Abstract
Anti-Hu antibody–associated paraneoplastic neurological syndromes (Hu-PNSs) are severe and often precede the detection of a malignancy, usually small-cell lung cancer. In Hu-PNS, it is hypothesized that neuronal cells are destroyed by T cells targeted against HuD, a protein expressed by small-cell lung cancer cells and neurons. There is only limited evidence for the existence of HuD-specific T cells. To detect these T cells in the blood of Hu-PNS patients, we employed 3 highly sensitive assays that included T cell stimulation with dendritic cells (DCs) to specifically expand the number of any HuD-specific T cells. A total of 17 Hu-PNS patients were tested with 1 or more of the following 3 assays: (1) tetramer staining after stimulation of T cells with conventionally generated DCs (n = 9), (2) interleukin (IL)-13 enzyme-linked immunosorbent spot (ELISpot; n = 3), IL-4 and IL-5 and interferon (IFN)–γ multiplex cytokine bead array (n = 2) to assay cytokine production by T cells after stimulation with conventionally generated DCs, and (iii) IFN-γ ELISpot and tetramer staining after T cell stimulation with accelerated co-cultured DCs (n = 11). No circulating HuD-specific T cells were found. We suggest that either autoaggressive T cells in Hu-PNS are not targeted against HuD or that their numbers in the blood are too low for detection by highly sensitive techniques.
Keywords: anti-Hu, CD8+ T cell, HuD-specific T cell, immune response, paraneoplastic neurological syndrome
Anti-Hu antibody (Hu-Ab)–associated paraneoplastic neurological syndromes (Hu-PNSs) are severe, have no effective treatment, and often precede the detection of a malignancy, usually small-cell lung cancer (SCLC).1 Hu-PNSs are thought to be caused by an immune response against the HuD protein that is normally exclusively expressed by neuronal cells and is aberrantly expressed by SCLC cells.2 Although patients with Hu-PNS have high titers of autoantibodies against the HuD protein, there is no evidence that these autoantibodies directly cause neuronal damage.3 The intracellular localization of HuD makes it inaccessible to autoantibodies, and therefore it is likely that neuronal destruction in Hu-PNS is caused by HuD-specific cytotoxic T cells. The hypothesis that these T cells cause neuronal damage is supported by autopsy studies that show the presence of cytotoxic T cells around neurons in the nervous system tissue of Hu-PNS patients.4,5
Previous studies that aimed to detect circulating HuD-specific T cells showed different, often conflicting results.6–13 First, it is uncertain whether the HuD protein is immunogenic. One study reported that the HuD protein can elicit a T cell response in healthy subjects and mice,10 whereas another study, in mice, demonstrated tolerance to the HuD protein.11 Second, it is unclear which epitopes within the HuD protein are the targets of this hypothetical T cell response. Previously, we12,13 could not confirm T cell responses to T cell epitopes that were identified by others.6,10 More recently, Roberts et al.9 described in 3 patients T cell responses to the HuD-derived T cell epitopes Hu133 and Hu157 that have not been confirmed by others until now. Third, it is unclear which cytokines are produced by HuD-specific T cells. Previously, classical CD8+ T cells producing interferon (IFN)–γ were described,6 whereas Roberts et al. also reported “type 2” CD8+ T cells that secreted robust amounts of the type 2 cytokines interleukin (IL)-4, IL-5, and IL-13.9 The detection and further characterization of any HuD-specific T cells potentially could help develop specific therapies for Hu-PNS.
In this study, we aimed to confirm the presence of HuD-specific T cells in a relatively large group of 17 Hu-PNS patients. We used HuD-peptide loaded tetramer staining to detect CD8+ T cells, a combination of IL-13 enzyme-linked immunosorbent spot (ELISpot) and a flow cytometric multiplex bead array to detect type 2 CD8+ T cells, and IFN-γ ELISpot to test for classic “type 1” cytotoxic T cells. All procedures included the use of dendritic cells (DCs) to specifically expand the number of any HuD-specific T cells and to gain maximal sensitivity.
Materials and Methods
Sample Collection and Storage
Heparinized blood was drawn from 17 Hu-PNS patients who met the following criteria: presence of high-titer anti–Hu-Abs, a definite diagnosis of PNS,14 and the presence of the human leukocyte antigen (HLA)–A*0201 and/or HLA-A*0301 restriction element. As procedural controls, 3 healthy subjects were tested who were cytomegalovirus (CMV) seropositive and HLA-A*0201 and/or HLA-A*0301 positive. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density-gradient centrifugation and cryopreserved in liquid nitrogen as described.15 Absolute numbers of lymphocytes in the blood (expressed as 109 cells/L) and distribution of T cell subsets (expressed as % of lymphocytes) were determined by flow cytometry using a whole-blood stain, lyse, no-wash method based on counting beads (all patients except nos. 7, 10, and 14) or by a hematology analyzer and a flow cytometric method that included washing steps (the remaining 3 patients).16 The local ethical review committee approved the study, and written informed consent was obtained from all participants.
