Abstract
Melanoma brain metastasis that develops as the isolated first visceral site challenges the current paradigm of tumor progression in which brain metastasis is regarded as the final stage. Here we test the hypothesis that melanoma patients who develop brain metastasis as the isolated first visceral site have distinct clinicopathological features at the time of primary melanoma diagnosis. Cutaneous melanoma patients enrolled in 2 prospectively collected databases were studied (Cohort 1: 1972–1982, Cohort 2: 2002–2009). Patients who developed brain metastasis as isolated first visceral site were compared with (1) all other patients, (2) patients who developed visceral metastasis: extracranial only or extracranial and brain, and (3) patients who progressed to other isolated visceral sites first. Two hundred seven of 2280 (9.1%) patients developed brain metastasis (median follow–up, 5.2 y). Seventy–four of 207 (35.7%) brain metastasis patients progressed to brain metastasis as the isolated first visceral site. These patients presented with primaries that were thinner and had no mitosis compared with all other visceral metastasis patients (Fisher's combined P = .02, .05, respectively), and there was a significant difference in American Joint Committee on Cancer stage distribution at initial melanoma diagnosis (combined P = .02). Post–visceral metastasis survival, however, was shorter in patients with brain metastasis as isolated first visceral site than in patients with visceral metastasis: extracranial and brain (combined P = .03). Brain metastasis as isolated first visceral site is a distinct clinicopathological entity. Studies are needed to better understand the biological factors driving this phenotype at the time of primary melanoma diagnosis and to determine its clinical implications.
Keywords: brain neoplasms, melanoma, neoplasm metastasis, neoplasm staging
Melanoma incidence and mortality rates are on the rise.1,2 An estimated 70 000 new invasive melanoma cases will be diagnosed in 2011 with 9000 melanoma deaths expected to occur,2 half of which will be due to brain metastasis.3 Yet, there continues to be a paucity of effective treatments against this common complication of metastatic melanoma4 generally viewed as the terminal event in the natural history of melanoma metastasis.5,6 Melanoma brain metastases that develop concomitant with or subsequent to other visceral metastases support this current understanding of brain metastasis as the final stage of tumor progression.5,6 The impermeability of the blood–brain barrier is believed to both protect against intracranial seeding and prevent drug entry into the brain,7 creating a sanctuary site for metastasis to occur provided that patients with systemic disease survive long enough for the integrity of the blood–brain barrier to become compromised.5,6 Advances in the ability to detect and to treat extracranial metastases earlier and more effectively5–8 appear consistent with this theory, as they parallel the rising incidence of brain metastases in solid tumor patients.
Progression to brain metastasis as the isolated first site of visceral metastasis, however, challenges the prevailing paradigm and has been reported in melanoma and other solid tumors.9–24 Evidence of this brain metastasis phenotype comes primarily from case reports/series9–12,14–18 and data on extracranial sites of involvement at brain metastasis diagnosis in the tables of larger cohort studies on brain metastasis risk factors and treatment outcomes.19–24 To our knowledge, only 2 studies (one in breast cancer and the other in ovarian cancer) have attempted to address the clinical relevance of brain metastasis as the isolated first visceral site, yet both reported on fewer than 50 patients with this particular phenotype.12,13
In this study, our first objective was to determine the prevalence of brain metastasis as the isolated first visceral site as a distinct brain metastasis phenotype in a large, prospectively accrued melanoma patient population. We then attempted to examine the clinicopathological characteristics of those primary melanoma patients who developed brain metastasis as the isolated first site of visceral metastasis.
Patients and Methods
Study Population
The study population comprised cutaneous melanoma patients prospectively enrolled in 2 databases at New York University (NYU) Medical Center: Cohort 1 (November 1972–November 1982)25 and Cohort 2 (August 2002–December 2009).26 Informed consent was obtained from all patients at the time of enrollment. Cohorts 1 and 2 are both well–characterized, population–based cohorts for which 415 and 371 fields of clinicopathological information, respectively were prospectively recorded, and despite differences in the information collected, the same fields were abstracted for the purpose of this study: year of and age at pathological diagnosis, gender, primary tumor thickness (mm), ulceration status, mitosis (absence vs presence), histologic subtype, anatomic site, first recurrence date and site, presence and date of brain metastasis, and date and site of first visceral metastasis. All patients were then restaged according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system27 to minimize inherent differences in the staging of melanoma patients diagnosed in the 1970s to 2000s. Differences in visceral metastasis detection in the pre–CT/MRI era nonetheless remain such that the method by which brain metastases were diagnosed (clinical vs histologic) as well as the presence of neurological symptoms at brain metastasis diagnosis were collected for patients in Cohort 2. Cohorts 1 and 2 were followed through October 1993 and October 2010, respectively.
Statistical Analysis
Continuous variables were summarized using median and range and compared across groups using the Wilcoxon–Mann–Whitney test or Kruskal–Wallis test. Categorical variables were summarized by counts and proportions. After excluding unclassified subjects, categorical variables were compared across groups using the chi–square test or Fisher's exact test. Cross–sectional and time–to–event statistical analyses were performed within each of the 2 cohorts (ie, stratified by cohort). Fisher's method was used to obtain the combined P value from the P values of Cohort 1 and Cohort 2, respectively.
The main event of interest was brain metastasis as the isolated first site of visceral metastasis. Time to the event of interest was calculated from the date of initial melanoma diagnosis. Competing events included visceral metastasis: extracranial only or extracranial and brain and death not related to melanoma and before metastasis. Cumulative incidence functions between groups stratified by each potential prognostic factor were compared using Gray's test,28 which accounts for competing risks. Multivariate evaluation of risk factors was also performed using a semiparametric Cox proportional hazards model for the subdistribution, as proposed by the Fine–Gray model.29 Post–visceral metastasis survival data were summarized using median survival and Kaplan–Meier survival curves. The comparison of survival curves between patients with brain metastasis as the isolated first site of visceral metastasis and patients with visceral metastasis: extracranial and brain was performed using the log–rank test. Statistical significance was claimed when the P value was less than .05, and all statistical analyses were performed using SAS version 9.2 or R.
