Abstract
Objective
To investigate the effect of a primary care model for COPD on process of care and patient outcome.
Design
Controlled study with delayed intervention in control group.
Setting
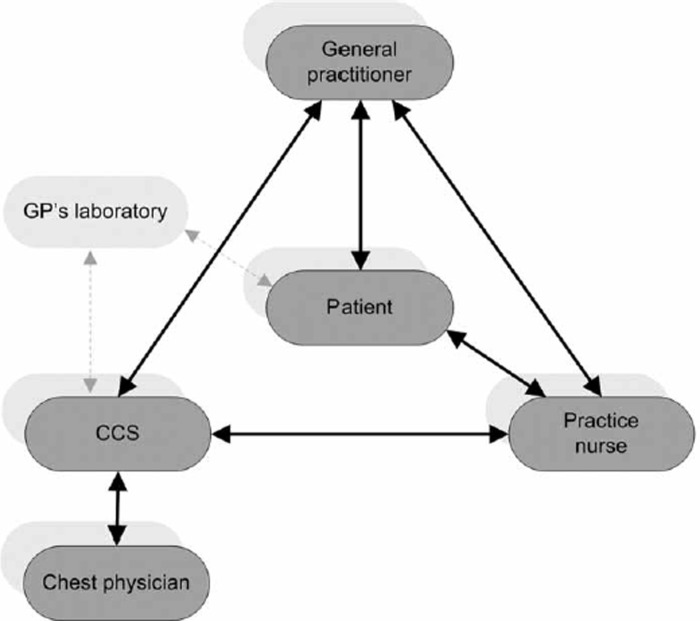
The GP delegates tasks to a COPD support service (CSS) and a practice nurse. The CSS offers logistic support to the practice through a patient register and recall system for annual history-taking and lung function measurement. It also forms the link with the chest physician for diagnostic and therapeutic advice. The practice nurse's most important tasks are education and counselling.
Subjects
A total of 44 practices (n =22 for intervention and n =22 for control group) and 260 of their patients ≥40 years with obstructive lung diseases.
Results
Within the intervention group planned visits increased from 16% to 44% and from 19% to 25% in the control condition (difference between groups p =0.014). Annual lung function measurement rose from 17% to 67% in the intervention and from 11% to 18% in the control group (difference between groups p =0.001). Compared with control, more but not statistically significant smokers received periodic advice to quit smoking (p =0.16). At baseline 41% of the intervention group were using their inhalers correctly and this increased to 54% after two years; it decreased in the control group from 47 to 29% (difference between groups p =0.002). The percentage of patients without exacerbation did not change significantly compared with the control condition. The percentage of the intervention group not needing emergency medication rose from 79% to 84% but decreased in the controls from 81 to 76% (difference between groups p =0.08).
Conclusion
Combining different disciplines in one model has a positive effect on compliance with recommendations for monitoring patients, and improves the care process and some patient outcomes.
Keywords: COPD, family practice, guidelines adherence, integrated healthcare system, practice nurse, primary care
To organize general practice care in a way that planned care can be delivered to patients with chronic diseases, a number of facilities are required which are often lacking in usual care practices.
For implementation of the national guidelines for the treatment of asthma and COPD, the care has to meet stringent requirements and should involve various disciplines, ranging from non-medical staff to medical specialists.
Combining different disciplines in one integrated care model has a positive effect on compliance with the recommendations on monitoring patients, and improves the process of care and some patient outcomes.
By far the majority of patients with COPD are treated in general practice. To that end, international and national guidelines have been developed [1–4]. Recommendations include using lung function measurement in diagnostics and monitoring, checking symptoms periodically and if necessary adjusting medication, and educating patients to take responsibility for the daily management of their disease. However, care is often still not being given according to the guidelines [5], [6]. In particular lung function measurements [7], periodic check-ups [8], and supervision of inhalation techniques [9] have proved to be difficult. To implement guidelines, care must meet stringent requirements and should involve various disciplines, from non-medical staff to medical specialists. This means new disciplines are needed in general practice and/or specific disciplines outside general practice should be called in for task delegation and consultation [10]. Various interventions have been tested over the last few years. It appears that a recall system can be successfully organized on a scale that goes beyond the individual general practice and can be run by someone without a medical background [11]. Nurses can fulfil a key role in clinics by providing patient education and counselling [12–15]. Delegation of medical tasks and the presence of a practice nurse are significant associated with spirometry utilization [16]. A combination of spirometry and smoking cessation advice by a nurse appears to increase smoking cessation rates in smokers with mild COPD [17]. According to Bodenheimer et al. [18] the possibility of consultation with a specialist without a full referral contributes fundamentally to the care of people with a chronic disease.