Proteins and Peptides
Recombinant HuD and Yo proteins were produced in Escherichia coli and purified using metal affinity chromatography, essentially as described before.3 Endotoxins were removed by Triton-X114 phase separation.17 A HuD protein-spanning peptide mix (HuDm) that consisted of 93 15-mers, with an 11-amino-acid overlap and a CMV phosphoprotein-65 (pp65) protein-spanning 15-mers mix (pp65m), were obtained from Jerini Peptide Technologies. The single 9-mers Hu133 (NLYVSGLPK) and Hu157 (RIITSRILV), selected based on the observations of Roberts et al.,9 and NLVPMVATV (NLV, a CMV pp65-derived peptide) were obtained from Pepscan. Tetanus toxoid (TTX) was kindly provided by Dr. R. Rappuoli.
Conventionally Generated DCs
After thawing the PBMCs, we isolated CD14+ cells by magnetic separation (Miltenyi Biotec) and cultured them in RPMI (Roswell Park Memorial Institute) 1640 medium with GlutaMAX (Invitrogen), supplemented with 1% l-glutamine, 10% heat-inactivated human AB serum, 1% penicillin/streptomycin, 100 U/mL IL-4 (R&D Systems), and 100 U/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunotools).18 To induce DC maturation, 1 µg/mL prostaglandin (PG)E2 and 50 ng/mL tumor necrosis factor (TNF)–α were added after 6 days (R&D Systems). After 2 additional days of culture (day 8), these conventionally generated DCs (cDCs) were used for in vitro stimulation of CD8+ T cells.
In Vitro Stimulation of CD8+ T Cells with cDCs
In parallel with the generation of cDCs, the CD14− T cell fraction was cultured for 8 days prior to stimulation using a feeder system, as described.13 CD8+ T cells were isolated from the CD14− fraction by magnetic separation (Miltenyi Biotec). Depending on the number available, cDCs were added to the CD8+ T cells at ratios of 1:10–1:30. The CD8+ T cells and cDCs were cultured in complete culture medium (RPMI-1640 with 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid, 1% l-glutamine, 10% human AB serum, and 1% penicillin/streptomycin). Peptides (Hu133, Hu157, or NLV) were added at a final concentration of 10μg/mL. One day after addition of cDCs and peptides, 10 IU/mL IL-2 (R&D Systems) was added to the cultures.
In Vitro Stimulation by Accelerated Co-cultured DCs
Thawed PBMCs were incubated for 24–48 h with peptides or proteins together with DC-activating agents to induce DCs and stimulate T cells as described.19,20 PBMCs were cultured in AIM-V (Adoptive Immunotherapy Media–Vero; Invitrogen) medium with 1000 U/mL of GM-CSF and 500 U/mL of IL-4 (R&D Systems). Proteins (Yo, HuD) or peptide mixes (HuDm, pp65m) were added at 10 μg/mL or 2 μg/mL, respectively. After 24 h, we added DC maturation stimuli (2000 U/mL TNF-α, 20 ng/mL IL-1ß [R&D Systems], and 2 μM PGE2 [Merck Calbiochem]), 1 ng/mL IL-7 (R&D Systems), and single peptides (Hu133, Hu157, or NLV) at 10 μg/mL. After 48 h, nonadherent cells were collected, washed, and used for IFN-γ ELISpot and tetramer staining.
Tetramer Staining
Up to 2 × 106 cells were stained with phycoerythrin-conjugated tetramers, anti-CD3 fluorescein isothiocyanate, anti-CD8 allophycocyanin (Becton Dickinson), and 7-amino-actinomycin-d (7AAD; Sigma-Aldrich) as described.15 The tetramers Hu133, HLA-A*0301, and Hu157 HLA-A*0201, selected based on the observations of Roberts et al.,9 and NLV HLA-A*0201 were obtained from Beckman Coulter. Irrelevant tetramers loaded with glycoprotein 100–derived peptides or HIV-derived peptides were obtained from Beckman Coulter or provided by Dr. W.A.F. Marijt (Leiden University Medical Center, the Netherlands). Listmode data were acquired on a FACSCalibur or FACSCanto flow cytometer (Becton Dickinson). We gated on viable T cells (7AAD−, CD3+ cells with appropriate side and forward scatter properties). A positive response was defined as (1) a distinct population of CD8+ tetramer-positive cells and (2) a higher percentage of CD8+ tetramer-positive cells than irrelevant tetramer-positive cells.
