Abstract
Medulloblastomas account for 20% of pediatric brain tumors. With an overall survival of 40%–70%, their treatment is still a challenge. The majority of medulloblastomas lack p53 mutations, but even in cancers retaining wild-type p53, the tumor surveillance function of p53 is inhibited by the oncoprotein MDM2. Deregulation of the MDM2/p53 balance leads to malignant transformation. Here, we analyzed MDM2 mRNA and protein expression in primary medulloblastomas and normal cerebellum and assessed the mutational status of p53 and MDM2 expression in 6 medulloblastoma cell lines. MDM2 expression was elevated in medulloblastomas, compared with cerebellum. Four of 6 medulloblastoma cell lines expressed wild-type p53 and high levels of MDM2. The tumor-promoting p53-MDM2 interaction can be inhibited by the small molecule, nutlin-3, restoring p53 function. Targeting the p53-MDM2 axis using nutlin-3 significantly reduced cell viability and induced either cell cycle arrest or apoptosis and expression of the p53 target gene p21 in these 4 cell lines. In contrast, DAOY and UW-228 cells harboring TP53 mutations were almost unaffected by nutlin-3 treatment. MDM2 knockdown in medulloblastoma cells by siRNA mimicked nutlin-3 treatment, whereas expression of dominant negative p53 abrogated nutlin-3 effects. Oral nutlin-3 treatment of mice with established medulloblastoma xenografts inhibited tumor growth and significantly increased survival. Thus, nutlin-3 reduced medulloblastoma cell viability in vitro and in vivo by re-activating p53 function. We suggest that inhibition of the MDM2-p53 interaction with nutlin-3 is a promising therapeutic option for medulloblastomas with functional p53 that should be further evaluated in clinical trials.
Keywords: MDM2, medulloblastoma, nutlin-3, p21, wild-type p53
Medulloblastoma is the most common malignant brain tumor of childhood.1 With an overall survival of 40%–70%, despite multimodal therapy approaches (surgery, radiation, and chemotherapy), medulloblastoma still represents a major clinical challenge in pediatric oncology. Long-term sequelae due to the aggressive treatment regimens also demand the development of targeted approaches with fewer adverse effects. This aspect is also of importance for patients with relapsed medulloblastoma, who are in urgent need of additional effective therapies.
A major factor contributing to treatment failure in refractory or recurrent medulloblastoma is primary or secondary resistance to chemotherapy, in which dysfunction of the p53 pathway plays a key role. Functional p53 is needed to induce multiple anti-proliferative processes, including cell cycle arrest and apoptosis, thereby effectively limiting the survival of potentially pre-neoplastic cells. Indeed, loss of p53 facilitates medulloblastoma development in mouse models,2–4 and up to 40% of medulloblastomas show p53 protein expression indicative of a dysfunctional p53 pathway.5 An important role for p53 inactivation in medulloblastoma pathogenesis is also suggested by the increased incidence of medulloblastomas in patients with Li Fraumeni syndrome, which is caused by a germ-line mutation in p53.6
Dysfunction of p53 because of mutations in the TP53 tumor suppressor gene is rare in neuroectodermal embryonal tumors, including medulloblastomas.7,8 Fewer than 10% of sporadic medulloblastomas display TP53 mutations,9,10 which are associated with adverse outcome in pediatric patients.11,12 The mechanism underlying the inactivation of the p53 pathway in the majority of medulloblastomas and other brain tumors has remained unclear for many years. It has been more recently observed in other cancers with wild-type p53 that p53 inactivation can be achieved by different alternative routes.7 One of these alternative routes of potential clinical significance for medulloblastoma is the rapid proteasomal degradation of p53 mediated by direct interaction of p53 with the E3 ubiquitin ligase, MDM2, which is promoted by the ubiquitination factor E4B.13,14 Amplification or overexpression of MDM2, leading to increased degradation of p53, is frequently observed in tumors with wild-type TP53.15 Although MDM2 gene amplification occurs in approximately 7% of human malignancies that lack TP53 mutations,14 it has not been detected in medulloblastomas.16,17 However, MDM2 protein overexpression has been observed at least in a subset of adult medulloblastomas.15 More significantly, the discovery that the loss of MDM2 in Ptch1+/− mice, a model for sonic hedgehog–mediated human medulloblastoma, impedes cerebellar tumorigenesis adds substantial evidence to the hypothesis of an important role for MDM2 in medulloblastoma pathogenesis.18
On the basis of the existing data, functional reactivation of p53 and/or inhibition of the p53/MDM2 axis in medulloblastoma are widely considered to be promising therapeutic options for this most common brain tumor in children. As early as 1995, Rosenfeld et al. successfully restored p53 function in medulloblastoma with use of wild-type TP53 gene transfer.19 However, this initial approach was too complex for transfer to the clinic. No other therapeutic attempts for p53 reactivation in medulloblastoma have been described to date.
The biological and clinical importance of p53 function in medulloblastoma and other tumors have motivated the quest for inhibitors of the MDM2-p53 interaction to restore p53 function in tumors with wild-type p53. One of the first inhibitors identified was nutlin-3, which binds tightly and selectively to the p53-interaction domain of MDM2. Nutlin-3 competitively blocks the interaction of MDM2 and p53, thereby preventing ubiquitination and degradation of p53. Treatment with nutlin-3 has been shown to restore p53 activation and subsequent induction of apoptosis, senescence, or reversible cell cycle arrest in various model systems.20,21 Thus, restoration of p53 function using nutlins could open new avenues for the successful treatment of tumors that have retained wild-type p53 in the presence of high MDM2 activity. To provide proof-of-principle that the p53-MDM2 interaction is therapeutically useful in medulloblastoma, we analyzed the potential effect of nutlin-3 on medulloblastoma cells grown in cell culture models and as xenografts in nude mice.
Materials and Methods
Cell Lines and Nutlin-3 Treatment
The human medulloblastoma cell lines, DAOY, HD-MB3, ONS-76, UW-228, and D-341, were grown in RPMI 1640 supplemented with 10% FCS, L-glutamine, and antibiotics. Medium for cells used for xenografting into mice was also supplemented with 1% NEAA. D-283 cells were cultured in Eagle's Minimum Essential Medium supplemented with 10% FCS and antibiotics. All cell lines were authenticated by STR DNA typing by the DSMZ (Braunschweig, Germany) prior to experiments. The HD-MB3 cell line was a gift from H. Deubzer, who generated this cell line in the DKFZ (Heidelberg, Germany). Nutlin-3 (Cayman Chemical Europe) was dissolved in ethanol and stored as a 10 mmol/L stock solution in small aliquots at −20°C. Cells were exposed to 0–32 µmol/L nutlin-3 for the period indicated, with the final ethanol concentration kept constant in each experiment. For rescue experiments, ONS-76 cells (p53 wt) were electroporated with a plasmid encoding dominant negative p53 (donated by Prof. M. Eilers) containing the Val135 p53 mutation or EGFP as a control. Selection of transfected cells was achieved by addition of puromycin (1 g/ml) to the medium.