Results
Demographic and primary tumor characteristics of all patients are shown in Table 1 stratified by cohort and presence of brain metastasis. A total of 2280 melanoma patients were identified, and 207 (9.1%) developed brain metastasis during follow–up (median, 5.2 y). Median time to brain metastasis from the date of initial melanoma diagnosis was 2.7 and 2.1 years in brain metastasis patients from Cohorts 1 and 2, respectively. Brain metastasis was diagnosed clinically in all 90 (100%) brain metastasis patients in Cohort 1 as well as in 90/117 (76.9%) brain metastasis patients in Cohort 2. Furthermore, 46.1% (54/117) of the brain metastasis patients in Cohort 2 presented at brain metastasis diagnosis with neurological symptoms, including altered mental status, headache, nausea/vomiting, seizure, vision/speech/balance disorder, weakness, paresthesias, and focal neurology (Table 2). Of the 207 brain metastasis patients in Cohorts 1 and 2 combined, 74 (35.7%) (95% confidence interval: 0.292–0.422) progressed to brain metastasis as the isolated first site of visceral metastasis: 40/90 (44.4%) in Cohort 1 and 34/117 (29.1%) in Cohort 2.
Table 1.
Demographic and primary tumor characteristics of 2280 NYU melanoma patients with varying metastatic patterns
| Characteristic | Cohort 1 (1972–1982) |
Cohort 2 (2002–2009) |
||
|---|---|---|---|---|
| Brain Metastasis | No Brain Metastasis | Brain Metastasisa | No Brain Metastasisb | |
| (n= 90) | (n = 953) | (n = 117) | (n = 1120) | |
| n (%) | n (%) | n (%) | n (%) | |
| Age at pathological diagnosis (y) | ||||
| Median (range) | 54 (16–88) | 52 (4–91) | 57 (27–86) | 59 (6–98) |
| Age at brain metastasis diagnosis (y) | ||||
| Median (range) | 59 (18–88) | — | 60 (27–88) | — |
| Gender | ||||
| Male | 54 (60.0%) | 459 (48.2%) | 72 (61.5%) | 608 (54.3%) |
| Female | 36 (40.0%) | 494 (51.8%) | 45 (38.5%) | 512 (45.7%) |
| Primary tumor thickness (mm) | ||||
| Median (range) | 2.70 (0.5–15) | 1.20 (0.1–14) | 2.18 (0.21–30) | 0.93 (0.12–30) |
| Primary tumor ulceration status | ||||
| Absent | 43 (48.3%) | 736 (79.4%) | 49 (50.0%) | 903 (83.1%) |
| Present | 46 (51.7%) | 191 (20.6%) | 49 (50.0%) | 183 (16.9%) |
| Primary tumor mitosis | ||||
| Absent | 19 (23.5%) | 353 (41.5%) | 14 (14.6%) | 381 (36.7%) |
| Present | 62 (76.5%) | 497 (58.5%) | 82 (85.4%) | 656 (63.3%) |
| Primary tumor histologic subtype | ||||
| Superficial spreading | 52 (68.4%) | 685 (79.8%) | 34 (35.1%) | 628 (60.3%) |
| Nodular | 19 (25.0%) | 98 (11.4%) | 53 (54.6%) | 268 (25.7%) |
| Other | 5 (6.6%) | 75 (8.7%) | 10 (10.3%) | 145 (13.9%) |
| Primary tumor anatomic site | ||||
| Head/neck | 13 (14.4%) | 136 (14.3%) | 27 (27.0%) | 170 (15.6%) |
| Axial | 42 (46.7%) | 364 (38.2%) | 40 (40.0%) | 420 (38.7%) |
| Extremity | 35 (38.9%) | 453 (47.5%) | 33 (33.0%) | 497 (45.7%) |
| AJCC stage at pathological diagnosis | ||||
| I | 26 (29.2%) | 608 (64.3%) | 30 (25.6%) | 758 (67.7%) |
| II | 34 (38.2%) | 246 (26.0%) | 28 (23.9%) | 195 (17.4%) |
| III | 29 (32.6%) | 88 (9.3%) | 42 (35.9%) | 155 (13.8%) |
| IV | 0 (0.0%) | 4 (0.4%) | 17 (14.5%) | 12 (1.1%) |
Number of patients may not sum to total due to unclassified/unavailable data.
a17 unknown primaries.
b33 unknown primaries.
Table 2.
Detection of brain metastasis in patients from Cohort 2
| Variable | Brain Metastasis as Isolated First Visceral Site | Visceral Metastasis: Extracranial and Brain |
|---|---|---|
| (n = 34) | (n = 83) | |
| Presentation | ||
| Asymptomatic | 10 (29.4%) | 44 (53.0%) |
| Symptomatica | 24 (70.6%) | 30 (36.1%) |
| Altered mental status/cognitive impairment | 6 | 9 |
| Headache | 9 | 7 |
| Nausea/vomiting | 3 | 3 |
| Seizure | 2 | 1 |
| Vision disorder | 2 | 3 |
| Speech disorder | 2 | 5 |
| Balance disorder | 6 | 7 |
| Weakness | 7 | 5 |
| Paresthesias | 2 | 2 |
| Focal neurology | 1 | 4 |
| Unknown | 0 (0.0%) | 9 (10.8%) |
| Basis of brain metastasis diagnosis | ||
| Clinical | 20 (58.8%) | 70 (84.3%) |
| Histologic | 14 (41.2%) | 13 (15.7%) |
aPatients may have presented with more than one symptom.