IFN-γ ELISpot
After stimulation with accelerated co-cultured (ac)DCs, PBMCs were assayed for 6 h as described previously.19 Spots were counted with a Bioreader 3000 (BioSys). A positive response was defined as (1) a weak response (3–4 SDs above the mean number of spot-forming cells [SFCs] in wells without peptide) that could be reproduced in a second experiment or (2) an intermediate to strong response (more than 4 SDs above the mean number of SFCs in wells without peptide). Previously, these cutoff values were shown to yield a high sensitivity (86.4%) and specificity (90.9%) for detecting autoreactive T cells in type 1 diabetes.21
Tests for the Detection of Type 2 CD8+ T Cells
CD8+ T cells that were stimulated with cDCs for 8 days were plated in triplicates of 100 000 cells/100 μL/well in polyvinylidene fluoride plates (Millipore) coated with anti–IL-13 Abs (Mabtech). Subsequently, 25 000 peptide-pulsed T2 cells in 100 μL per well were added and incubated for 20 h. Culture supernatants were collected and stored at −80°C. ELISpot plates were processed according to the manufacturer's instructions (Mabtech). Cytokine concentrations of culture supernatants were determined with the Th1/2 cytometric bead array kit from Becton Dickinson, according to the manufacturer's instructions. Since receiver-operator characteristics data are not available for these methods under these specific conditions, we used a more stringent definition of a positive response than for the IFN-γ ELISpot assay: (1) a number of SFCs or cytokine level more than 2× the background level in wells with T2 cells but without peptides and (2) an increase in the number of SFCs or the cytokine level after stimulation with peptide-pulsed cDCs.
Results and Discussion
Seventeen Hu-PNS patients were included who tested positive for the HLA-A*0201 restriction element and/or the HLA-A*0301 restriction element (Table 1). The median age of the patients was 68 years (range, 46–77). In 14 patients an underlying tumor was detected, mostly SCLC (n = 10). All but 3 patients (nos. 2, 6, and 17) had progressive neurological symptoms in the 4 weeks prior to study entry, indicating ongoing neuronal destruction. Most patients had not received immunomodulatory or cancer treatment prior to blood withdrawal and showed normal numbers of lymphocytes in the blood. Two patients who had received chemotherapy showed subnormal lymphocyte counts (nos. 3 and 6, Table 1).
Table 1.
Patient characteristics
| Hu-PNS Patient | Age/Gender | Hu-Ab Titer | PNS | Tumor | Therapy prior to blood withdrawal | Symptom study (months) | HLA-A | Peripheral blood lymphocytes |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (109/L) | CD3+ (%) | CD3+, 4+ (%) | CD3+, 8+ (%) | ||||||||
| 1 | 76/F | 3200 | EM | SCLC | None | 5 | 0201 | 1.95 | 82 | 51 | 29 |
| 2 | 66/F | 3200 | SN | NSCLC | Chemo + RTa | 33 | 0201 | 1.90 | 73 | 55 | 14 |
| 3 | 75/M | 3200 | SN | SCLC | Chemo + RT | 5 | 0201,0301 | 0.50 | 81 | 62 | 17 |
| 4 | 68/M | 6400 | LE, CD | SCLC | Chemo | 8 | 0201 | 1.12 | 80 | 46 | 27 |
| 5 | 61/F | 12 800 | EM | SCLC | Chemo | 2 | 0301 | 1.36 | 77 | 64 | 11 |
| 6 | 46/M | 6400 | EM | SCLC | Chemo | 3 | 0301 | 0.65 | 62 | 47 | 14 |
| 7 | 72/F | 12 800 | SN | No | None | 4 | 0201,0301 | 1.80 | 54 | 42 | 12 |
| 8 | 74/F | 800 | CD | Lungb | None | 2 | 0201 | 1.22 | 72 | 56 | 14 |
| 9 | 59/M | 12 800 | CD | No | None | 6 | 0201 | 1.65 | 67 | 56 | 10 |
| 10 | 68/F | 1600 | SN | SCLC | None | 2 | 0201 | 1.16 | 48 | 28 | 20 |
| 11 | 66/M | 25 600 | SN | NSCLC | None | 3 | 0301 | 1.55 | 73 | 52 | 19 |
| 12 | 61/M | 6400 | CD | SCLC | None | 4 | 0301 | 1.85 | 81 | 64 | 16 |
| 13 | 77/M | 800 | CD | Prostatec | None | 8 | 0201 | 1.24 | 70 | 43 | 22 |
| 14 | 61/F | 800 | CD | No | None | 6 | 0201 | ND | 55 | 35 | 20 |
| 15 | 73/F | 6400 | SN | SCLC | None | 2 | 0201 | 2.05 | 78 | 40 | 32 |
| 16 | 71/F | 12 800 | EM | SCLC | Steroids | 1 | 0301 | 1.26 | 68 | 37 | 27 |
| 17 | 73/M | 800 | AN | SCLC | Chemo | 9 | 0201 | 1.26 | 64 | 52 | 11 |
aPatient received chemotherapy 2 years prior to study entry and was in complete remission.