TP53 Mutation, Global Genomic, and Expression Analysis
Mutation analysis of TP53 was done according to established protocols in the laboratory of H.-P. Seelig (Karlsruhe, Germany). For array comparative genome hybridization (array CGH), samples were profiled on 60K or 180K human whole-genome oligoarrays (Agilent Technologies). Data were uploaded to a publicly available database (http://medgen.ugent.be/arraycghbase) and analyzed as previously described.22,23 MDM2 and p21 gene expression was monitored using real-time polymerase chain reaction (PCR) using Assays on Demand (Applied Biosystems). Expression values were normalized to the geometric mean of GAPDH.24 For all these experiments, total RNA was isolated from cells with use of the RNeasyMini kit (Qiagen) and cDNA synthesis was performed using the SuperScript reverse transcription kit (Invitrogen).
Western Blot Analysis
Protein lysates were extracted from cells treated with 4 µmol/L nutlin-3 for 24, 48, or 72 h and blotted as described in Kahl et al.25 Transfer membranes were incubated for 1–12 h using the following antibodies and dilutions: p21 (polyclonal, Abcam), 1:500; p53 (clone 2B2.68, Santa Cruz), 1:500; MDM2 (clone IF2, Zymed Laboratories), 1:800; β-actin (Sigma-Aldrich), and 1:2000.
Cell Proliferation, Viability, and Cycle Analysis
Cells were seeded onto 96-well plates (2 × 10³ per well) in triplicate, incubated for 6 h to permit surface adherence, and treated with 0–32 µmol/L nutlin-3 for 24, 48, or 72 h. Medium was replaced daily, and nutlin-3 and ethanol concentrations were constant throughout the experiment. Cell viability was analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche), performed according to the manufacturer's protocol. The IC50 was calculated using GraphPad Prism 5.0 (GraphPad Incorporation). For analysis of cell cycle distribution, cell lines were cultured in the presence of 4 µmol/L nutlin-3 or an equivalent amount of ethanol for 24, 48, or 72 h; trypsinized; washed with PBS; and incubated with propidium iodide for 15 min to stain DNA. Cellular DNA content was analyzed in an FC500 flow cytometer (Beckman Coulter). Apoptosis was assessed using the Cell Death enzyme-linked immunosorbent assay (ELISA; Roche), cell proliferation was assayed using the BrdU ELISA (Roche), and both were performed according to the manufacturer's protocols. For both assays, cells were seeded onto 96-well plates (7 × 10³ per well) in duplicate, incubated for 6 h to permit surface adherence, and treated with 4 µmol/L nutlin-3 for 24, 48, or 72 h. Medium was replaced daily, and nutlin-3 concentration was constant throughout the experiment. To assess cellular survival in real time, HD-MB3 cells (7 × 10³ per well) were plated onto 96-well Xcelligence microelectronic cell sensor plates (Roche) in triplicate and cultured overnight in antibiotic-free complete media. Cells were transiently transfected with siRNAs directed against MDM2 (QIAGEN, Cat. No. SI02653392 and SI00300846) or control siRNA at a final concentration of 20 pmol, and adherence was continuously monitored for 144 h to assess cellular survival. All experiments were independently performed at least 3 times, if not otherwise indicated.
Immunohistochemistry
Tissue microarrays (TMA) were prepared from paraffin-embedded tissue specimens of 86 primary medulloblastomas and 10 cerebellum samples selected from archived files in the Department of Pediatric Oncology and Hematology, University Children's Hospital, Zurich, Switzerland. Three different tissue cores in a single tumor were arrayed from formalin-fixed, paraffin-embedded tissue blocks using a manual device (Beecher Instruments). Two micrometer paraffin sections were cut from every tissue microarray and used for subsequent immunohistochemical analyses. In addition, xenografted tumors harvested from nu/nu mice treated either with nutlin-3 or with control were paraffin embedded, and 5 µm sections were cut from each block. For both TMA and xenograft tumors, immunohistochemical staining was conducted as previously described.25 In brief, formalin-fixed paraffin-embedded tissue sections were deparaffinized by routine techniques and placed in 200 mL of target retrieval solution (pH, 6.0; Envision Plus Detection Kit; Dako) for 20 min at 100°C. After cooling for 20 min, slides were quenched with 3% H2O2 for 5 min before incubating with primary antibody using a Dako Autostainer (Dako Cytomation). Primary antibodies used were: p21 (1:25, Cytomed), p53 (1:250, DAKO), MDM2 (1:100, Invitrogen), cleaved caspase 3 (1:200, Cell Signaling), and Mib-1 (1:25, DAKO). Slides were developed with EnVisionTM (Dako). Nuclear immunostaining for p53 and MDM2 was evaluated on the TMA with use of a semiquantitative scoring system by 2 independent researchers. In brief, the number and intensity of positive cells were counted and scored between 0 and 3 (0, no positive nuclei; 1, <20% nuclei display intense staining or more nuclei display weak staining; 2, 20%–80% intense staining or more nuclei display moderate staining; 3, 80%–100% nuclei display intense staining). Samples with a score of 2 or 3 were considered to be positive.
Growth of Xenograft Tumors in Nude Mice
HD-MB3 medulloblastoma cells were cultured to 80% confluency, harvested, and suspended in 200 µL Matrigel (BD Bioscience) for subcutaneous inoculation (1 × 107 cells per mouse, n= 10 mice) into the right flank of 4-week-old female athymic NCR (nu/nu) mice. Mice were randomly assigned to either nutlin-3 or vehicle control groups (n= 5 mice per group) after tumors reached 100–300 mm³ in size. Nutlin-3 or vehicle control was administered by oral gavage twice daily at a dose of 200 mg per kg body weight in 2% Klucel and 0.2% Tween-80 (both from Fagron). Tumor growth was monitored using a caliper, and tumor volume was calculated using the formula (breadth × length × height):2. Mice were euthanized by cervical dislocation when tumor size exceeded 1000 mm³. The tumor, liver, spleen, brain, and kidneys were removed, and half of each tissue was snap-frozen in liquid nitrogen then stored at −80°C, and the other half of tissue was formalin fixed and paraffin embedded. To examine the effects of nutlin-3 treatment on reactivation of the p53 pathway, nu/nu mice with established HD-MB3 xenografts were treated orally with 3 doses of nutlin-3 (200 mg per kg body weight) over a course of 24 h (doses were given at 0, 12, and 24 h; n= 2). Mice were euthanized by cervical dislocation 4 h after the last dose, and tumors were excised then snap frozen (half) and formalin fixed (half). All animal experiments were performed in accordance with the Council of Europe guidelines for accommodation and care of laboratory animals and protocols were approved by the Institutional Ethical Commission for Animal Experimentation.
