight (kg) |
Baseline post- prandial C-peptide (nmol/l) |
Insulin dose (IU) |
||||||||||||||
| n | Baseline | 12 months | change1 | CI | Baseline | 12 months | change1 | CI | Baseline | 12 months | change1 | CI | CI | 12 months | CI | Min–max | ||
| A (fasting hyperglycaemia) | ||||||||||||||||||
| Insulin only | 6 | 15.4 | 10.5 | −4.9 | −10.6,0.8 | 9.7 | 8.5 | −1.2 | −2.9,0.4 | 89.6 | 95.9 | 6.3 | 0.7,11.9 | 1.53 | 1.1,2.0 | 58.5 | 41.9,75.1 | 40–82 |
| Glipizide + insulin | 11 | 13.7 | 8.9 | −4.8 | −7.7,−1.8 | 9.4 | 8.7 | −0.8 | −1.7,0.2 | 88.9 | 93.6 | 4.7 | 2.1,7.2 | 1.48 | 1.1,2.0 | 38.6 | 24.8,52.5 | 18–82 |
| Metformin + insulin | 13 | 15.0 | 8.4 | −6.5 | −8.5,−4.6 | 10.1 | 8.6 | −1.5 | −2.0,−1.0 | 86.2 | 90.1 | 4.0 | 1.4,6.6 | 1.15 | 0.9,1.4 | 37.7 | 25.6,49.8 | 14–82 |
| All patients | 30 | 14.6 | 9.1 | −5.5 | −7.1,−3.9 | 9.8 | 8.6 | −1.2 | −1.6,−0.8 | 87.9 | 92.6 | 4.6 | 3.0,6.3 | 1,35 | 1.2,1.5 | 42.2 | 34.5,49.9 | 14–82 |
| B (overall hyperglycaemia) | ||||||||||||||||||
| Insulin only | 5 | 10.9 | 9.4 | −1.4 | −9.0,6.2 | 9.9 | 7.5 | −2.7 | −5.6,0.2 | 79.7 | 88.4 | 6.3 | −0.5,13.0 | 1.62 | 0.8,2.4 | 90.0 | −10.3,190.3 | 30–160 |
| Glipizide + insulin | 4 | 9.3 | 10.5 | 1.2 | −4.5,6.9 | 10.2 | 8.7 | −1.5 | −3.8,0.8 | 85.2 | 90.1 | 4.9 | −4.2,13.9 | 0.88 | 0.5,1,3 | 36.0 | 15.4,56.6 | 22–48 |
| Metformin + insulin | 13 | 9.4 | 6.8 | −2.3 | −4.0,−1.1 | 9.7 | 8.3 | −1.3 | −2.2,−0.5 | 77.2 | 79.7 | 2.4 | 0.2,4.7 | 1.15 | 0.9,1.5 | 28.5 | 14.0,42.9 | 10–103 |
| All patients | 22 | 9.5 | 8.0 | −1.3 | −3.0,−0.7 | 9.9 | 8.2 | −1.6 | −2.3,−0.7 | 79.9 | 83.8 | 3.9 | 1.9,5.9 | 1.20 | 1.0,1.4 | 41.6 | 23.7,59.6 | 10–160 |
A= fasting plasma glucose/HbA1c ≥ 1.3; B= fasting plasma glucose/HbA1c < 1.3; CI= 95% confidence interval. 1Baseline vs. 12 months.
Impact of initial C-peptide level
When the patients were grouped by the initial postprandial C-peptide level and all three insulin treatment groups were combined for analysis, the fasting plasma glucose decreased in the high C-peptide group less than in the low C-peptide group (Table IV). The HbA1c values declined significantly in both C-peptide strata with various insulin regimens, with the exception of a subgroup of patients with high C-peptide treated with a combination of insulin and glipizide.
Table IV.
