Abstract
We sought to assess the feasibility and estimate the benefit of sparing the neurogenic niches when irradiating the brain of pediatric patients with medulloblastoma (MB) based on clinical outcome data. Pediatric MB survivors experience a high risk of neurocognitive adverse effects, often attributed to the whole-brain irradiation that is part of standard management. Neurogenesis is very sensitive to radiation, and limiting the radiation dose to the hippocampus and the subventricular zone (SVZ) may preserve neurocognitive function. Radiotherapy plans were created using 4 techniques: standard opposing fields, intensity-modulated radiotherapy (IMRT), intensity-modulated arc therapy (IMAT), and intensity-modulated proton therapy (IMPT). Mean dose to the hippocampus and SVZ (mean for both sites) could be limited to 88.3% (range, 83.6%–91.0%), 77.1% (range, 71.5%–81.3%), and 42.3% (range, 26.6%–51.2%) with IMAT, IMRT, and IMPT, respectively, while maintaining at least 95% of the prescribed dose in 95% of the whole-brain target volume. Estimated risks for developing memory impairment after a prescribed dose of 23.4 Gy were 47% (95% confidence interval [CI], 21%–69%), 44% (95% CI, 21%–65%), 41% (95% CI, 22%–60%), and 33% (95% CI, 23%–44%) with opposing fields, IMAT, IMRT, and IMPT, respectively. Neurogenic niche sparing during cranial irradiation of pediatric patients with MB is feasible and is estimated to lower the risks of long-term neurocognitive sequelae. Greatest sparing is achieved with intensity-modulated proton therapy, thus making this an attractive option to be tested in a prospective clinical trial.
Keywords: CNS, medulloblastoma, neurocognitive sparing, radiotherapy, risk modeling
Radiotherapy is one of the most effective therapeutic modalities for malignant central nervous system (CNS) tumors. Medulloblastoma (MB) accounts for about 20% of CNS tumors in children, and the peak incidence is at 5 years of age.1 The prognosis of MB, which is a primitive neuro-ectodermal tumor (PNET) located in the cerebellum or the fourth ventricle, improved considerably with the introduction of adjuvant radiotherapy.1 This success has generated a growing population of children surviving their cancer. Irradiation of the CNS is, however, associated with a risk of severe adverse effects, including neurocognitive sequelae. Younger age at treatment is correlated with more severe cognitive deficits.2,3 The detailed pathogenesis of cognitive dysfunction after radiotherapy is yet unknown, but several mechanisms likely play a role.4 Post-irradiation MRI reveals multiple changes, including white matter microstructure disruptions,5 decreased size of corpus callosum and subregions,6 and abnormal hippocampal development.7 In mammals, neurogenesis occurs at 2 major sites, the subgranular zone (SGZ) in the dentate gyrus of the hippocampus8,9 and the subventricular zone (SVZ) of the lateral ventricles.10,11 The stem and progenitor cells in these niches are sensitive to irradiation, and recent discoveries in neural stem cell biology and brain plasticity have provided clues toward a deeper understanding of the effects of ionizing radiation of the developing brain.12–19 In rodents, neurogenesis has been shown to be important for hippocampal-dependent memory formation.20,21 Several human studies demonstrate a relationship between absorbed dose to the brain and cognitive outcome,22–25 and recent studies show more specifically a correlation between temporal lobe irradiation and neurocognitive sequelae.26,27 Thus, because neurogenesis is important for hippocampal-dependent memory and the hippocampus is situated in the temporal lobe, it seems reasonable to hypothesize that the hippocampus is the main critical structure for radiotherapy-related cognitive function impairment. The role of SVZ irradiation for cognitive outcome shown is less clear; however, because of the proposed regenerative features of neurogenesis, the SVZ is included as an organ at risk (OAR) in this study.
New radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT), intensity-modulated arc therapy (IMAT), and intensity-modulated proton therapy (IMPT), have facilitated the delivery of highly conformal dose distributions. Defining the hippocampus and the SVZ as OARs on a pre-radiotherapy magnetic resonance (MR) examination fused with CT scan facilitates reducing the dose to these regions during craniospinal irradiation (CSI). This would be particularly relevant for the hippocampus, because this region is important for memory function.28 With a dose prescription of 23.4–36 Gy, which is used for patients with MB, neurocognitive dysfunction is reported to be a common adverse effect.29,30 If a significant reduction in dose to the OARs can be achieved, a reduction in late cognitive adverse effects would be expected. A steep dose gradient would be needed to achieve a homogeneous dose to the rest of the brain while sparing the hippocampus and SVZ.