bPET and CT scanning suspect for lung tumor not pathologically confirmed.
cProstate carcinoma 10 years prior to neurological symptoms.
Abbreviations: PNS, paraneoplastic neurological syndrome; F, female; M, male; EM, encephalomyelitis; SN, sensory neuronopathy; LE, limbic encephalitis; CD, cerebellar degeneration; AN, autonomic neuropathy; SCLC, small-cell lung cancer; NSCLC, non-SCLC; No, no tumor found after tumor workup; Chemo, chemotherapy; RT, radiotherapy; ND, not determined.
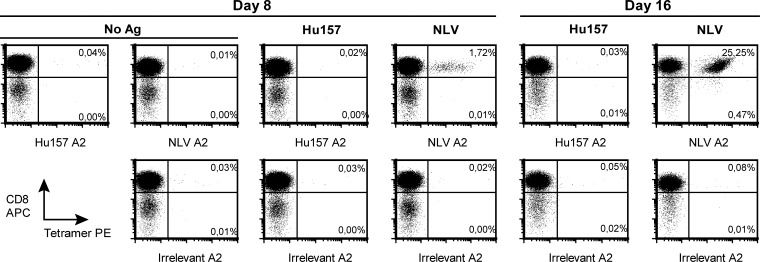
First, we stimulated CD8+ T cells of 9 Hu-PNS patients (nos. 1–9) with cDCs pulsed with the HuD-derived HLA-A*0301–binding peptide Hu133 or the HLA-A*0201–binding peptide Hu157 and used HLA-peptide tetramers to reproduce the results of Roberts et al.9 Staining of CD8+ T cells from Hu-PNS patient no. 1 with Hu157-loaded tetramers after 2 cycles of stimulation with peptide-pulsed cDCs did not show any Hu157-specific T cells, while stimulation with the CMV pp65-derived positive control peptide NLV resulted in a dramatic increase in NLV-specific T cells from 0.01% to 25% of the T cells (Fig. 1). The other 8 patients were stimulated for 1 cycle prior to tetramer staining and did not show any Hu133- or Hu157-specific T cells (data not shown). Since the median number of acquired CD8+ T cells was 124 000 (range, 12 000–811 000) and a count of 100 tetramer-positive cells is needed for a reliable positive result,22 we reached a median sensitivity of 0.08% (range, 0.01%–0.85%) of stimulated CD8+ T cells. Therefore, the sensitivity of our assay should have been sufficient to detect similar frequencies as reported by Roberts et al. (i.e., 0.26%–0.79%).
Fig. 1.
Tetramer staining of T cells after stimulation with cDCs. Tetramer staining of T cells from patient no. 1 after 1 cycle (day 8) and 2 cycles (day 16) of stimulation with cDCs. CD8-enriched T cells were stimulated with cDCs pulsed with Hu157, the CMV pp65-derived peptide NLV, or no antigen (No Ag) as indicated in boldface. This indication applies to both rows of panels. Then, the stimulated T cells were stained with HLA-A*0201 tetramers loaded with Hu157, NLV, or irrelevant peptide as indicated by the x-axis labels of each panel. The percentage of CD8+ NLV-tetramer-positive T cells (upper right quadrants) increased from 0.01% of the T cells (No Ag, day 8) to 25% of the T cells (NLV, day 16). The percentage of Hu157-tetramer-positive T cells remained similar to that of the negative control tetramer (Irrelevant A2). Abbreviations: Hu157, cDCs pulsed with Hu157 peptide; NLV, cDCs pulsed with the CMV pp65-derived peptide NLV; Hu157 A2, HLA-A*0201 tetramer loaded with Hu157; NLV A2, HLA-A*0201 tetramer loaded with NLV; Irrelevant A2, tetramer loaded with irrelevant peptide.