Subjects stratified by treatment-specific1 baseline C-peptide levels in the three insulin treatment groups of type 2 diabetic patients at baseline and at 12 month follow-up visit.
| Fasting glucose |
HbA1c |
Body weight |
|||||||||||
| n | Baseline | 12 months | change | CI | Baseline | 12 months | Change | CI | Baseline | 12 months | change | CI | |
| Low C-peptide | |||||||||||||
| Insulin only | 5 | 13.5 | 11.2 | −3.3 | −10.9,4.4 | 9.1 | 8.5 | −0.9 | −2.6,0.9 | 82.9 | 91.6 | 5.9 | −0.1,11.9 |
| Glipizide + insulin | 9 | 12.9 | 8.6 | −3.7 | −7.7,0.4 | 9.9 | 8.4 | −1.5 | −2.3,−0.8 | 83.0 | 87.8 | 5.4 | 1.4,9.4 |
| Metformin + Insulin | 17 | 12.4 | 7.9 | −4.1 | −5.9,−2.3 | 9.9 | 8.6 | −1.4 | −1.9,−0.8 | 81.6 | 85.0 | 3.4 | 1.3,5.4 |
| All patients | 31 | 12.6 | 8.3 | −4.5 | −5.4,−2.3 | 9.8 | 8.5 | −1.3 | −1.7,−0.9 | 82.1 | 86.4 | 3.9 | 2.7,6.0 |
| High C-peptide | |||||||||||||
| Insulin only | 5 | 13.2 | 9.9 | −4.7 | −13.5,4.0 | 10.2 | 7.0 | −2.8 | −4.8,−0.8 | 86.4 | 93.5 | 6.7 | 0.4,13.0 |
| Glipizide + insulin | 6 | 11.7 | 10.4 | −1.9 | −6.9,3.0 | 9.4 | 9.0 | −0.8 | −1.8,1.7 | 93.6 | 98.3 | 3.7 | 2.1,5.3 |
| Metformin + insulin | 7 | 12.5 | 7.4 | −4.8 | −7.6,−1.9 | 10.3 | 8.1 | −1.7 | −2.9,−0.5 | 81.9 | 86.5 | 3.5 | −0.3,7.3 |
| All patients | 18 | 12.3 | 9.2 | −3.1 | −6.1,−1.3 | 9.9 | 8.3 | −1.4 | −2.4,−0.5 | 87.3 | 93.1 | 5.2 | 2.6,6.4 |
1Stratification by median value: insulin only; low C-peptide <1.8 nmol/l, INS + GLI and INS + MET, low C-peptide <1.3 nmol/l. The number of patients may vary slightly at different time points.
Discussion
The patients in this study represent typical type 2 diabetic patients with poor metabolic control. No separate analysis for gender was performed because of the relatively small number of patients. The majority of the patients were moderately overweight; one-third had an initial fasting plasma glucose >14 mmol/l, and one-third HbA1c values >10%. The overall glucose control improved significantly, by a minimum of 1.0 HbA1c percentage points, in all treatment regimens studied. The importance of a sufficiently long follow-up time must be emphasized. The improvement in the metabolic control did not stabilize until after 6 months of treatment. There was a significant weight increase, smallest in patients treated with metformin and insulin. This is in line with the results of an earlier study [16]. Both combination treatments resulted in a lower need for insulin; the final insulin dose was almost double in patients who were treated with insulin only.
To define the type of hyperglycaemia, we used the ratio fasting plasma glucose/HbA1c. We did not measure postprandial glucose levels. It has been shown that postprandial glucose levels in patients with poor metabolic control contribute to approximately 30% of HbA1c levels. HbA1c gives a surrogate measure of both fasting and postprandial glucose levels [17]. If we had measured postprandial glucose levels, the importance of the type of hyperglycaemia would probably have been even more apparent.
The most interesting new finding in this study was the importance of hyperglycaemia type with regard to insulin regimen. Using insulin twice daily seemed beneficial in overall hyperglycaemia (Group B), but not in fasting hyperglycaemia (Group A). This is logical as the postprandial hyperglycaemic values cannot be efficiently managed with bedtime insulin, even with OHAs. Use of bedtime insulin alone has never been a practice in Finland, and was thus not included in the study. Treatment with glipizide combined with bedtime insulin tended to be the least effective in lowering HbA1c in patients with fasting hyperglycaemia.
In the whole study group, the initial C-peptide concentration did not seem to predict either the improvement in metabolic control or the amount of weight gain. However, patients with a high C-peptide level, suggesting that they were highly insulin-resistant, showed an improvement in the HbA1c when treated with sulphonylurea plus bedtime insulin. In contrast, patients with a relatively low C-peptide (but above 0.6 nmol/l) level benefited only modestly from treatment with sulphonylurea plus insulin.