Statistical Analysis
Re-analysis of microarrays data was performed using R2 (http://hgserver1.amc.nl/cgi-bin/r2/main.cgi). SPSS, version 18.0 (SPSS) was used for further statistical analysis. Student’s 2-sided t test was used for the comparison of all interval variables, and χ2 test was used for the comparison for all categorical variables. Graph Pad Prism 5.0 was used to calculate IC50 concentrations and to perform Kaplan Meier survival analysis with log rank statistics for the mouse cohorts.
Results
MDM2 mRNA and Protein Expression Is Elevated in Primary Medulloblastomas and Medulloblastoma Cell Lines
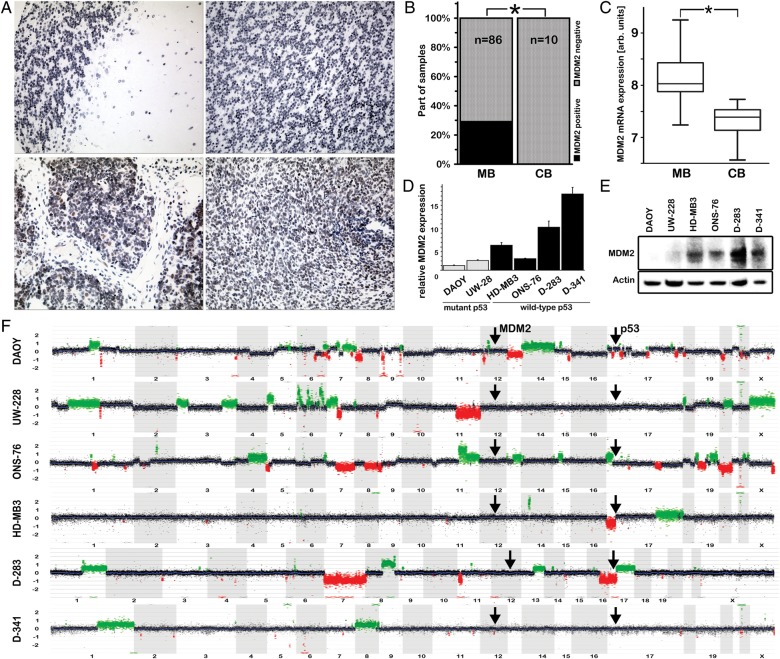
Inactivation of p53 via mutation is known to occur in only in a small percentage of primary medulloblastomas. MDM2 overexpression is an alternative mechanism to inactivate p53 function, which has not been investigated in pediatric medulloblastomas to date. We therefore analyzed MDM2 protein expression using immunohistochemistry on tissue arrays consisting of 86 primary medulloblastomas and 10 cerebellum samples, as a normal tissue control (Fig. 1A and B). MDM2 protein expression was detected in 25 of 86 primary medulloblastomas (29%), whereas no MDM2 protein expression was detected in normal cerebellum samples (P= .041). Kool, et al. previously published global mRNA expression profiles of 62 primary medulloblastomas and 9 samples of normal cerebellum performed on Affymetrix chips.26 We re-analyzed these data and detected significantly higher MDM2 mRNA expression levels in medulloblastomas, compared with cerebellum (P< .001) (Fig. 1C).
Fig. 1.
MDM2 expression is elevated in primary medulloblastomas and medulloblastoma cell lines with wild-type p53. (A and B) MDM2 expression was analyzed by immunohistochemistry in 86 medulloblastomas and 10 tissue samples of normal cerebellum on tissue arrays. MDM2 protein was expressed in medulloblastomas but not in normal cerebellum. (C) Re-analysis of published microarray data26 revealed that MDM2 mRNA expression was significantly higher in medulloblastomas, compared with cerebellum. (D and E) MDM2 mRNA and protein expression was higher in medulloblastoma cell lines with wild-type p53 in comparison with medulloblastoma cell lines with TP53 mutations. (F) MDM2 amplification was not detected in any of the 6 medulloblastoma cell lines using array CGH.
We then analyzed the mutational status of p53 in 6 established medulloblastoma cell lines and identified TP53 mutations in DAOY (C242F, previously reported27) and UW-228 (T155N) cells. HD-MB3 and ONS-76 medulloblastoma cells possess the R72P SNP in TP53, which affects neither p53 expression nor function. TP53 was not mutated in D-283 and D-341 medulloblastoma cells. Because mutant p53 is reported to accumulate at higher levels than wt-p53, we also analyzed p53 protein expression using immunohistochemistry on the tissue arrays described above (Supplementary material, Fig. S1). Expression of p53 protein was detectable in only 11 of 86 primary medulloblastomas (13%), whereas p53 protein expression was absent in all analyzed normal cerebellum samples. Of interest, MDM2 mRNA expression was higher in the 4 medulloblastoma cell lines (Fig. 1D), all of which expressed wild-type p53 but demonstrated p53 dysfunction indicated by low p21 expression. MDM2 protein expression was also higher in these 4 medulloblastoma cell lines without TP53 mutations, compared with the 2 with TP53 mutations (Fig. 1E). A frequent reason for elevated MDM2 mRNA expression in cancers is the amplification of the MDM2 gene.28 However, MDM2 amplification was not detected in any of the 6 medulloblastoma cell lines using array CGH (Fig. 1F and Supplementary material, Fig. S2). Taken together, we detected elevated MDM2 mRNA and protein expression in all medulloblastoma cell lines with wild-type p53 and in primary medulloblastomas. On the basis of these results and the published literature, we hypothesized that (1) elevated MDM2 expression is a potential mechanism for p53 inactivation in medulloblastoma cells with wild-type p53, which might ultimately lead to tumorigenesis, and (2) p53 tumor suppressor function could be restored in medulloblastoma cells by blocking the interaction between MDM2 and p53 with the small molecule, nutlin-3.