We have developed a care model that fits into such an approach to chronic disease management, one in which various disciplines (general practitioner – (GP), practice nurse, logistic COPD Support Service (CSS) and chest physician (see Figure 1)) are integrated. All these participating elements were financed by the regular health insurance system. The GP can delegate tasks to the nurse and the CSS. The chest physician can support the diagnostic and therapeutic decision-making without actually seeing the patient. We assumed that the management of chronic obstructive lung diseases could be improved by the introduction of this model. The model was focused on the ≥ 40 age group with chronic obstructive lung diseases covering asthma as well as COPD, as the difference between COPD and asthma is not always clearly established in the primary care population. We performed a study to test the effects of the care model for COPD and/or asthma on the process of care and on patient outcomes.
Figure 1.
Actors primary care model COPD.
Material and methods
Design and study population
The effect of the care model on COPD and/or asthma was examined in a controlled study with delayed intervention in the control group. With an interval of two years, pre-test and post-test measurements were performed. The care model was introduced in a region in the south of the Netherlands in 2002 with a running-in period of over a year. During that period the practice nurses were trained and the CCS (a logistic support service, linked to the regional primary care laboratory with a specialized lung nurse and some administrative staff) was set up. GPs qualified for a practice nurse if they had working space for a nurse and an electronic patient register. Due to limited funding, not all interested practices could start at the same time. Based on regional distribution criteria (division between sub-regions and between urban–rural) the first cohort of practices was selected to start with the care model in 2002. These practices formed the intervention group (n = 22); the practices on the waiting list formed the control group (n = 22). By October 2003, 11 practice nurses had been appointed. Before the nurse started, a random sample of patients per practice was drawn: patients ≥ 40 years with a documented lung condition and using inhalation medicines (137 in the intervention group and 123 in the control group).
Intervention
In each practice, the nurse (without knowing who had been selected in the random sample) made a survey of all patients ≥ 40 years with chronic lung obstruction on the basis of diagnostic data and medication use. The CSS called up all these patients for extensive history-taking and lung function measurement. The results were sent to a chest physician for assessment, diagnosis (or confirming or adjusting an earlier diagnosis), and advice on treatment. The CSS maintained a register of patients qualifying for annual history-taking and lung function measurement. Patients visited their GP to discuss the results and determine whether the medication was still adequate. They also visited the practice nurse who checked their inhalation technique, and gave education and counselling (smoking cessation). Patients who, according to the GP, met the criteria for referral were referred to the chest physician.
In the control group the patients received the usual care, which generally meant that they were seen only when they consulted the GP about their symptoms.
Variables and instruments
To study whether patients received care according to the guidelines, we collected data on planned consultations, periodic lung function measurements, and smoking cessation advice. To measure the effect of the care model on patient outcomes, we collected data on smoking status, inhalation technique, exacerbations, and emergency medication. Furthermore, some general characteristics from GPs and patients were noted.
In more detail:
Process of care: contact with the general practice (when symptoms deteriorated/or at fixed moments); periodic lung function measurements (no/yes, in the surgery/yes, by the laboratory); smoking cessation advice (yes/no).
Smoking habits were assessed by asking patients about current smoking behaviour (yes/no).
The inhalation technique was checked with inhalation-specific checklists from the Netherlands Asthma Foundation (Table I).
Exacerbations were assessed by asking the patient about the duration of symptoms or changes in phlegm, cough, dyspnoea, wheezing, and bronchodilator use in the past 3 months. An exacerbation was defined as an episode of > 3 days with > three of the above-mentioned five items.
Emergency medication: prescriptions for systemic corticosteroids (Anatomic Therapeutic Chemical Classification System (ATC) code A07EA).
General characteristics of the general practice: number of GPs per location, degree of urbanization (more or less than 80 000 inhabitants), size of practice (number of registered patients), number of active shifts per GP; number of patients with documented asthma/COPD at baseline, active recall system offering planned care (yes/no).