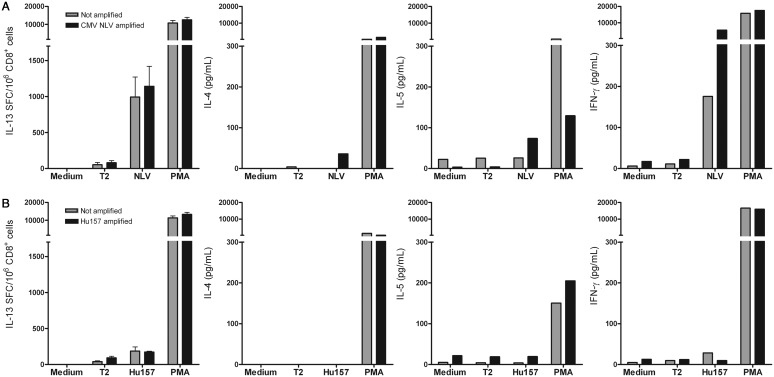
Since Roberts et al. also reported robust secretion of the type 2 cytokines IL-4, IL-5, and IL-13 in stimulated bulk CD8+ T cells, we additionally tested for the presence of these “type 2” CD8+ T cells in 3 patients (nos. 7–9). Figure 2 shows the results of an IL-13 ELISpot and an IL-4, IL-5, and IFN-γ bead array after stimulation with cDCs in Hu-PNS patient no. 7 and an HLA-A*0201-positive CMV-seropositive healthy control. CD8+ T cells of patient no. 7 did not show IL-13, IL-4, IL-5, or IFN-γ secretion in response to the Hu157 peptide. CD8+ T cells of the healthy control showed a significant increase in the number of IL-13 SFCs and IFN-γ concentration in culture supernatant in response to the NLV peptide. We additionally tested 2 Hu-PNS patients (nos. 8 and 9) with IL-13 ELISpot and 1 of these patients (no. 9) also with the flow cytometric bead array and did not detect any type 2 HuD-specific T cells.
Fig. 2.
Secretion of type 2 cytokines and IFN-γ by CD8+ T cells after stimulation with cDCs. CD8+ T cells were tested for secretion of type 2 cytokines and IFN-γ in response to peptide-pulsed T2 cells after stimulation with peptide-pulsed cDCs (amplified, black bars) or cDCs without peptides (not amplified, grey bars). Upper panels (A) show the results of T cells obtained from a CMV-seropositive healthy donor, lower panels (B) show the results for Hu-PNS patient no. 7. CD8+ T cells were tested in medium and against T2 cells (T2), T2 cells pulsed with the CMV pp65-derived peptide NLV, T2 cells pulsed with the HuD-derived peptide Hu157, or T2 cells that were added simultaneously with PMA plus ionomycin (PMA). Panels on the left show the numbers of IL-13 SFC/106 CD8+ T cells, the other panels show cytokine concentrations in culture supernatants (pg/mL) of IL-4, IL-5, and IFN-γ. The CD8+ T cells of the CMV-seropositive healthy donor (upper panels) secreted both IL-13 and IFN-γ. CD8+ T cells of Hu-PNS patient no. 7 (lower panels) did not secrete the type 2 cytokines IL-4, IL-5, or IL-13 or IFN- γ. Abbreviations: cDCs, conventionally generated dendritic cells; CMV, cytomegalovirus; PMA, phorbol myristate acetate plus ionomycin; IL, interleukin; PNS, paraneoplastic neurological syndromes.