Nutlin-3 Reduces Viability of Medulloblastoma Cells with Wild-Type p53 In Vitro
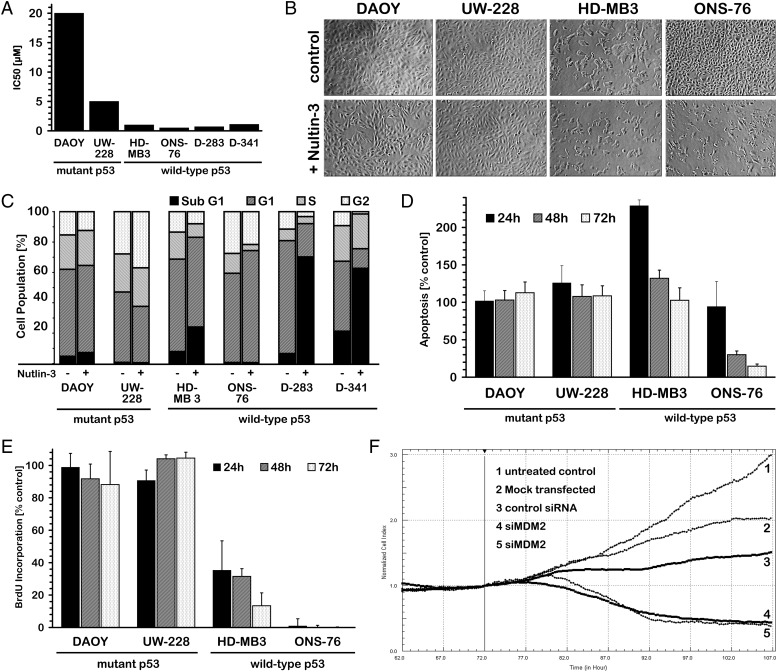
To test this hypothesis, we first assessed the effect of the small molecule, nutlin-3, in medulloblastoma cell lines with and without TP53 mutations. All 6 cell lines were treated with varying concentrations of nutlin-3 in vitro. Nutlin-3 treatment significantly reduced viability of the 4 medulloblastoma cell lines with wild-type p53 (D-283, D-341, HD-MB3, and ONS-76), whereas the DAOY and UW-228 cell lines harboring mutated p53 were remarkably less sensitive to nutlin-3 treatment, as indicated by the significantly higher IC50 values (Fig. 2A). We also observed that nutlin-3 treatment inhibited growth of HD-MB3 and ONS-76 cell lines with wild-type p53 but did not affect the growth of medulloblastoma cell lines with mutated p53 (Fig. 2B). This experiment was technically feasible only for the cell lines growing in monolayers, not for the D-283 and D-341 cell lines growing in suspension.
Fig. 2.
Nutlin-3 treatment reduces viability of wild-type p53 medulloblastoma cells similarly to MDM2 knockdown. (A and B) Sensitivity to nutlin-3 was much higher in medulloblastoma cell lines with functional p53, as is shown by their lower IC50 and their reduced growth under treatment with nutlin-3 in comparison with the medulloblastoma cell lines with TP53 mutation (DAOY, UW-228). (C–E) Proliferation was inhibited and the fraction of apoptotic cells increased after nutlin-3 treatment in cells with wild-type p53, but not in cells with mutated p53. This is shown by cell cycle analysis, cell death, and BrdU ELISA performed after treatment of 2 medulloblastoma cell lines with and 2 without TP53 mutations with nutlin-3 for 24, 48, and 72 h. (F) MDM2 knockdown in HD-MB3 cells harboring functional p53 using siRNA mimicked the effect of nutlin-3 treatment and inhibited cell growth.
FACS analysis revealed that nutlin-3 treatment increased the fraction of apoptotic cells, as indicated by the higher percentage of sub-G1 cells in 3 of 4 medulloblastoma cell lines with wild-type p53, but not in the 2 cell lines with mutant p53 (Fig. 2C). These results were also confirmed using a cell death ELISA that detected mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates (Fig. 2D). Proliferation of HD-MB3 and ONS-76, measured by a BrdU ELISA, was dramatically reduced after 72 h of nutlin-3 treatment, whereas proliferation of the p53 mutant cell lines, DAOY and UW-228, remained unaffected (Fig. 2E). Of interest, siRNA-mediated knockdown of MDM2 performed representatively in HD-MB3 cells mimicked the effect of nutlin-3 treatment and inhibited cell growth, whereas transfection of scrambled control siRNA or mock transfection did not affect cell growth (Fig. 2F). These results indicate an important role of MDM2 and its interaction with p53 for the viability and proliferation of medulloblastoma cells.
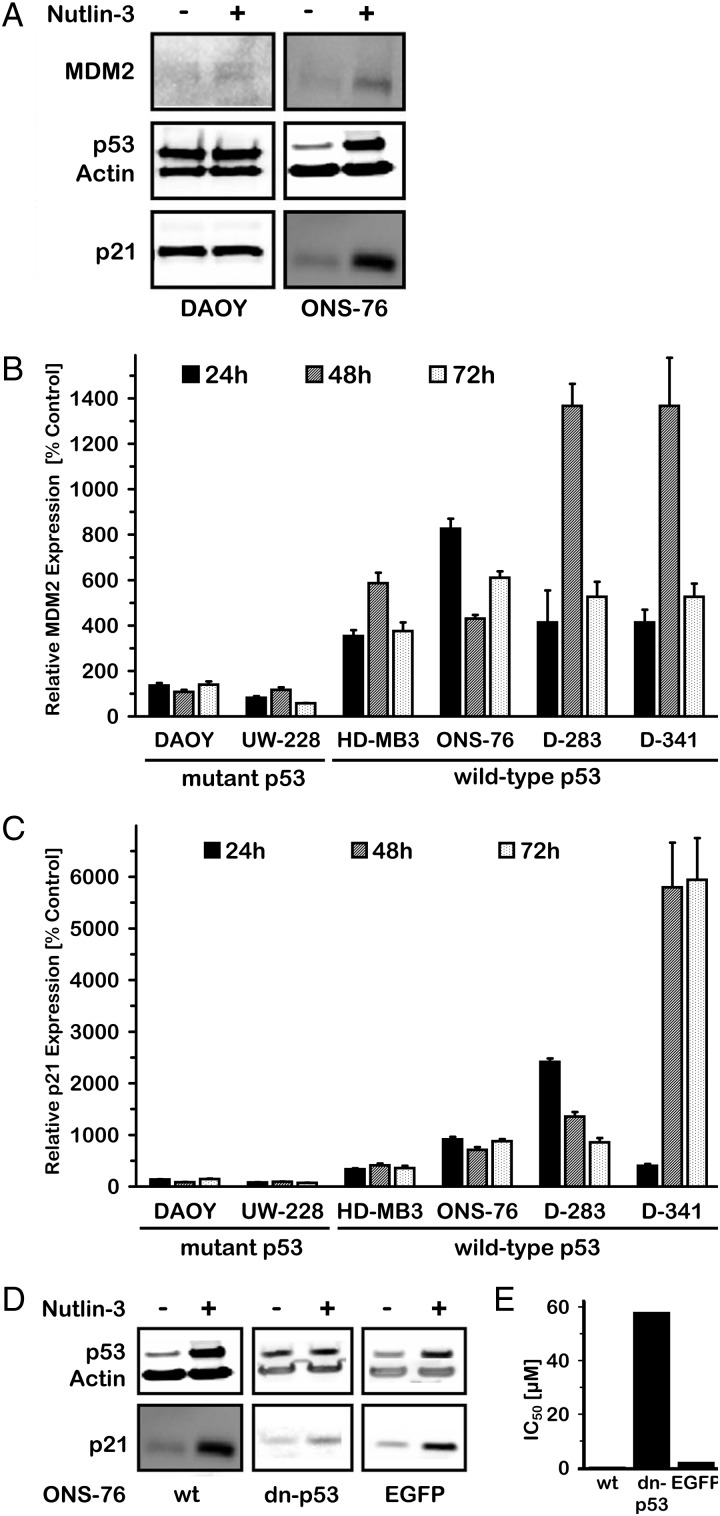
As further support for our hypothesis, we next investigated expression of p53 and its downstream target, p21, on both protein and mRNA levels to examine whether the p53 pathway is indeed dysfunctional or inhibited in medulloblastoma cells with wild-type p53 and whether it can be restored by nutlin-3 treatment. Baseline expression of p21 protein was very low in medulloblastoma cell lines with wild-type p53. Nutlin-3 treatment of these cells significantly elevated p21 and p53 protein expression in contrast to medulloblastoma cell lines without wild-type p53 (Fig. 3A and C). In real-time RT-PCR, mean p21 expression peaked at different times after the start of treatment (HD-MB3: 4.1-fold at 48 h, ONS-76: 9.2-fold at 24 h, D-283: 24.1-fold at 24 h, D-341: 59.4-fold for 72 h) but was significantly increased by nutlin-3 treatment in all cell lines expressing wild-type p53. Nutlin-3 treatment did not significantly affect p21 expression in DAOY or UW-288 cells with mutant p53. Nutlin-3 also significantly induced MDM2 expression on both mRNA and protein levels in medulloblastoma cells with functional p53 (Fig. 3A–C). Mean MDM2 mRNA expression peaked at different times after the start of treatment (HD-MB3: 5.9-fold at 48 h, ONS-76: 8.3-fold at 24 h, D-283: 9.9-fold at 72 h, D-341: 13.7-fold at 48 h). Nutlin-3 treatment had no significant effect on MDM2 expression in DAOY and UW-288 medulloblastoma cells with mutant p53. Taken together, these results indicate that nutlin-3 treatment leads to pharmacological activation of the p53 pathway, which induces cell cycle arrest and apoptosis in medulloblastoma cells with functional p53.