General characteristics of the patients: age, gender. To assess the seriousness of the symptoms we used the MRC dyspnoea scale [19], which comprises five statements: 1 = breathless only on strenuous exercise; 2 = short of breath when hurrying on the level or going up a slight hill; 3 = walking slower than their peers on the level because of breathlessness or having to stop for breath when walking at own pace on the level; 4 = stopping for breath after walking 100 metres or after a few minutes on the level; 5 = too breathless to leave the house.
Table I.
Inhalation checklist.
| Inhaler | Inhaler with spacer | Ingelheim inhaler |
|---|---|---|
|
|
Open mouthpiece
|
| Turbuhaler | Discus | Diskhaler |
Remove white protective cap
|
Put thumb on thumbgrip and push your thumb away from you until Discus clicks
|
Remove coverPull the cartridge out using both handsPush cartridge back in
|
• = essential in evaluation.
All data were collected by means of questionnaires completed by the GP and the patients [20], except inhalation technique and emergency medication. Inhalation technique was checked pre-test and post-test by the same laboratory assistant. Data on emergency medication were obtained from community pharmacists.
Power calculation
As primary outcome variable we chose the correct use by the patient of the inhaler because it has been shown that change in organization of care integrating non-physicians can influence this patient outcome [21]. An earlier study showed that 60% of COPD patients used their inhalers correctly [22]. With the intervention, we expected an increase of 20%. Based on an alpha of 5% and a beta of 80% a random sample survey of 39 general practices with 5 patients each was needed (195 patients), taking into account clustering of patients per GP. With an expected dropout of 10%, the total number of patients needed was 215.
Analysis
Differences between the intervention and control group were tested with mixed logic model repeated measures (Glimmix procedure SAS V8.2). In all tests, corrections were made for the random/cluster effect caused by patient and GP.
Results
The 44 general practices approached were all included; there were no dropouts. At baseline the practices in the control group were comparable with those in the intervention group regarding number of GPs, population size, and average number of asthma/COPD patients (Table II). None of the practices had an active recall system offering planned care to COPD and/or asthma patients. The intervention practices were more often located in a city (>80 000 inhabitants) and were less often single-handed than the controls.
Table II.
Characteristics of practices and patients (SD).
| Intervention group | Control group | |
| Characteristics of practices | ||
| Number of practices | 22 | 22 |
| Number of general practitioners | 29 | 28 |
| Urban practice (>80 000 inhabitants) (%) | 38 | 27 |
| Single-handed practices (%) | 27 | 52 |
| Mean population/FTE | 2519 (346) | 2746 (414) |
| Mean number of asthma/COPD patients/1000 patients1 | 47 (25) | 51 (19) |
| Practices with active recall system (%) | 0 | 0 |
| Characteristics of patients in sample | ||
| Number of patients | 137 | 123 |
| Mean age | 59 (12) | 58 (10) |
| Males (%) | 42 | 48 |
| Dyspnoea score 1 or 2 (%) | 62 | 61 |
1Twelve intervention practices and 11 control practices could not supply these data.
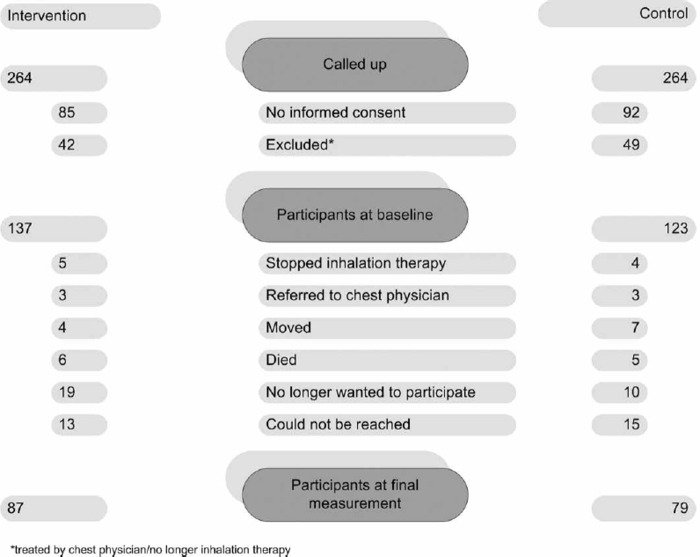
Data of 260 patients were collected at baseline. The intervention group was comparable with the controls regarding age, gender, and dyspnoea score (Table II). During the intervention 94 patients (37% of the intervention and 36% of the control group) dropped out (stopped inhalation therapy, moved, died, were referred to a chest physician, or no longer wanted to participate, Figure 2), resulting in 87 in the intervention and 79 in the control group for the final measurement. Analysis showed that patients who dropped out did not differ in gender, age, and dyspnoea score from patients who underwent a second measurement. That is why we included all the patients in the effect measurement (with more patients in the pre-test than in the post-test).