Clinicopathological Features at Initial Presentation of Melanoma Differ in Patients who Developed Brain Metastasis as the Isolated First Site of Visceral Metastasis Compared with All Other Patients
The clinicopathological features of primary melanoma patients who developed brain metastasis as the isolated first site of visceral metastasis were first compared with those of all other patients, a group that included patients without visceral metastasis, patients with visceral metastasis: extracranial only, and patients with visceral metastasis: extracranial and brain at the time of brain metastasis diagnosis. Significant differences were observed in primary tumor thickness, ulceration, histotype, and distribution of AJCC stage at pathological diagnosis between patients with brain metastasis as the isolated first visceral site and all other patients in Cohort 1 (P= .0017, .0002, .04, .007, respectively) and Cohort 2 (P = .0002, <.0001, .0002, .001, respectively) (Table 3) . Fisher's combined P values for these 4 clinicopathological features were also significant (P < .0001, <.0001, .0001, <.0001, respectively). Patients who developed brain metastasis as the isolated first site of visceral metastasis had thicker primaries and a higher rate of ulcerated tumors and nodular melanomas than all other patients. In addition, the cumulative incidence of brain metastasis as isolated first visceral site in the presence of competing risks, namely visceral metastasis: extracranial only or extracranial and brain and death not related to melanoma and before metastasis, is significantly increased according to Gray's test in these same patients (i.e., those whose tumors were >1 mm, ulcerated, or of the nodular histologic subtype) as well as in patients with advanced disease (stage III/IV) at pathological diagnosis on univariate analysis (Fisher's combined P values for Cohorts 1 and 2: .0003, <.0001, .0008, .0007, respectively) (Table 4). It is important to note, however, that 40.0% (16/40) and 38.2% (13/34) of patients with brain metastasis as isolated first visceral site in Cohorts 1 and 2, respectively (Table 3), presented at initial melanoma diagnosis with stage I disease compared with 61.6% (618/1003) and 64.4% (775/1203) of patients in the other group, which included all other patients. However, only 2 of the 4 clinicopathological predictors of time to brain metastasis as the isolated first visceral site remained statistically significant in a multivariate competing–risk Cox model (Fine–Gray model) fitted to data from Cohort 1 due to the relatively small number of cases of brain metastasis as the isolated first site of visceral metastasis, namely primary tumor ulceration status and AJCC stage at pathological diagnosis (log–hazard ratio = .97, .83; P = .0027, .023, respectively). These same 2 predictors were no longer jointly significant in a similar multivariate model fitted to data from Cohort 2 as patients in Cohort 2 were followed for a shorter period of time, but their log–hazard ratios were both positive and thus in the same direction as Cohort 1 (log–hazard ratio = 1.20, .32; P = .0021, .46 for primary tumor ulceration status and AJCC stage at pathological diagnosis, respectively).
Table 3.
Comparison of melanoma patients with brain metastasis as isolated first visceral site versus all other patients
| Characteristic | Cohort 1 |
Pd | Cohort 2 |
Pd | Pce | ||
|---|---|---|---|---|---|---|---|
| Brain Metastasis as Isolated First Visceral Site | Other Metastasis or No Metastasisa | Brain Metastasis as Isolated First Visceral Site | Other Metastasis or No Metastasisa | ||||
| (n= 40) | (n = 1003) | (n = 34)b | (n = 1203)c | ||||
| n (%) | n (%) | n (%) | n (%) | ||||
| Primary tumor thickness (mm) | .0017f | .0002f | <.0001 | ||||
| Median (range) | 2.20 (0.50–10) | 1.30 (0.10–15) | 1.61 (0.52–30) | 0.96 (0.12–30) | |||
| Primary tumor ulceration status | .0002 | <.0001 | <.0001 | ||||
| Absent | 21 (52.5%) | 758 (75.6%) | 15 (50.0%) | 937 (81.0%) | |||
| Present | 19 (47.5%) | 218 (21.7%) | 15 (50.0%) | 217 (18.8%) | |||
| Unclassified | 0 (0.0%) | 27 (2.7%) | 0 (0.0%) | 3 (0.3%) | |||
| Primary tumor mitosis | .20 | .10 | .10 | ||||
| Absent | 11 (27.5%) | 361 (36.0%) | 6 (20.0%) | 389 (33.6%) | |||
| Present | 26 (65.0%) | 533 (53.1%) | 23 (76.7%) | 715 (61.8%) | |||
| Unclassified | 3 (7.5%) | 109 (10.9%) | 1 (3.3%) | 53 (4.6%) | |||
| AJCC stage at pathological diagnosis | .007 | .001 | <.0001 | ||||
| I | 16 (40.0%) | 618 (61.6%) | 13 (38.2%) | 775 (64.4%) | |||
| II | 13 (32.5%) | 267 (26.6%) | 8 (23.5%) | 215 (17.9%) | |||
| III | 11 (27.5%) | 106 (10.6%) | 9 (26.5%) | 188 (15.6%) | |||
| IV | 0 (0.0%) | 4 (0.4%) | 4 (11.8%) | 25 (2.1%) | |||
| Unable to assess | 0 (0.0%) | 8 (0.8%) | 0 (0.0%) | 0 (0.0%) | |||
| Primary tumor histologic subtype | .04 | .0002 | .0001 | ||||
| Nodular | 8 (20.0%) | 109 (10.9%) | 17 (56.7%) | 304 (26.3%) | |||
| Superficial spreading/other | 25 (62.5%) | 792 (79.0%) | 12 (40.0%) | 805 (69.6%) | |||
| Unclassified | 7 (17.5%) | 102 (10.2%) | 1 (3.3%) | 48 (4.1%) | |||
Unclassified data were removed before the chi–square test or Fisher's exact test was applied.
aIncludes patients with visceral metastasis: extracranial only or extracranial and brain as well as patients with no visceral metastasis.
b4 unknown primaries.
c46 unknown primaries.
dBy the Wilcoxon–Mann–Whitney test or Kruskal–Wallis test for continuous variables and the chi–square test or Fisher's exact test for categorical variables.
eCombined Fisher P value for comparing patients with brain metastasis as isolated first visceral site and all other patients.
fAnalyzed as a continuous variable.