Fig. 3.
Treatment of medulloblastoma cells with nutlin-3 activated the p53 pathway in wild-type p53 cells. (A–C) Activation of the p53 pathway was shown by induction of p21 and MDM2 protein and mRNA in a medulloblastoma cell line that harbors wt TP53 (ONS-76) in comparison to a cell line with p53 mutated (DAOY). (D and E) Induction of p21 by nutlin-3 in p53-wt ONS-76 cells was abrogated by transfection with dominant negative p53 (dn-p53), but not by a control-transfected plasmid encoding GFP, as shown by Western blotting. Cell viability assays (MTT) confirmed that dn-p53 rescues ONS-76 from nutlin-3 induced cell death.
To show the critical role of functional p53 for nutlin-3 activity, we investigated whether expression of a dominant negative mutant of p53 (dn-p53) was able to abrogate nutlin-3 effects in medulloblastoma cells lines with wild-type p53. Stable, ectopic expression of dn-p53 in the ONS-76 cell line as a representative example increased basal levels of the p53 protein. However, nutlin-3 treatment of dn-p53 expressing ONS-76 cells did not significantly induce p21 protein expression, indicating that dn-p53 inactivated intrinsic p53. Induction of p21 was achieved in parental ONS-76 cells or ONS-76 cells expressing GFP from the same vector used to express the dn-p53 construct (Fig. 3D). Furthermore, the sensitivity to nutlin-3 was significantly reduced in ONS-76 cells expressing dn-p53 (IC50: ONS-76 dn-p53 = 58.2 µM vs, ONS-76 = 0.5 µM, ONS-76 EGFP = 2.3 µM, Fig. 3E). Expression of dominant negative mutant p53 blocked nutlin-3 action and further supports that the effect of nutlin-3 is based on reactivation of functional p53 via blocking the p53-MDM2 interaction.
Nutlin-3 has Antitumoral Activity Against Human Medulloblastoma Cells Grown as Xenografts in Mice
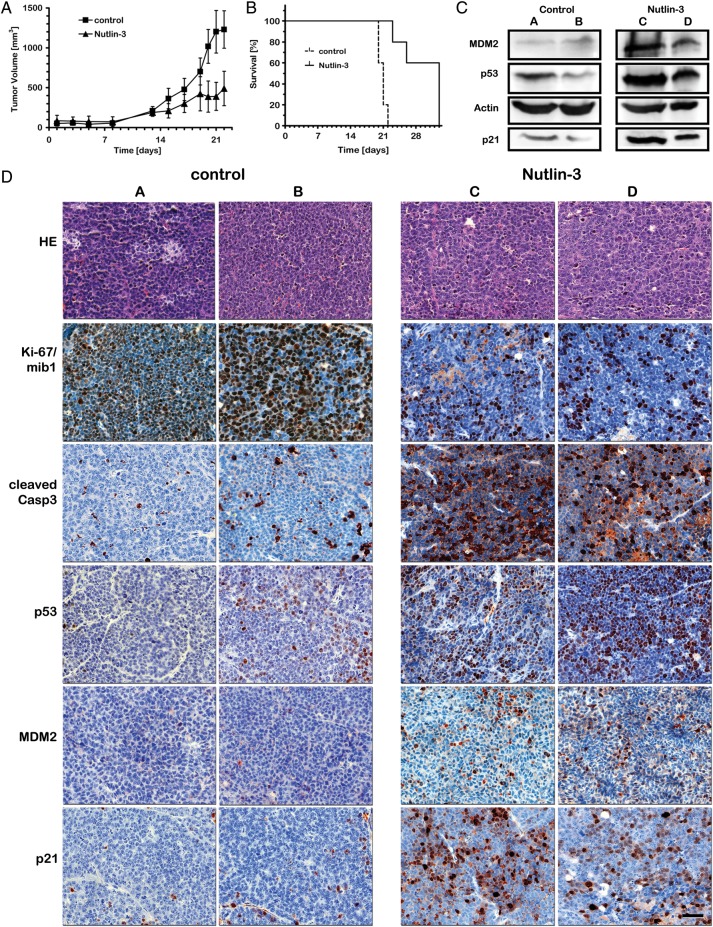
To add further evidence for a therapeutic benefit of nutlin-3 for medulloblastoma, we treated mice with established HD-MB3 xenografts orally with nutlin-3 to assess its in vivo efficacy against medulloblastoma cells with functional p53. The treatment regimen was well tolerated and did not alter the physical status or behavior of the mice, induce weight loss, or produce any other obvious signs of toxicity. The growth of HD-MB3 xenografts was significantly suppressed in mice treated with nutlin-3 (n= 5), compared with mice treated with vehicle control (n= 5) (Fig. 4A). After 8 days of treatment, the mean volumes of HD-MB3 xenografts were 388 mm³ for nutlin-3-treated mice and 1018 mm³ for vehicle-treated mice (difference in mean tumor volume between treatment groups = 630 mm³, 95% CI = 195–212 mm³, P= .004). With a primary end point defined as a tumor volume > 1000 mm³, the survival of the mice treated with nutlin-3 was significantly prolonged when compared to mice treated with vehicle control (Fig. 4B, Supplementary material, Table S1). Nutlin-3 treatment of the mice activated the p53 pathway in the xenograft tumor tissue, as we showed for medulloblastoma cell lines grown in vitro. Both p53 and p21 proteins were elevated in comparison with the xenograft tumors from nontreated mice (Fig. 4C and D). Also consistent with our in vitro data, immunohistochemical analysis of the xenograft tumors indicated increased apoptosis and decreased proliferation after nutlin-3 treatment by an increase of cleaved caspase 3 and reduced KI-67, respectively. Nutlin-3 treatment also induced MDM2 expression in the xenograft tumors through a feed-back loop. Thus, nutlin-3 activated the p53 pathway in vivo, decreased xenograft tumor growth, and increased mouse host survival.