This study was performed before the launch of glitazones, glinides, and long-acting insulin analogues. However, our results are still relevant for current clinical practice for the following reasons. The use of glitazones with insulin was prohibited in the EU until December 2006 and this combination increases the risk of cardiac failure. Glinides are mainly used in early diabetes. NPH insulin is still widely used in type 2 diabetic patients [18] because of its similar effect on HbA1c and lower price compared with insulin analogues, insulin glargine, and insulin detemir [19], [20].
In conclusion, the diurnal glucose profile is useful in selecting the form of insulin therapy in type 2 diabetes. Patients with overall type hyperglycaemia benefit most when treated with insulin administered twice daily. Hence, our analysis suggests that bedtime insulin should not automatically be the first choice when starting insulin treatment for patients in poor metabolic control with maximal oral drugs.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Poskiparta M, Kasila K, Kiuru P. Dietary and physical activity counselling on type 2 diabetes and impaired glucose tolerance by physicians and nurses in primary healthcare in Finland. Scand J Prim Health Care. 2006;24:206–10. doi: 10.1080/02813430600866463. [DOI] [PubMed] [Google Scholar]
- 3.Klimt CR, Knatterud GL, Meinert CL, Prout TE, (UGDP) A study of the effects of hypoglycaemic agents on vascular complications in patients with adult-onset diabetes. Diabetes. 1970;19:747–830. [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;12:837–53. [PubMed] [Google Scholar]
- 5.Yki-Järvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758–67. doi: 10.2337/diacare.24.4.758. [DOI] [PubMed] [Google Scholar]
- 6.Wandell PE. Quality of life of patients with diabetes mellitus: An overview of research in primary health care in the Nordic countries. Scand J Prim Health Care. 2005;23:68–74. doi: 10.1080/02813430510015296. [DOI] [PubMed] [Google Scholar]
- 7.Yki-Järvinen H, Kauppila M, Kujansuu E, et al. Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1992;327:1426–33. doi: 10.1056/NEJM199211123272005. [DOI] [PubMed] [Google Scholar]
- 8.Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with non-insulin-dependent diabetes mellitus. Diabetologia. 1999;42:406–12. doi: 10.1007/s001250051172. [DOI] [PubMed] [Google Scholar]
- 9.DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 10.Gaude P, Vedel P, Larsen N, Jensen GVH, Parving H, Pedersen O. Multifactorial interventions and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 11.Qiao Q, Tuomilehto J, Borch-Johnsen K. Post-challenge hyperglycaemia is associated with premature death and macrovascular complications. Diabetologia. 2003;46((Suppl 1)):17–21. doi: 10.1007/s00125-002-0932-4. [DOI] [PubMed] [Google Scholar]
- 12.Ceriello A. Postprandial hyperglycaemia and diabetes complications: Is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Madsbad S, Krarup T, McNair P, Christiansen C, Faber OK, Transbol I, Binder C. Practical clinical value of the C-peptide response to glucagon stimulation in the choice of treatment in diabetes mellitus. Acta Med Scand. 1981;210:153–6. doi: 10.1111/j.0954-6820.1981.tb09793.x. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes mellitus. Report of a WHO Study Group. Geneva: World Health Organization; 1985. World Health Organization Technical Report Series 727. [PubMed] [Google Scholar]
- 15.Yki-Järvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758–67. doi: 10.2337/diacare.24.4.758. [DOI] [PubMed] [Google Scholar]
- 16.Yki-Järvinen H, Ryysy L, Nikkilä K, Tulokas T, Vanamo R, Heikkilä M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus: A randomized, controlled trial. Ann Intern Med. 1999;130:389–96. doi: 10.7326/0003-4819-130-5-199903020-00002. [DOI] [PubMed] [Google Scholar]
- 17.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–5. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 18.Riddle MC. Rosenstock J. Gerich J Insulin Glargine 4002 Study Investigators. The treat-to-target trial: Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 19.Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, Vähätalo M, Virtamo H, Nikkilä K, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: The LANMET study. Diabetologia. 2006;49:442–51. doi: 10.1007/s00125-005-0132-0. [DOI] [PubMed] [Google Scholar]
- 20.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–74. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]