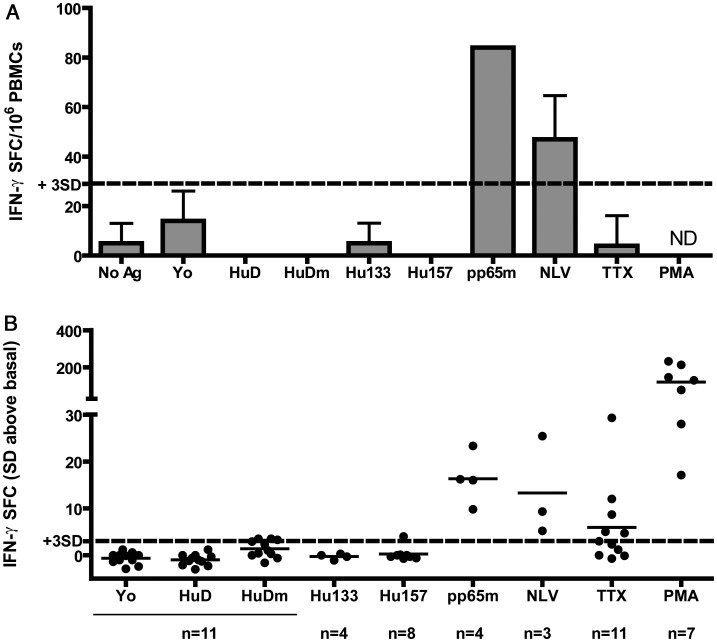
Because of these negative results, we decided to test 11 patients (nos. 7–17) using an alternative approach based on methods that have successfully detected low numbers of circulating autoreactive T cells in type 1 diabetes. This approach involved 48-h stimulation with acDCs and IFN-γ ELISpot with IL-7 co-stimulation. Advantages of these methods are reduction of culture time and of number of manipulations, which may therefore offer a better representation of the in vivo situation, and a high sensitivity due to stimulation with acDCs, reduction of T cell background activity by using serum-free culture media, and an increase in the amplitude of the antigen-specific response by IL-7 co-stimulation.19 In order to test for other HuD epitopes than Hu133 and Hu157, we also tested against the entire HuD protein and a HuD protein-spanning 15-mers mix (HuDm). Figure 3 shows the results of the IFN-γ ELISpot assays after stimulation with acDCs in 11 Hu-PNS patients. Testing against the HuD protein, HuDm, Hu133 and Hu157 showed either negative results (less than 3 SDs above background) or weak responses (3–4 SDs above background) that could not be reproduced and were therefore considered negative. All 4 CMV-seropositive patients tested positive against the CMV pp65 peptide mix (pp65m). Three of them were HLA-A*0201 and responded to the CMV pp65-derived HLA-A*0201–binding peptide NLV. In addition, we performed Hu133- and Hu157-loaded tetramer staining ex vivo and after 12 days of stimulation with acDCs. We acquired a median number of 147 000 (range, 15 000–645 000) T cells ex vivo and 9000 (range, 1000–166 000) T cells after acDC stimulation, corresponding to median sensitivities of T cells of 0.07% (range, 0.02%–0.67%) and 1.14% (range, 0.06%–9.58%), respectively; no HuD-specific T cells were detected.
Fig. 3.
IFN-γ ELISpot of PBMCs after stimulation with acDCs. In panel A the numbers of IFN-γ SFC/106 PBMCs are presented after stimulation with acDCs in Hu-PNS patient no. 8. Panel B shows the summary for all 11 tested patients as a relative value representing the mean number of SFCs in wells with antigen minus the mean number of SFCs in wells without antigen divided by the SDs of wells without antigen. The cutoff value for a weak positive response (+3 SDs) is indicated by the dotted line. Responses to CMV antigens are shown for CMV-seropositive patients only. Responses to peptides predicted to bind HLA-A*0201 or HLA-A*0301 molecules are shown for individuals with the corresponding HLA-A phenotype only. Stimulation with positive control peptides (pp65m and NLV) and PMA with ionomycin (PMA) resulted in a significantly higher number of IFN-γ spot-forming cells (more than 4 SDs) in CMV-seropositive patients and other patients. Stimulation with HuD or HuD-derived peptides (HuDm, Hu133, and Hu157) resulted in either no response (less than 3 SDs) or weak responses (3–4 SDs) that could not be reproduced. Abbreviations: No Ag, no antigen; Yo, Yo protein; HuD, HuD protein; HuDm, HuD 15-mers mix; Hu133, Hu133 peptide; Hu157, Hu157 peptide; pp65m, CMV-derived pp65 15-mers mix; NLV, CMV-derived NLV peptide; PMA, phorbol myristate acetate plus ionomycin; TTX, tetanus toxoid; ND, not determined.
There are several possible explanations for our negative results and the discrepancies between our results and those of Roberts et al. First, methodological differences could have been responsible. We tried to limit these differences by using exactly the same protocols as Roberts et al. for stimulation of CD8+ T cells with cDCs and using the same reagents for tetramer staining, IL-13 ELISpot, and the multiplex bead array. However, Roberts et al. used separate vials for separated cryopreservation of monocytes and T cells, while we used single vials with PBMCs. Hence, we had to keep the T cells in culture for 8 additional days during the generation of cDCs. Theoretically, the numbers of HuD-specific T cells may have decreased during this period. However, we also used stimulation by acDCs as an alternative approach to reduce culture time and the number of manipulations, and this approach did not result in detectable levels of any HuD-specific T cells.