Table 4.
Univariate analysis of clinicopathological predictors of time to brain metastasis as isolated first visceral site in the context of competing risks
| Variable | P (Cohort 1)a | P (Cohort 2)a | Pb |
|---|---|---|---|
| Primary tumor thickness (mm) | |||
| ≤1 vs >1 | .002 | .015 | .0003 |
| Primary tumor ulceration status | |||
| Absent vs present | .002 | .0002 | <.0001 |
| Primary tumor mitosis | |||
| Absent vs present | .13 | .27 | .23 |
| AJCC stage at pathological diagnosis | |||
| I/II vs III/IV | .0013 | .05 | .0007 |
| Primary tumor histologic subtype | |||
| Nodular vs superficial spreading/other | .036 | .002 | .0008 |
| Gender | |||
| Male vs female | .65 | .35 | .56 |
aBy Gray's test.
bCombined P value.
Clinicopathological Features at Initial Presentation of Melanoma Differ in Patients who Developed Brain Metastasis as the Isolated First Site of Visceral Metastasis Compared with All Other Visceral Metastasis Patients
Significant differences were observed in primary tumor thickness and the distribution of clinical stage at initial melanoma diagnosis between Cohort 1 patients with brain metastasis as the isolated first site of visceral metastasis and those with visceral metastasis: extracranial only (P = .03, .03, respectively) (Table 5). Patients who progressed to brain metastasis as the isolated first visceral site had thinner primaries and an increased incidence of stage I melanoma at pathological diagnosis than patients who progressed to visceral metastasis: extracranial only. A greater proportion of primaries without mitosis was also observed among patients with brain metastasis as isolated first visceral site (P = .06). Differences in primary tumor thickness, mitosis, and the distribution of AJCC stage at pathological diagnosis trended in the same direction in the comparison of Cohort 2 patients (P = .10, .14, .12, respectively).
Table 5.
Comparison of melanoma patients with brain metastasis as isolated first visceral site vs visceral metastasis: extracranial only or extracranial and brain
| Characteristic | Cohort 1 |
P1d | P2e | Cohort 2 |
P1d | P2e | Pcf | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Metastasis as Isolated First Visceral Site | Visceral Metastasis: Extracranial and Brain | Visceral Metastasis: Extracranial Only | Brain Metastasis as Isolated First Visceral Site | Visceral Metastasis: Extracranial and Brain | Visceral Metastasis: Extracranial Only | ||||||
| (n = 40) | (n = 50) | (n = 107) | (n = 34)a | (n = 83)b | (n = 121)c | ||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||||
| Primary tumor thickness (mm) | .03g | .02g | .10g | .15g | .02 | ||||||
| Median (range) | 2.20 (0.50–10) | 2.85 (0.78–15) | 2.80 (0.50–12) | 1.61 (0.52–30) | 2.44 (0.21–22) | 3.00 (0.20–30) | |||||
| Primary tumor mitosis | .06h | .06h | .14h | .14h | .05 | ||||||
| Absent | 11 (27.5%) | 8 (16.0%) | 14 (13.1%) | 6 (20.0%) | 8 (11.4%) | 9 (8.8%) | |||||
| Present | 26 (65.0%) | 36 (72.0%) | 78 (72.9%) | 23 (76.7%) | 59 (84.3%) | 80 (78.4%) | |||||
| Unclassified | 3 (7.5%) | 6 (12.0%) | 15 (14.0%) | 1 (3.3%) | 3 (4.3%) | 13 (12.7%) | |||||
| AJCC stage at pathological diagnosis | .03i | .03i | .12i | .12i | .02 | ||||||
| I | 16 (40.0%) | 10 (20.0%) | 18 (16.8%) | 13 (38.2%) | 17 (20.5%) | 25 (20.7%) | |||||
| II | 13 (32.5%) | 21 (42.0%) | 45 (42.1%) | 8 (23.5%) | 20 (24.1%) | 29 (24.0%) | |||||
| III | 11 (27.5%) | 18 (36.0%) | 40 (37.4%) | 9 (26.5%) | 33 (39.8%) | 55 (45.5%) | |||||
| IV | 0 (0.0%) | 0 (0.0%) | 4 (3.7%) | 4 (11.8%) | 13 (15.7%) | 12 (9.9%) | |||||
| Unable to assess | 0 (0.0%) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||||
Unclassified data were removed before the chi–square test or Fisher's exact test was applied.
a4 unknown primaries.
b13 unknown primaries.
c19 unknown primaries.
dBrain metastasis as isolated first visceral site vs visceral metastasis: extracranial only.
eBrain metastasis as isolated first visceral site vs visceral metastasis: extracranial only or extracranial and brain.
fCombined Fisher P value for comparing patients with brain metastasis as isolated first visceral site and visceral metastasis: extracranial only or extracranial and brain.
gAnalyzed as a continuous variable using the Wilcoxon–Mann–Whitney test.
hCompared using the chi–square test.
iCompared using Fisher's exact test.