Fig. 4.
Nutlin-3 has antitumoral activity against medulloblastoma tumor xenografts in mice. (A) Tumor growth was significantly delayed and (B) survival was significantly prolonged in mice treated with nutlin-3 (n= 5), compared with controls (n= 5). (C) Induction of MDM2, p53, and p21 protein was detectable in tumors from mice treated with nutlin-3 (n= 2, tumor C + D) but not in controls (n= 2, tumor A + B). (D) Immunohistochemical analysis also showed induction of MDM2, p53, and p21 protein, in addition to increased apoptosis (cleaved caspase 3) and decreased proliferation (KI-67) in tumors from mice treated with nutlin-3 (tumor C + D) but not in controls (tumor A + B).
Discussion
We report here that nutlin-3 shows anti-tumoral activity against medulloblastoma cells with wild-type p53 grown both in vitro and as mouse xenograft tumors in vivo. Nutlin-3 exerted its effect through re-activation of the p53 pathway, an effect that was mimicked by MDM2 knockdown. Our data confirmed elevated expression of MDM2 mRNA and protein in primary medulloblastomas in comparison with cerebellum. MDM2 mRNA expression was also elevated in medulloblastoma cell lines with wild-type p53, but we detected no amplification of the MDM2 gene in medulloblastoma cell lines as a possible explanation for elevated expression. Our data confirm 2 smaller previous studies analyzing MDM2 expression in primary medulloblastomas, in which also no amplification of the MDM2 gene was detected, whereas overexpression of MDM2 protein in a subset of adult medulloblastomas could be shown.15,16 Our data indicate that MDM2 is mainly upregulated at the transcriptional level in medulloblastoma cells and that high levels of MDM2 were at least partly responsible for functional inactivation of p53 in medulloblastoma cell lines with wild-type p53.
Mechanisms that lead to upregulation of MDM2 in the absence of gene amplification might include changes in the expression levels of upstream regulators of MDM2 transcription but might also include epigenetic changes at the MDM2 locus or downregulation of miRNAs that target MDM2.29,30 Further research to elucidate the mechanisms that lead to increased MDM2 expression is warranted, because targeting these regulatory pathways could be alternative or even synergistic to targeting MDM2 with nutlin, as analyzed in this study.
A series of genetic studies in mouse models have shown that loss of p53 induces tumor formation and that restoration of p53 leads to a rapid regression of established tumors, providing strong evidence for designing anticancer drugs that restore p53 function.31–33 Several different therapeutic approaches have been attempted with this goal.21,34–36 Among these, targeting the MDM2-p53 interaction by small molecules for the reactivation of p53 has emerged as a promising approach for the treatment of cancers that retain wild-type p53.21,37–39 The atomic-level understanding of the MDM2-p53 interaction through X-ray crystallography provided the solid foundation for structure-based design of small-molecule antagonists of this interaction.40 A breakthrough in the design of potent small-molecule MDM2 inhibitors was obtained through the discovery of small molecules, termed nutlins by Vassilev et al.21
The cis-imidazoline, nutlin-3, has a high binding affinity and specificity to MDM2 and a highly desirable pharmacokinetic profile.21,39 It has previously been shown to reduce the viability of several tumor cell lines expressing wild-type p53 in vitro. The cell lines assessed were derived from the neuroectodermal tumor, neuroblastoma, and from Ewing sarcoma, osteosarcoma, melanoma, and several carcinomas.21,39,41–45 Here, we show, for the first time to our knowledge, that nutlin-3 was effective at reducing the viability of medulloblastoma cell lines with wild-type p53 in vitro and in vivo. Nutlin-3 induced either cell cycle arrest characterized by depletion of S-phase cells and accumulation at G1/S phase boundaries of the cell cycle or p53-dependent apoptosis in medulloblastoma cell lines with functional p53. For the cell line in which nutlin-3 did not induce apoptosis, ONS-76, proliferation was suppressed, suggesting that nutlin-3 induced senescence in ONS-76 cells. In addition to upregulation of MDM2 expression in medulloblastoma cells with wild-type p53, activation of p53 by nutlin-3 also caused upregulation of the p53 target gene, the p21Waf1/Cip1 cyclin-dependent kinase inhibitor, which is an essential mediator of p53-induced cell cycle arrest. These results are consistent with previously published results that nutlin-1 treatment of tumor cell lines with wild-type p53 lead to accumulation of p53 and subsequent elevation of MDM2 and p21 expression, consistent with activation of the p53 pathway.21 Of interest, the levels of p53 or MDM2 do not increase steadily over the time points analyzed. Because nutlin-3 activates p53 immediately after treatment, the observed levels of apoptotic cells can be interpreted by an early p53 reactivation, causing a peak of apoptosis at 48 h after nutlin-3 treatment. As a compensatory mechanism to activation of p53, MDM2 levels may increase with a different kinetic than activation of pathways downstream of p53, and these effects can be cell line specific. Knockdown of MDM2 expression using siRNA phenocopied the effect of nutlin-3 on cell viability in medulloblastoma cells, further emphasizing the central role of MDM2 in the tumorigenic effect of the MDM2/p53 axis and in the targeting mechanism of nutlins. In contrast, enforced expression of dominant-negative mutant p53 abrogated the nutlin-3 effect, indicating that an intact p53 pathway is needed for sensitivity of medulloblastoma cells to MDM2 inhibitors. Conclusive evidence of the dependence of the activity of MDM2 inhibitors on p53 status has previously been provided by a recent study with 100 B-CLL patients treated with nutlin-3, in which the p53 status was identified as the major determinant of response.46 We showed in the first part of our study that treatment with the MDM2 inhibitor, nutlin-3, is effective against medulloblastoma cell lines with wild-type p53 and that this effect occurred through re-activation of the p53 pathway.