Second, differences in patient characteristics between the 2 studies may exist. Theoretically, a delay in our patient inclusion could have resulted in more chronically ill patients with stable symptoms in whom certain autoaggressive T cells may have disappeared. Of note, Roberts et al. could detect Hu133-specific T cells in only a single patient with progressive symptoms for 5 months, and not in 2 chronically ill patients who had had symptoms for more than 10 months. However, since 14 of our 17 patients had progressive neurological symptoms and 13 of the 17 patients were included not later than 6 months after onset of their neurological symptoms, we consider it unlikely that a delay in patient inclusion could account for our negative results.
Third, the number of circulating HuD-specific T cells in our study may have been too low for detection with even the most sensitive techniques. HuD-specific T cells may be preferentially found in or around target tissues such as CNS, dorsal roots, or SCLC rather than in peripheral blood.
Fourth, our negative results suggest that HuD itself might not be the target of the hypothetical autoaggressive T cells in Hu-PNS. Although the high expression of HuD in both neurons and SCLC cells makes HuD an attractive target of the autoaggressive T cells in Hu-PNS, other proteins may be involved. For example, another Hu protein, HuB, is also expressed by neurons and SCLC cells.23 Even proteins that do not belong to the family of Hu proteins may be involved, despite the presence of high titers of Hu-Abs in our patients. In celiac disease, for example, a B cell response targets the enzyme tissue transglutaminase itself, while T cell responses target the products of this enzyme.24
Finally, the high titers of immunoglobulin G1 anti–Hu-Abs in patients with Hu-PNS suggest the help of CD4+ T cells. In this paper, we focus on CD8+ T cells because these cells are believed to directly cause neuronal damage by the release of cytotoxins (classic CD8+ T cells) and because HuD-specific type 2 CD8+ T cells have been reported in the literature.9 Although we could also have detected CD4+ T cells with the third assay, further research is needed to specifically address the role of CD4+ T cells in Hu-PNS.
In summary, we propose either that autoaggressive CD8+ T cells in Hu-PNS target not HuD but other antigens (eg, HuB) or that these cells are extremely rare in the blood, which makes their detection not amenable to clinical application.
Funding
This research was supported by The Gratama Foundation (Harlingen, the Netherlands), and JDRF (grant 1-2008-106), the European Foundation for the Study of Diabetes (EFSD/JDRF/Novo Nordisk European Programme in Type 1 Diabetes Research 2007), the Fondation Recherche Médicale (grant Installation Nouvelle Equipe), and the Ile-de-France CODDIM (grant Soutien aux Jeunes Equipes), to R.M. R.M. is an INSERM Avenir Investigator and recipient of an APHP-INSERM Contrat Hospitalier de Recherche Translationelle.
Acknowledgments
The authors thank P. Maat for his help with the generation of the HuD protein; P.M.M.L. van Elzakker, C.M. Groot-van Ruijven, S.C.L. van Steenbergen-Langeveld, and G. Naselli for sharing their technical expertise with us; and Dr. N.M. van Besouw, G.J.M. Zuijderwijk-Roest, and Dr. C.C. Baan for their help with the automated read-out of the ELISpot plates.
Conflict of interest statement. None declared.
References
- 1.de Beukelaar JW, Sillevis Smitt PA. Managing paraneoplastic neurological disorders. Oncologist. 2006;11(3):292–305. doi: 10.1634/theoncologist.11-3-292. doi:10.1634/theoncologist.11-3-292. [DOI] [PubMed] [Google Scholar]
- 2.Roberts WK, Darnell RB. Neuroimmunology of the paraneoplastic neurological degenerations. Curr Opin Immunol. 2004;16(5):616–622. doi: 10.1016/j.coi.2004.07.009. doi:10.1016/j.coi.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Sillevis Smitt PA, Manley GT, Posner JB. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology. 1995;45(10):1873–1878. doi: 10.1212/wnl.45.10.1873. [DOI] [PubMed] [Google Scholar]
- 4.Bernal F, Graus F, Pifarre A, Saiz A, Benyahia B, Ribalta T. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta neuropathologica. 2002;103(5):509–515. doi: 10.1007/s00401-001-0498-0. doi:10.1007/s00401-001-0498-0. [DOI] [PubMed] [Google Scholar]
- 5.Wanschitz J, Hainfellner JA, Kristoferitsch W, Drlicek M, Budka H. Ganglionitis in paraneoplastic subacute sensory neuronopathy: a morphologic study. Neurology. 1997;49(4):1156–1159. doi: 10.1212/wnl.49.4.1156. [DOI] [PubMed] [Google Scholar]