Patients who developed brain metastasis as the isolated first visceral site were then compared with patients with visceral metastasis: extracranial only combined with patients who had progressed to visceral metastasis: extracranial and brain at the time of brain metastasis diagnosis. There were no significant differences in clinicopathological characteristics between patients with visceral metastasis: extracranial only and patients with visceral metastasis: extracranial and brain (P > .05). Significant differences in primary tumor thickness and the distribution of AJCC stage at pathological diagnosis were shown on univariate analysis of patients with brain metastasis as the isolated first site of visceral metastasis and patients with visceral metastasis: extracranial only or extracranial and brain in Cohort 1 (P = .02, .03, respectively). Patients who progressed to brain metastasis as the isolated first visceral site had thinner tumors, and a greater percentage presented with stage I disease at initial melanoma diagnosis. Differences in both tumor thickness and stage distribution trended in the same direction in the comparison within Cohort 2 patients, and combined Fisher P values for primary tumor thickness, distribution of clinical stage at melanoma diagnosis, and primary tumor mitosis were statistically significant (P = .02, .02, .05, respectively). In the comparison of patients with brain metastasis as the isolated first visceral site and patients with visceral metastasis: extracranial and brain at the time of brain metastasis diagnosis, the combined Fisher P values for the same clinicopathological features did not reach statistical significance, but differences trended in the same direction (P = .09, .15, .22, respectively) (Supplementary material, Table S1).
Brain Metastasis as the Isolated First Site of Visceral Metastasis Is a Distinct Isolated Visceral Metastasis Phenotype
To determine whether brain metastasis as the isolated first site of visceral metastasis is a distinct phenotype of site-specific visceral metastasis, the clinicopathological features of these brain metastasis patients were compared with those of patients who developed other isolated first sites of visceral metastasis, namely the lung (n= 143), liver (n = 34), and bone (n= 39) (Table 6). These 4 groups differed in primary tumor thickness, mitosis, and distribution of stage at pathological diagnosis (P = .04, .06, .03, respectively), but there was no significant difference in any of the characteristics examined between the groups with varying isolated extracranial sites at visceral metastasis diagnosis (P > .05). In comparing patients with brain metastasis versus lung metastasis as the isolated first site of visceral metastasis, significant differences were found in primary tumor thickness, mitosis, and distribution of clinical stage at presentation of melanoma (P= .01, .013, .008, respectively). Patients who progressed to brain metastasis as the isolated first visceral site had thinner primaries (median, 1.75 vs 2.70 mm) and a higher rate of melanomas with no mitosis (24.3% vs 9.8%), and 39.2% (29/74) of these patients presented at initial melanoma diagnosis with stage I disease compared with 17.5% (25/143) of patients with lung metastasis as the isolated first site of visceral metastasis. Patients with brain metastasis as the isolated first site of visceral metastasis were then compared with patients with other site–specific visceral metastasis combined. Differences in primary tumor thickness, mitosis, and distribution of AJCC stage at pathological diagnosis were statistically significant and in the same direction as the comparison of brain metastasis versus lung metastasis as the isolated first visceral site (P= .005, .006, .006, respectively).
Table 6.
Comparison of melanoma patients with varying isolated first sites of visceral metastasis
| Characteristic | Brain Metastasis as Isolated First Visceral Site | Lung Metastasis as Isolated First Visceral Site | Liver Metastasis as Isolated First Visceral Site | Bone Metastasis as Isolated First Visceral Site | P1e | P2f | P3g |
|---|---|---|---|---|---|---|---|
| (n = 74)a | (n = 143)b | (n = 34)c | (n = 39)d | ||||
| n (%) | n (%) | n (%) | n (%) | ||||
| Primary tumor thickness (mm) | .04h | .01h | .005h | ||||
| Median (range) | 1.75 (0.50–30) | 2.70 (0.32–30) | 3.70 (0.50–13) | 2.94 (0.45–28) | |||
| Primary tumor mitosis | .06i | .013j | .006j | ||||
| Absent | 17 (24.3%) | 13 (9.8%) | 3 (9.1%) | 4 (11.1%) | |||
| Present | 49 (70.0%) | 101 (75.9%) | 27 (81.8%) | 28 (77.8%) | |||
| Unclassified | 4 (5.7%) | 19 (14.3%) | 3 (9.1%) | 4 (11.1%) | |||
| AJCC stage at pathological diagnosis | .03i | .008i | .006i | ||||
| I | 29 (39.2%) | 25 (17.5%) | 7 (20.6%) | 8 (20.5%) | |||
| II | 21 (28.4%) | 53 (37.1%) | 7 (20.6%) | 12 (30.8%) | |||
| III | 20 (27.0%) | 55 (38.5%) | 18 (52.9%) | 14 (35.9%) | |||
| IV | 4 (5.4%) | 9 (6.3%) | 2 (5.9%) | 5 (12.8%) | |||
| Unable to assess | 0 (0.0%) | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) |
Unclassified data were removed before the chi–square test or Fisher's exact test was applied.
a4 unknown primaries.
b10 unknown primaries.
c1 unknown primary.
d3 unknown primaries.
eComparing all 4 groups.
fBrain metastasis as isolated first visceral site vs lung metastasis as isolated first visceral site.
gBrain metastasis as isolated first visceral site vs other isolated first visceral sites combined (lung, liver, bone).
hAnalyzed as a continuous variable using the Wilcoxon–Mann–Whitney test or Kruskal–Wallis test as appropriate.
iCompared using Fisher's exact test.
jCompared using the chi–square test.