Figure 2.
Patient flow.
Process of care
After two years of intervention, the percentage of patients included in planned care at the general practice rose from 16% to 44% (Table III). The difference in change compared with the control group is statistically significant. The percentage undergoing periodical lung function measurements rose from 17% to 67% (to 75% if only patients involved in planned care are counted). All smokers were periodically advised to quit.
Table III.
Effect model on process of care and patient outcome.
| Intervention |
Control |
|||||
| Before | After | Before | After | Difference in change between intervention and control group Odds [CI] | p-value | |
| Process of care | ||||||
| Planned visits (% patients) | 16 | 44 | 19 | 25 | 1.08 [1.2, 6.9] | 0.014 |
| Periodical lung function measurement (% patients) | 17 | 67 | 11 | 18 | 5.54 [1.9, 16.2] | 0.001 |
| Periodical smoking cessation advice (% smokers) | 60 | 100 | 61 | 58 | 17.41 [0.3, 971.4] | 0.16 |
| Patient outcome | ||||||
| Non-smokers (% patients) | 70 | 81 | 70 | 74 | 1.03 [0.5, 1.8] | 0.9 |
| Correct inhalation technique (% patients) | 41 | 54 | 47 | 29 | 3.68 [1.5, 8.5] | 0.002 |
| No exacerbation in 3 months (% patients) | 79 | 81 | 77 | 69 | 1.75 [0.7, 5.0] | 0.24 |
| No emergency medication in 12 months (% patients) | 79 | 84 | 81 | 76 | 1.96 [08, 5.0] | 0.08 |
Patient outcomes
The percentage of non-smokers rose 11% in the intervention and 4% in the control group; the difference in change was not statistically significant. Regarding inhalation technique, the percentage of patients handling their inhalers correctly rose from 41% to 54% in the intervention group while it decreased in the control group from 47% to 29%. The percentage of patients without exacerbation in the previous three months rose from 79% to 81% in the intervention group; it decreased in the controls from 77% to 69%. The difference in change was not statistically significant.
We also noticed a difference in change regarding emergency medication. The percentage of patients not needing emergency medication rose from 79% to 84% in the intervention group, while it decreased in the control group; this difference was not statistically significant.
Discussion
A model for integrated primary COPD and/or asthma management was evaluated in this study. The model proved to have a positive effect on planned care and periodic lung function measurement. A positive effect on patient outcomes was also found. The percentage of patients who used their inhalers correctly rose, but the gain in preventing deterioration was even greater, as seen in the control group where the inhalation technique was not checked periodically (we hypothesized that if you assess inhalation technique at a random moment in a cross-sectional population, you also include people who have recently started on medication and have received instructions on how to use the inhaler and have a good technique. If you follow that same population, few people appear to retain the good technique.) The data on patients in our study correspond to those of another Dutch study GPs (70% with a dyspnoea score ≤ 2) [23]. At baseline we found fewer patients with a correct inhalation technique than in a comparable study among a Dutch population (45% vs. 72% with the correct technique). The high score there may have been due to extra attention to inhalation technique in a previous study by the same researchers, as they suggest themselves [24]. The difference between the intervention and control group in our study may be substantial but still half of all users do not handle their inhalers correctly, meaning it is unclear whether they inhale the correct dose of medication. Further studies are thus needed to find out whether this can be improved by shortening the intervals between inhaler checks – for example, a check at every prescription renewal, because research shows that mistakes occur shortly after the instructions are given, arguing in favour of short cyclic check [25]. The treatment of acute COPD exacerbations is shifting to general practitioners [26]. Patient recognition of exacerbations and prompt treatment improves exacerbation recovery, reduces risks of hospitalization, and is associated with a better health-related quality of life [27]. From this perspective, the question is whether we should have expected less or maybe even more emergency drug use as positive effect of the care model. The decrease we found is not significantly different from the control group, but we believe it is a positive effect because we also saw a decrease in self-reported exacerbations.