Translating a promising MDM2 inhibitor with excellent in vitro properties into an anticancer drug requires that the inhibitor have excellent pharmacological properties and be nontoxic for normal tissues. Previously published pharmacokinetic studies have shown that oral administration of nutlin-3 induced robust accumulation and activation of p53 in osteosarcoma and prostate cancer xenografts,21,45,47 as was also demonstrated in the tumor tissue of our medulloblastoma mouse model. Of most importance, the nutlin-3 effect appears to be selective for cancer over normal cells, as revealed by the lack of toxicity to peripheral blood mononuclear cells or bone marrow-derived hematopoietic progenitors and bone marrow stromal epithelial cells in patients with leukemia.48,49 In addition, nutlin-3 has been shown to protect normal cells against S-phase and M-phase–specific chemotherapeutic drugs without altering their activity against cancer cells.50,51 However, the latter data from in vitro studies await confirmation in vivo. We performed an initial evaluation of the efficacy of nutlin-3 treatment in vivo in a medulloblastoma xenograft mouse model, using a dosage regimen that has previously been shown to be safe for control mice and effective against osteosarcoma tumor xenografts.21 Nutlin-3 potently inhibited growth of medulloblastoma tumor xenografts expressing wild-type p53 and significantly prolonged mouse survival. These findings were consistent with previous studies of the drug in several xenograft models of human cancer with wild-type p53.21,43,44 Radiosensitive tissues, such as the thymus and crypts of the small intestine, are extremely susceptible to p53-induced apoptosis.52,53 Restoration of p53 by a genetic approach in the absence of MDM2 resulted in severe pathological damage to radiosensitive mouse tissues and death of all animals within 5 days,54 raising concern that p53 activation by MDM2 inhibitors could be toxic to healthy tissues. However, in our mouse model, nutlin-3 achieved its antitumor activity against medulloblastoma xenografts without causing visible signs of toxicity in the animals, as assessed by necropsy studies and assessment of body weight. Nutlin-3 had previously also shown little toxicity to animals at therapeutically efficacious dose schedules against osteosarcoma xenografts.21 In this study by Vassilev et al., nutlin-3 was well tolerated at doses of 200 mg/kg administered twice daily orally for 20 days.
Here, we provide first evidence that the p53 pathway can be re-activated by nutlin-3 treatment in medulloblastoma xenograft tumors and that targeting the MDM2/p53 axis using nutlins represents a potentially attractive therapeutic strategy for medulloblastomas retaining wild-type p53, because nutlin-3 has promising anti-tumoral activity against medulloblastoma cells with wild-type p53 both in vitro and in vivo. The effects of nutlin-3 against medulloblastoma cells occur through re-activation of the p53 pathway by prevention of p53 degradation through disruption of the p53-MDM2 interaction and lead either to cell death or to cell cycle arrest. Intensive research efforts in the past decade have yielded nutlin-3 as a potent and specific inhibitor of the MDM2-p53 interaction with desirable pharmacological properties. Nutlin-3, meanwhile, has progressed to advanced preclinical development or early-phase clinical trials for several tumor entities.40 In light of the fact that about 30% of patients with medulloblastoma experience relapses and that relapse tumors are often resistant to chemotherapeutic agents, it may be interesting to see whether extended treatment of human medulloblastoma xenografts for several months will create resistance to nutlin-3 or provoke changes associated with drug resistance in medulloblastoma cells. Testing nutlin-3 in an orthotopic xenograft model could also provide additional information about whether nutlin-3 is able to cross the blood-brain barrier, easing treatment in patients with medulloblastoma. Ultimately, clinical testing of nutlin-3 against medulloblastoma is necessary to provide the ultimate proof of the usefulness of this therapeutic strategy for the treatment of this highly aggressive pediatric malignancy.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online (http://neuro-oncology.oxfordjournals.org/).
Funding
This work was supported by FWO-Flanders (to K. De P.), German Research Foundation (SFB 876 to A. S.), EU (FP7-HEALTH-2010 “ASSET” to A. E.), and Stiftung für krebskranke Kinder in Essen.
Supplementary Material
Acknowledgments
We thank M. Baumann, A. Odersky, and E. Mahlow, for excellent technical assistance; K. Astrahantseff, for proofreading the manuscript; and H. Stephan, for figure preparation.
Conflict of interest statement. None declared.
References
- 1.McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39(3):190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- 2.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61(2):513–516. [PubMed] [Google Scholar]
- 3.Uziel T, Zindy F, Xie S, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev. 2005;19(22):2656–2667. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Momota H, Shih AH, Edgar MA, Holland EC. c-Myc and beta-catenin cooperate with loss of p53 to generate multiple members of the primitive neuroectodermal tumor family in mice. Oncogene. 2008;27(32):4392–4401. doi: 10.1038/onc.2008.81. [DOI] [PubMed] [Google Scholar]
- 5.de Bont JM, Packer RJ, Michiels EM, den Boer ML, Pieters R. Biological background of pediatric medulloblastoma and ependymoma: a review from a translational research perspective. Neuro Oncol. 2008;10(6):1040–1060. doi: 10.1215/15228517-2008-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleihues P, Schauble B, zur Hausen A, Esteve J, Ohgaki H. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol. 1997;150(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 7.Benard J, Douc-Rasy S, Ahomadegbe JC. TP53 family members and human cancers. Hum Mutat. 2003;21(3):182–191. doi: 10.1002/humu.10172. [DOI] [PubMed] [Google Scholar]
- 8.Saylors RL, 3rd, Sidransky D, Friedman HS, et al. Infrequent p53 gene mutations in medulloblastomas. Cancer Res. 1991;51(17):4721–4723. [PubMed] [Google Scholar]
- 9.Biegel JA. Cytogenetics and molecular genetics of childhood brain tumors. Neuro Oncol. 1999;1(2):139–151. doi: 10.1215/15228517-1-2-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison D. Classifying the medulloblastoma: insights from morphology and molecular genetics. Neuropathol Appl Neurobiol. 2002;28(4):257–282. doi: 10.1046/j.1365-2990.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 11.Tabori U, Baskin B, Shago M, et al. Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol. 2010;28(8):1345–1350. doi: 10.1200/JCO.2009.23.5952. [DOI] [PubMed] [Google Scholar]
- 12.Pfaff E, Remke M, Sturm D, et al. TP53 mutation is frequently associated with CTNNB1 mutation or MYCN amplification and is compatible with long-term survival in medulloblastoma. J Clin Oncol. 2010;28(35):5188–5196. doi: 10.1200/JCO.2010.31.1670. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Pomeroy SL, Ferreira M, et al. UBE4B promotes Hdm2-mediated degradation of the tumor suppressor p53. Nat Med. 2011;17(3):347–355. doi: 10.1038/nm.2283. [DOI] [PubMed] [Google Scholar]