Post–Visceral Metastasis Survival Is Shorter in Patients who Developed Brain Metastasis as Isolated First Visceral Site Compared with Other Brain Metastasis Patients
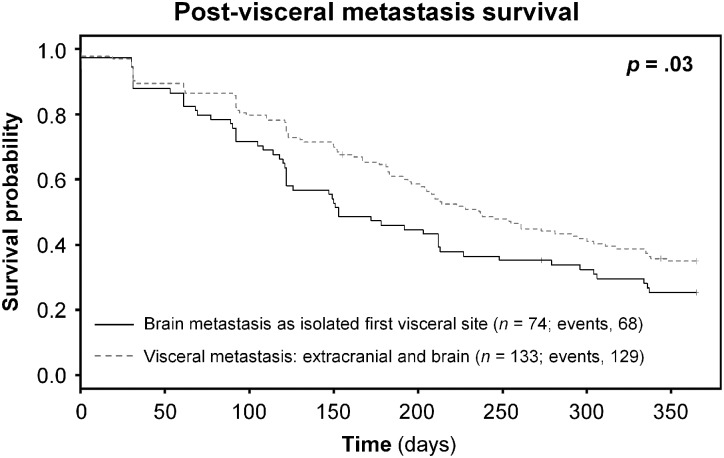
At the time of analysis, 197/207 (95.2%) brain metastasis patients from Cohorts 1 and 2 had died of melanoma: 68/74 (91.9%) with brain metastasis as the isolated first site of visceral metastasis, and 129/133 (97.0%) with visceral metastasis: extracranial and brain at the time of brain metastasis presentation. Patients who developed brain metastasis as isolated first visceral site were found to have significantly shorter post–visceral metastasis survival times compared with patients who progressed to brain metastasis concomitant with or subsequent to other visceral metastases; the Kaplan–Meier estimate of survival after visceral metastasis diagnosis for Cohorts 1 and 2 is shown in Fig. 1 (combined P = .03).
Fig. 1.
Survival after visceral metastasis diagnosis in patients with brain metastasis as isolated first visceral site compared with patients with visceral metastasis: extracranial and brain at brain metastasis diagnosis
Discussion
To our knowledge, this is the first study to distinguish melanoma patients who develop brain metastasis as the isolated first visceral site from other brain metastasis patients, to characterize different brain metastasis patterns based on clinicopathological features present at initial melanoma diagnosis, and to examine the potential clinical implications of these differences. Melanoma patients who develop brain metastasis as the isolated first visceral site were hypothesized to have distinct clinicopathological features present at primary melanoma diagnosis, and our findings support this hypothesis, which represents a major departure from the current understanding of brain metastasis as the final stage of tumor progression.5,6 Only 2 studies have focused on brain metastasis as the isolated first site of visceral metastasis, one in breast cancer12 and the other in ovarian cancer.13 However, limiting the generalizability of the findings of these 2 studies is their small sample size and the lack of a population–based cohort, in contrast to our study of over 2000 primary melanoma patients who presented with early– or late–stage melanoma at initial melanoma diagnosis.
Some considerations need to be addressed prior to interpreting our data. Melanoma patients diagnosed in the 1970s and 1980s were included, many of whom were treated before the first AJCC melanoma staging system was established in 1978. To address this issue, all patients were restaged according to the 2009 AJCC guidelines27 such that the homogeneity of clinicopathological parameters between the 2 cohorts would improve. Advances in the clinical management of melanoma, however, have inevitably impacted the diagnosis and treatment of patients in Cohort 2, particularly as they relate to the detection of brain metastasis on CT/MRI. Yet, even with advanced imaging techniques available, over a third of melanoma brain metastasis patients are symptomatic at presentation,30 an estimate based on the incidence of symptomatic brain metastasis in general and not by brain metastasis phenotype. The diagnosis of brain metastasis is nonetheless made clinically as in the pre–CT/MRI era with or without confirmatory brain imaging. The quoted incidence of symptomatic brain metastasis in the literature is consistent with the 36.1% of patients in Cohort 2 who not only progressed to visceral metastasis: extracranial and brain at the time of brain metastasis diagnosis but were also symptomatic. There was, however, an estimated 2–fold increase in the incidence of symptomatic brain metastasis in patients with brain metastasis as the isolated first visceral site—70.6% versus 36.1%—that may have introduced lead-time bias into the survival analysis performed. Patients who developed brain metastasis as the isolated first site of visceral metastasis from Cohorts 1 and 2 were combined in our post–visceral metastasis survival analysis as were patients with visceral metastasis: extracranial and brain at brain metastasis presentation, and as a result, we see that the majority of patients in either brain metastasis group were symptomatic at the time of brain metastasis diagnosis: 64/74 (86.5%) versus 80/133 (60.2%), respectively. Changes in adjuvant and metastatic treatment regimens over the last 4 decades are additional confounding factors in relation to progression to brain metastasis as the isolated first visceral site. Analyses were therefore stratified by database to assess whether our conclusions would differ as the standard of care changed and given the differences in data collected for Cohorts 1 and 2. Patient–to–patient treatment differences nevertheless remain even within each cohort, both of which were accrued at a single institution. Despite the potential limitations of even a large single–institution study, both early– and late–stage melanoma patients were represented, as NYU Medical Center has the largest dermatology department in the United States, the National Cancer Institute–designated NYU Cancer Institute, and a world–renowned neurological surgery department. With active research and clinical trials in both oncology and neurosurgery, NYU serves as an important tertiary referral center such that the effect of referral bias may be seen in our findings. Nonetheless, even with over 2000 cutaneous melanoma patients prospectively accrued at our institution over a 40–year period, the incidence of brain metastasis as the isolated first visceral site remained similar across the 2 study cohorts.