The number of patients willing to take part in the study was relatively low (intervention: 68%, control group: 65%) and the dropout rate was very high. This can be considered a weakness of the study (for data collection patients had to visit a laboratory twice to check the inhalation technique and fill in the questionnaire) but not a weakness of the care model. In fact 88% of all patients being treated by the GP were included in the care model [28].
Although the number of patients with both measurements was lower than the calculated number needed in the power analyses, we do not think that our study is under-powered. In the repeated measurement analysis (PROC MIXED, SAS) all patients are included. This means that data on patients with only one measurement were also analysed. We did not study the cost-effectiveness of the model, but we would like to make a few points here. A great deal of the efforts (and thus also the costs) in the intervention group were put into surveying the target group. These efforts will always be needed if the GP is going to provide planned care for patients with asthma or COPD, and therefore should not be accounted to this specific model. The same applies to setting up the call-up system. On the other hand, paper consultations by chest physicians are model-specific. Consultation in this way is cheap, has proved to be valid [29], and increases the number of patients who can be treated in primary care.
We conclude that this study has shown that combining various disciplines in an integrated model as described here improves care processes and patient outcomes in primary care for COPD and/or asthma. The care model is especially interesting in those settings in which chronic disease management is general practice based.
Acknowledgements
This study was funded by the Netherlands Asthma Foundation and the Health Insurance Companies CZ and VGZ.
References
- 1. GOLD. Available at: http://www.goldcopd.com. [Google Scholar]
- 2.Buist AS. Guidelines for the management of chronic obstructive pulmonary disease (review) Respir Med. 2002;96:S11–16. doi: 10.1016/s0954-6111(02)80029-4. [DOI] [PubMed] [Google Scholar]
- 3.Geijer RMM, Thiadens HA, Smeele IJM, Sachs APE, Bottema BJAM, Van Hensbergen W, et al. NHG-Standaard COPD en Astma bij Volwassenen: Diagnostiek [Dutch College of General Practitioners Guidelines for COPD and Asthma in Adults: Diagnostics] Huisarts Wet. 2001;44:107–17. [Google Scholar]
- 4.Geijer RMM, Van Schayck CP, Van Weel C, Sachs APE, Bottema BJAM, Smeele IJM, et al. NHG-standaard COPD: Behandeling [Dutch College of General Practitioners Guidelines for COPD: Treatment] Huisarts Wet. 2001;44:207–19. [Google Scholar]
- 5.Barr RG, Celli BR, Martinez FJ, Ries AL, Rennard SI, Reilly JJ, Jr, et al. Physician and patient perceptions in COPD: Rhe COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415. doi: 10.1016/j.amjmed.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 6.Kaminsky DA, Marcy TW, Bachand M, Irvin CG. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639–48. [PubMed] [Google Scholar]
- 7.Walters JA, Hansen E, Mudge P, Johns DP, Walters EH, Wood-Baker R. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201–3. [PubMed] [Google Scholar]
- 8.Jans MP, Schellevis FG, Le Coq EM, Bezemer PD, van Eijk JT. Health outcomes of asthma and COPD patients: The evaluation of a project to implement guidelines in general practice. Int J Qual Health Care. 2001;13:17–25. doi: 10.1093/intqhc/13.1.17. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Asthma guidelines: Recommendations versus reality. Respir Med. 2004;98((Suppl A)):S1–7. doi: 10.1016/j.rmed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Katon W, Von Korff M, Lin E, Simon G. Rethinking practitioner roles in chronic illness: The specialist, primary care physician, and the practice nurse. Gen Hosp Psychiatry. 2001;23:138–44. doi: 10.1016/s0163-8343(01)00136-0. [DOI] [PubMed] [Google Scholar]