- 6.Rousseau A, Benyahia B, Dalmau J, et al. T cell response to Hu-D peptides in patients with anti-Hu syndrome. J Neurooncol. 2005;71(3):231–236. doi: 10.1007/s11060-004-1723-1. doi:10.1007/s11060-004-1723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benyahia B, Liblau R, Merle-Beral H, Tourani JM, Dalmau J, Delattre JY. Cell-mediated autoimmunity in paraneoplastic neurological syndromes with anti-Hu antibodies. Ann Neurol. 1999;45(2):162–167. doi: 10.1002/1531-8249(199902)45:2<162::aid-ana5>3.0.co;2-r. doi:10.1002/1531-8249(199902)45:2<162::AID-ANA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Maruyama Y, Sugie M, Motizuki H, Kamakura K, Tanaka K. Cytotoxic T cell activity against peptides of Hu protein in anti-Hu syndrome. J Neurol Sci. 2002;201(1–2):9–12. doi: 10.1016/s0022-510x(02)00157-0. doi:10.1016/S0022-510X(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 9.Roberts WK, Deluca IJ, Thomas A, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest. 2009;119(7):2042–2051. doi: 10.1172/JCI36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plonquet A, Garcia-Pons F, Fernandez E, et al. Peptides derived from the onconeural HuD protein can elicit cytotoxic responses in HHD mouse and human. J Neuroimmunol. 2003;142(1–2):93–100. doi: 10.1016/s0165-5728(03)00269-8. doi:10.1016/S0165-5728(03)00269-8. [DOI] [PubMed] [Google Scholar]
- 11.DeLuca I, Blachere NE, Santomasso B, Darnell RB. Tolerance to the neuron-specific paraneoplastic HuD antigen. PLoS One. 2009;4(6):e5739. doi: 10.1371/journal.pone.0005739. doi:10.1371/journal.pone.0005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Beukelaar JW, Verjans GM, van Norden Y, et al. No evidence for circulating HuD-specific CD8+ T cells in patients with paraneoplastic neurological syndromes and Hu antibodies. Cancer Immunol Immunother. 2007;56(9):1501–1506. doi: 10.1007/s00262-007-0295-2. doi:10.1007/s00262-007-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Beukelaar JW, Milikan JC, Verjans GM, et al. No evidence for the presence of HuD-specific T cells in the cerebrospinal fluid of patients with Hu-associated paraneoplastic neurological syndromes. J Neurol. 2009;256(2):279–282. doi: 10.1007/s00415-009-0051-y. doi:10.1007/s00415-009-0051-y. [DOI] [PubMed] [Google Scholar]
- 14.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. doi:10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratama JW, van Esser JW, Lamers CH, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–1364. doi: 10.1182/blood.v98.5.1358. doi:10.1182/blood.V98.5.1358. [DOI] [PubMed] [Google Scholar]
- 16.de Beukelaar JW, Sillevis Smitt PA, Hop WC, et al. Imbalances in circulating lymphocyte subsets in Hu antibody associated paraneoplastic neurological syndromes. Eur J Neurol. 2007;14(12):1383–1391. doi: 10.1111/j.1468-1331.2007.01986.x. doi:10.1111/j.1468-1331.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 17.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256(4):1604–1607. [PubMed] [Google Scholar]
- 18.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exper Medicine. 1994;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. doi:10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinuzzi E, Afonso G, Gagnerault MC, et al. acDCs enhance human antigen-specific T-cell responses. Blood. 2011;118(8):2128–2137. doi: 10.1182/blood-2010-12-326231. doi:10.1182/blood-2010-12-326231. [DOI] [PubMed] [Google Scholar]
- 20.Fourlanos S, Perry C, Gellert SA, et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60(4):1237–1245. doi: 10.2337/db10-1360. doi:10.2337/db10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallone R, Martinuzzi E, Blancou P, et al. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56(3):613–621. doi: 10.2337/db06-1419. doi:10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. (2007) H42-A2. Available at http://www.clsi.org/source/orders/free/h42-a2.pdf. Accessed September 12, 2011.
- 23.King PH. Differential expression of the neuroendocrine genes Hel-N1 and HuD in small-cell lung carcinoma: evidence for down-regulation of HuD in the variant phenotype. Int J Cancer. 1997;74(4):378–382. doi: 10.1002/(sici)1097-0215(19970822)74:4<378::aid-ijc3>3.0.co;2-s. doi:10.1002/(SICI)1097-0215(19970822)74:4<378::AID-IJC3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137(6):1912–1933. doi: 10.1053/j.gastro.2009.09.008. doi:10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]