Similar to previous brain metastasis studies in which the comparison focused on brain metastasis versus non–brain metastasis patients, we first compared the clinicopathological features present at initial melanoma diagnosis of patients who developed brain metastasis as the isolated first visceral site versus all others. Melanoma patients who progressed to brain metastasis as the isolated first site of visceral metastasis had primaries that were thicker, ulcerated, and of the nodular histologic subtype, all consistent with the observation that these patients also presented with more advanced disease at pathological diagnosis and with increased risk for this particular brain metastasis phenotype after taking into account competing risks. These primary tumor characteristics, however, may reflect the association with metastatic potential in general rather than with the specific metastatic phenotype of brain metastasis as the isolated first visceral site because the comparison group consisted predominantly of patients who had not progressed in the competing–risks analyses. To address this potential confounding factor, subsequent analyses included only metastatic melanoma patients. Because our population–based cohort exceeded 2000 patients, it allowed for the comparison of patients who developed brain metastasis as the isolated first visceral site with multiple subgroups of metastatic melanoma patients – visceral metastasis: extracranial only, visceral metastasis: extracranial and brain at the time of brain metastasis diagnosis, visceral metastasis: extracranial only or extracranial and brain, lung metastasis as isolated first visceral site, liver metastasis as isolated first visceral site, bone metastasis as isolated first visceral site, and other isolated first sites of visceral metastasis combined. Of the 2 previous studies on brain metastasis as the isolated first site of visceral metastasis, only the one in ovarian cancer attempted to compare patients with this brain metastasis phenotype with all other metastatic patients.13 The comparison, however, fell short because it was unbalanced, with only 8 of the 150 patients in the study progressing to brain metastasis via this pattern, and these 8 patients were then compared with 142 patients who developed extracranial metastases with no mention of whether or not this included distant metastases in skin, lymph node, and/or viscera. There is clinical utility in examining the differences in the clinicopathological features of patients who progress to brain metastasis as the isolated first visceral site compared with those in other metastatic subgroups, which are revealed in our study.
Morbidity and mortality incurred from a delayed diagnosis of melanoma brain metastasis may be mitigated by identifying brain metastasis earlier on routine radiologic imaging. It remains to be determined, however, whether follow–up imaging would result in overall survival benefit in all melanoma patients who develop brain metastasis given the variable temporal course to brain metastasis and its potential biological implications. Our data show that a greater proportion of patients who developed brain metastasis as isolated first visceral site had primary tumors with no mitosis. The kinetics of melanoma brain metastasis development in patients who progress to brain metastasis as the isolated first visceral site may be particularly suitable for serial radiologic follow–up given the slow proliferative nature of their primaries.
Therapeutic implications of the different melanoma brain metastasis phenotypes identified and characterized for the first time in this study arise as well. Melanoma patients who develop brain metastasis as the isolated first site of visceral metastasis reveal some limitations of the current AJCC staging system with potentially relevant ramifications for adjuvant treatment. Their primaries are thinner compared with those of all other visceral metastases patients, and as thickness remains the single most important prognostic feature of primary melanomas, this finding is reflected in the equally unexpected staging distribution of these patients. A greater percentage of this particular subset of brain metastasis patients presented with stage I disease at initial melanoma diagnosis compared with the group with visceral metastasis: extracranial only or extracranial and brain. Although adjuvant therapy is currently not recommended for stage I patients, our data show that there is a subgroup of stage I melanoma patients who do progress to brain metastasis, in particular brain metastasis as the isolated first visceral site, yet forgo adjuvant treatment. It is therefore evident from our study that melanoma patients who have not received adjuvant therapy still progress to brain metastasis as the isolated first visceral site. This is in contrast to the consensus in previous reports of brain metastasis as the isolated first visceral site in which this phenotype is thought to be consistent with progression to brain metastasis as a consequence of prolonged survival following adjuvant therapy.9–18 It would be of great interest to develop models to identify these early–stage patients who are at high risk of developing brain metastasis. We have observed that patients in this subgroup tend to have more primaries arising on nonchronic sun–damaged skin as evidenced by the absence of solar elastosis (data not shown), melanomas previously shown to have a higher frequency of BRAF mutations. Given that BRAF inhibitors such as vemurafenib and GSK2118436 have already demonstrated efficacy against some melanoma brain metastases,31,32 it is logical that their clinical utility in the adjuvant setting be explored, especially in the prevention of brain metastasis as the isolated first visceral site because patients with this brain metastasis phenotype have a shorter post–visceral metastasis survival compared to other brain metastasis patients.
In conclusion, our study is the first to demonstrate that the clinicopathological profile of patients who progress to brain metastasis as the isolated first site of visceral metastasis is distinct from that of all other visceral metastases patients. Risk assessment models are therefore needed to identify those primary melanoma patients at risk for developing brain metastasis as isolated first visceral site. All future clinical trials assessing the efficacy and/or survival benefits of new brain metastasis treatments and routine brain imaging will also need to treat melanoma brain metastasis not as a single clinicopathological entity, but as a clinical state with real phenotypic and possibly genotypic and etiologic heterogeneity.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online (http://neuro-oncology.oxfordjournals.org/).
Funding
This work was supported by the Department of Defense Peer Reviewed Cancer Research Program (W81XWH-10-1-0804); the NYU Cancer Institute Cancer Center Support Grant (5P30CA016087); and the Marc Jacobs Campaign to support melanoma research.
Supplementary Material
Acknowledgments
This work was presented in part at the Annual Meeting of the American Society of Clinical Oncology, June 3–7, 2011, Chicago, IL and at Perspectives in Melanoma XV, September 16–17, 2011, New York, NY.
Conflict of interest statement. None declared.