- 11.Meulepas MA. Braspenning JCC. Grauw de WJ. Lucas AEM. Harms L. Akkermans RP, et al. 2006. Logistic support service improves processes and outcomes of diabetes care in general practice. (submitted) [DOI] [PubMed] [Google Scholar]
- 12.Boyle AH, Waters HF. COPD: focus on prevention: recommendations of the National Lung Health Education Program. Chronic obstructive pulmonary disease. Heart Lung. 2000;29:446–9. doi: 10.1067/mhl.2000.110990. [DOI] [PubMed] [Google Scholar]
- 13.Rothman AA, Wagner EH. Chronic illness management: What is the role of primary care? (review) Ann Intern Med. 2003;138:256–61. doi: 10.7326/0003-4819-138-3-200302040-00034. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg M, Ahlner J, Moller M, Ekstrom T. Asthma nurse practice: A resource-effective approach in asthma management. Respir Med. 1999;93:584–8. doi: 10.1016/s0954-6111(99)90159-2. [DOI] [PubMed] [Google Scholar]
- 15.Musto PK. General principals of management: Education (review) Nurs Clin North Am. 2003;38:621–33. doi: 10.1016/s0029-6465(03)00104-x. [DOI] [PubMed] [Google Scholar]
- 16.Poels PJ, Schermer TR, Jacobs A, Akkermans RP, Hartman J, Bottema BJ, van Weel C. Variation in spirometry utilization between trained general practitioners in practices equipped with a spirometer. Scand J Prim Health Care. 2006;24:81–7. doi: 10.1080/02813430500504362. [DOI] [PubMed] [Google Scholar]
- 17.Stratelis G, Molstad S, Jakobsson P, Zetterstrom O. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scand J Prim Health Care. 2006;24:133–9. doi: 10.1080/02813430600819751. [DOI] [PubMed] [Google Scholar]
- 18.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 19.Medical Research Council Committee on the Aetiology of Chronic Bronchitis. Standardized questionnaires on respiratory symptoms. BMJ. 1960;2:1665. [Google Scholar]
- 20.Smeele IJM, Jacobs JE, van Schayck CP, Grol RPTM, Maillé AR, Kaptein AA, et al. Quality of life of patients with asthma/COPD in general practice; impairments and correlations with clinical condition. Eur J Gen Pract. 1998;4:121–5. [Google Scholar]
- 21.Meulepas M. Lucas A. Jacobs J. Smeele I. Smeenk F. Bottema B, et al. Organisational interventions to improve asthma and COPD care. (submitted) [Google Scholar]
- 22.Van der Palen J, Klein JJ, Kerkhoff AH, Van Herwaarden CL, Zielhuis GA, Seydel ER. Inhalation technique of 166 adults asthmatics prior to and following a self-management program. J Asthma. 1999;36:441–7. doi: 10.3109/02770909909087286. [DOI] [PubMed] [Google Scholar]
- 23.Steuten LMG, Creutzberg EC, Vrijhoef HJM, Wouters EFM. COPD as a multi-component disease. Prim Care Respir J. 2006;15:84–91. doi: 10.1016/j.pcrj.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Palen J, Klein JJ, Kerkhoff AHM, Van Herwaarden CLA. Evaluation of the effectiveness of four different inhalers in patients with chronic obstructive pulmonary disease. Thorax. 1995;50:1183–7. doi: 10.1136/thx.50.11.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anani A, Higgins AJ, Crompton GK. Breath-actuated inhalers: Comparison of turbutaline turbohaler with salbutamol rotahaler. Eur Respir J. 1989;2:640–2. [PubMed] [Google Scholar]
- 26.Lampela P, Saynajakangas O, Keistinen T. Is the treatment of acute COPD exacerbations in Finland shifting to general practitioners? Scand J Prim Health Care. 2006;24:140–4. doi: 10.1080/02813430600830832. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:1298–303. doi: 10.1164/rccm.200310-1443OC. [DOI] [PubMed] [Google Scholar]
- 28.Meulepas M, Lucas A, Smeenk F, Smeele I, Jacobs J, Bottema B, et al. The feasibility of a primary care model for COPD. Prim Care Respir J. 2006;15:337–41. doi: 10.1016/j.pcrj.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas AEM. Smeenk FJWM. Smeele IJ. Brouwer T. van Schayck CP. (2006). Validity of diagnostic support of an asthma/COPD-service in primary care (submitted) [Google Scholar]