- 14.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6(12):909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 15.Giordana MT, Duo D, Gasverde S, et al. MDM2 overexpression is associated with short survival in adults with medulloblastoma. Neuro Oncol. 2002;4(2):115–122. doi: 10.1093/neuonc/4.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adesina AM, Nalbantoglu J, Cavenee WK. p53 gene mutation and mdm2 gene amplification are uncommon in medulloblastoma. Cancer Res. 1994;54(21):5649–5651. [PubMed] [Google Scholar]
- 17.Batra SK, McLendon RE, Koo JS, et al. Prognostic implications of chromosome 17p deletions in human medulloblastomas. J Neurooncol. 1995;24(1):39–45. doi: 10.1007/BF01052657. [DOI] [PubMed] [Google Scholar]
- 18.Malek R, Matta J, Taylor N, Perry ME, Mendrysa SM. The p53 inhibitor MDM2 facilitates Sonic Hedgehog-mediated tumorigenesis and influences cerebellar foliation. PLoS One. 2011;6(3):e17884. doi: 10.1371/journal.pone.0017884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfeld MR, Meneses P, Dalmau J, Drobnjak M, Cordon-Cardo C, Kaplitt MG. Gene transfer of wild-type p53 results in restoration of tumor-suppressor function in a medulloblastoma cell line. Neurology. 1995;45(8):1533–1539. doi: 10.1212/wnl.45.8.1533. [DOI] [PubMed] [Google Scholar]
- 20.Van Maerken T, Speleman F, Vermeulen J, et al. Small-Molecule MDM2 Antagonists as a New Therapy Concept for Neuroblastoma. Cancer Res. 2006;66(19):9646–9655. doi: 10.1158/0008-5472.CAN-06-0792. [DOI] [PubMed] [Google Scholar]
- 21.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 22.Menten B, Pattyn F, De Preter K, et al. arrayCGHbase: an analysis platform for comparative genomic hybridization microarrays. BMC Bioinformatics. 2005;6:124. doi: 10.1186/1471-2105-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olshen AB, Venkatraman ES, Lucito R, Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5(4):557–572. doi: 10.1093/biostatistics/kxh008. [DOI] [PubMed] [Google Scholar]
- 24.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahl P, Gullotti L, Heukamp LC, et al. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66(23):11341–11347. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 26.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffel C, Thomas GA, Tishler DM, Lassoff S, Allen JC. Absence of p53 mutations in childhood central nervous system primitive neuroectodermal tumors. Neurosurgery. 1993;33(2):301–305. doi: 10.1227/00006123-199308000-00018. discussion 305–306. [DOI] [PubMed] [Google Scholar]
- 28.Leach FS, Tokino T, Meltzer P, et al. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993;53(10 Suppl):2231–2234. [PubMed] [Google Scholar]
- 29.Pichiorri F, Suh SS, Rocci A, et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18(4):367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Xiao J, Lin H, Luo X, Luo X, Wang Z. miR-605 joins p53 network to form a p53:miR-605:Mdm2 positive feedback loop in response to stress. EMBO J. 2011;30(24):5021. doi: 10.1038/emboj.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventura A, Kirsch DG, McLaughlin ME, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445(7128):661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 32.Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127(7):1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2007;13(1):23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Issaeva N, Bozko P, Enge M, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10(12):1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 36.Bykov VJ, Issaeva N, Shilov A, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8(3):282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 37.Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14(17):5318–5324. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding K, Lu Y, Nikolovska-Coleska Z, et al. Structure-based design of potent non-peptide MDM2 inhibitors. J Am Chem Soc. 2005;127(29):10130–10131. doi: 10.1021/ja051147z. [DOI] [PubMed] [Google Scholar]
- 39.Shangary S, Qin D, McEachern D, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA. 2008;105(10):3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonnemann J, Palani CD, Wittig S, et al. Anticancer effects of the p53 activator nutlin-3 in Ewing's sarcoma cells. Eur J Cancer. 2011;47(9):1432–1441. doi: 10.1016/j.ejca.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Vassilev LT. Small-molecule antagonists of p53-MDM2 binding: research tools and potential therapeutics. Cell Cycle. 2004;3(4):419–421. [PubMed] [Google Scholar]
- 43.Van Maerken T, Ferdinande L, Taildeman J, et al. Antitumor activity of the selective MDM2 antagonist nutlin-3 against chemoresistant neuroblastoma with wild-type p53. J Natl Cancer Inst. 2009;101(22):1562–1574. doi: 10.1093/jnci/djp355. [DOI] [PubMed] [Google Scholar]
- 44.Endo S, Yamato K, Hirai S, et al. Potent in vitro and in vivo antitumor effects of MDM2 inhibitor nutlin-3 in gastric cancer cells. Cancer Sci. 2011;102(3):605–613. doi: 10.1111/j.1349-7006.2010.01821.x. [DOI] [PubMed] [Google Scholar]
- 45.Tovar C, Rosinski J, Filipovic Z, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA. 2006;103(6):1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saddler C, Ouillette P, Kujawski L, et al. Comprehensive biomarker and genomic analysis identifies p53 status as the major determinant of response to MDM2 inhibitors in chronic lymphocytic leukemia. Blood. 2008;111(3):1584–1593. doi: 10.1182/blood-2007-09-112698. [DOI] [PubMed] [Google Scholar]
- 47.Sarek G, Kurki S, Enback J, et al. Reactivation of the p53 pathway as a treatment modality for KSHV-induced lymphomas. J Clin Invest. 2007;117(4):1019–1028. doi: 10.1172/JCI30945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Secchiero P, Barbarotto E, Tiribelli M, et al. Functional integrity of the p53-mediated apoptotic pathway induced by the nongenotoxic agent nutlin-3 in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107(10):4122–4129. doi: 10.1182/blood-2005-11-4465. [DOI] [PubMed] [Google Scholar]
- 49.Kojima K, Konopleva M, Samudio IJ, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106(9):3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvajal D, Tovar C, Yang H, Vu BT, Heimbrook DC, Vassilev LT. Activation of p53 by MDM2 antagonists can protect proliferating cells from mitotic inhibitors. Cancer Res. 2005;65(5):1918–1924. doi: 10.1158/0008-5472.CAN-04-3576. [DOI] [PubMed] [Google Scholar]
- 51.Kranz D, Dobbelstein M. Nongenotoxic p53 activation protects cells against S-phase-specific chemotherapy. Cancer Res. 2006;66(21):10274–10280. doi: 10.1158/0008-5472.CAN-06-1527. [DOI] [PubMed] [Google Scholar]
- 52.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 53.Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15(2):82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- 54.Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell. 2006;10(6):501–514. doi: 10.1016/j.ccr.2006.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.