References
- 1.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–736. doi: 10.1093/jnci/djr077. doi:10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. doi:10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain MC. Brain metastases: a medical neuro-oncology perspective. Expert Rev Neurother. 2010;10(4):563–573. doi: 10.1586/ern.10.30. doi:10.1586/ern.10.30. [DOI] [PubMed] [Google Scholar]
- 4.Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16(3):248–255. doi: 10.1177/107327480901600307. [DOI] [PubMed] [Google Scholar]
- 5.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res. 2007;13(6):1644–1647. doi: 10.1158/1078-0432.CCR-07-0096. doi:10.1158/1078-0432.CCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 6.Deeken JF, Löscher W. The blood–brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. doi:10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Muñoz W, Kerbel RS. Preclinical approaches to study the biology and treatment of brain metastases. Semin Cancer Biol. 2011;21(2):123–130. doi: 10.1016/j.semcancer.2010.12.001. doi:10.1016/j.semcancer.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. doi: 10.1016/j.ijrobp.2009.08.025. doi:10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar LE, Chansky K, Albain KS, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol. 2005;23(13):2955–2961. doi: 10.1200/JCO.2005.08.026. doi:10.1200/JCO.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT) Ann Oncol. 2010;21(5):942–948. doi: 10.1093/annonc/mdp407. doi:10.1093/annonc/mdp407. [DOI] [PubMed] [Google Scholar]
- 11.Carey LA, Ewend MG, Metzger R, et al. Central nervous system metastases in women after multimodality therapy for high risk breast cancer. Breast Cancer Res Treat. 2004;88(3):273–280. doi: 10.1007/s10549-004-0999-3. doi:10.1007/s10549-004-0999-3. [DOI] [PubMed] [Google Scholar]
- 12.Boogerd W, Hart AA, Tjahja IS. Treatment and outcome of brain metastasis as first site of distant metastasis from breast cancer. J Neurooncol. 1997;35(2):161–167. doi: 10.1023/a:1005818323996. doi:10.1023/A:1005818323996. [DOI] [PubMed] [Google Scholar]
- 13.Kastritis E, Efstathiou E, Gika D, et al. Brain metastases as isolated site of relapse in patients with epithelial ovarian cancer previously treated with platinum and paclitaxel-based chemotherapy. Int J Gynecol Cancer. 2006;16(3):994–999. doi: 10.1111/j.1525-1438.2006.00596.x. doi:10.1111/j.1525-1438.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 14.Choo BA, Walji N, Spooner D, Barber P, Fernando IN. Prolonged relapse-free survival in two patients with an isolated brain metastasis from epithelial ovarian carcinoma. J Clin Oncol. 2010;28(17):e271–e272. doi: 10.1200/JCO.2009.26.4168. http://jco.ascopubs.org/content/28/17/e271.full.pdf . Accessed February 8, 2012 doi:10.1200/JCO.2009.26.4168. [DOI] [PubMed] [Google Scholar]
- 15.Shuch B, La Rochelle JC, Klatte T, et al. Brain metastasis from renal cell carcinoma: presentation, recurrence, and survival. Cancer. 2008;113(7):1641–1648. doi: 10.1002/cncr.23769. doi:10.1002/cncr.23769. [DOI] [PubMed] [Google Scholar]
- 16.Cohn DA, Stuart-Harris R. Isolated central nervous system relapse of non-seminomatous germ cell tumour of the testis. A case report and review of the literature. Oncology. 2001;61(3):184–188. doi: 10.1159/000055372. doi:10.1159/000055372. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Igarashi K, Tanizawa A, Terada Y, Sekine H. Brain metastasis as a sole recurrence of prostate cancer after total prostatectomy. Urol Int. 1998;60(2):121–123. doi: 10.1159/000030225. doi:10.1159/000030225. [DOI] [PubMed] [Google Scholar]
- 18.Arepally G, Kenyon LC, Lavi E. Late onset of isolated central nervous system metastasis of liposarcoma—a case report. Am J Clin Oncol. 1996;19(4):351–355. doi: 10.1097/00000421-199608000-00006. doi:10.1097/00000421-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42(2):660–668. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. doi:10.1002/1097-0142(197808)42:2<660::AID-CNCR2820420237>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Cohn-Cedermark G, Månsson-Brahme E, Rutqvist LE, Larsson O, Johansson H, Ringborg U. Central nervous system metastases of cutaneous malignant melanoma—a population-based study. Acta Oncol. 1998;37(5):463–470. doi: 10.1080/028418698430412. doi:10.1080/028418698430412. [DOI] [PubMed] [Google Scholar]
- 21.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. doi:10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 22.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–1300. doi: 10.1200/JCO.2004.08.140. doi:10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 23.Staudt M, Lasithiotakis K, Leiter U, et al. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer. 2010;102(8):1213–1218. doi: 10.1038/sj.bjc.6605622. doi:10.1038/sj.bjc.6605622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zakrzewski J, Geraghty LN, Rose AE, et al. Clinical variables and primary tumor characteristics predictive of the development of melanoma brain metastases and post-brain metastases survival. Cancer. 2011;117(8):1711–1720. doi: 10.1002/cncr.25643. doi:10.1002/cncr.25643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopf AW, Gross DF, Rogers GS, et al. Prognostic index for malignant melanoma. Cancer. 1987;59(6):1236–1241. doi: 10.1002/1097-0142(19870315)59:6<1236::aid-cncr2820590634>3.0.co;2-i. doi:10.1002/1097-0142(19870315)59:6<1236::AID-CNCR2820590634>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Wich LG, Hamilton HK, Shapiro RL, et al. Developing a multidisciplinary prospective melanoma biospecimen repository to advance translational research. Am J Transl Res. 2009;1(1):35–43. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2776290/?tool=pubmed . Accessed February 8, 2012. [PMC free article] [PubMed] [Google Scholar]
- 27.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. doi:10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. doi:10.1214/aos/1176350951. [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.2307/2670170. [Google Scholar]
- 30.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. doi:10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 31.Dummer R, Rinderknecht J, Goldinger SM, et al. An open-label pilot study of vemurafenib in previously treated metastatic melanoma patients with brain metastases [abstract] J Clin Oncol. 2011;29(15) (suppl-May 20, 2011):abstr 8548. [Google Scholar]
- 32.Long GV, Kefford RF, Carr PJA, et al. Phase 1/2 study of GSK2118436, a selective inhibitor of V600 mutant (mut) BRAF kinase: evidence of activity in melanoma brain metastases (mets) [abstract] Ann Oncol. 2010;21(suppl 8):viii12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.