Inverse-planned intensity-modulated therapy aims at optimizing the dose distribution inside the patient's body, guided by dose-volume objectives for tumor and OARs. The dose distribution is thus shaped around the target volume, often with a steep dose gradient to the neighboring tissue. The trade-off between treating the target to a sufficient and homogeneous dose and avoiding the OARs can be manipulated by the choice of planning dose-volume objectives. Conventional therapy, IMRT, and IMAT use photon beams for radiation dose deposition. Protons deposit their energy in tissue in a very different fashion than do photons, and their main characteristic is the sharp dose gradient at the distal edge of the beam. The IMPT technique therefore allows intensity modulation with a sharper dose fall-off, compared with the photon techniques.
Our aim with this retrospective dose planning study, focusing on the cranial component of the CSI course, was to evaluate how much modern radiation therapy techniques can reduce the absorbed dose to the hippocampus and SVZ and still treat the rest of the brain to an adequate radiation dose. We also evaluated the potential clinical benefit of this dose reduction based on dose-response data from a large clinical series with long-term follow-up on neurocognitive function of pediatric patients treated with radiation. In this study, we intend to explore the technological foundation for a prospective clinical trial based on dose-sparing of the hippocampus and SVZ.
Materials and Methods
Patients and Treatment Planning
Six patients with MB who all received CSI during 2002–2007 at Sahlgrenska University Hospital, Gothenburg, Sweden, were re-planned. Their age at time of treatment ranged from 6 to 11 years (median, 7.5 years). The clinical target volume (CTV) in this study comprised the whole brain, disregarding the spinal part of the target. The dose contribution to the hippocampus and SVZ from the boost treatment was assumed to be negligible in the present analysis. In reality, this dose contribution could be important depending on the size and location of the primary tumor, the treatment strategy used (treating the whole posterior fossa or only the tumor bed with a margin), and the treatment technique. Thus, the estimates presented in this study will apply to cases in which the assumption of a zero dose contribution to the hippocampus and SVZ from the boost is reasonable.
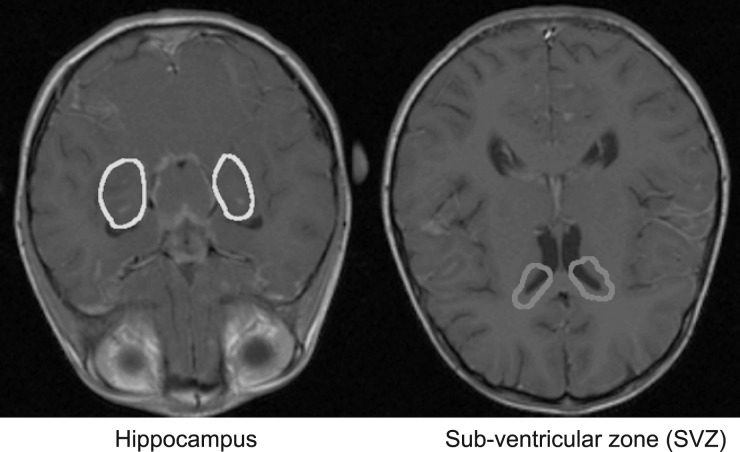
The OARs consisted of the hippocampus, the SVZ, and the eyes, which were delineated in each of the patients based on fused T1 and T2 MRI and CT scans. Target and OARs were delineated by an experienced neuroradiologist. The SVZ was defined as the lateral wall of the lateral ventricles with a margin of 2 mm. Figure 1 illustrates the SVZ and hippocampus overlaid on a transversal MRI scan. The prescribed dose was set to 36 Gy or 23.4 Gy, in 1.8 Gy/fraction. All treatment plans were generated using the Eclipse treatment planning system, version 10 (Varian Medical Systems).
Fig. 1.
The sub-ventricular zone (magenta) and hippocampus (yellow) overlaid on a transversal T1-weighted MRI scan.
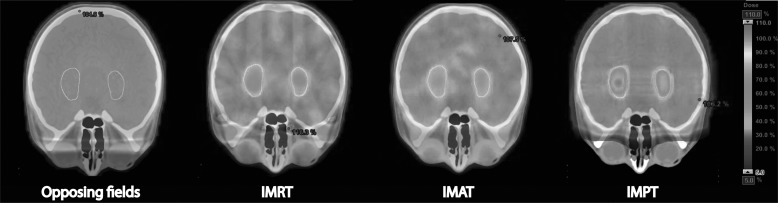
Treatment planning was performed with the aim of minimizing the mean absorbed dose to the hippocampus and the SVZ without compromising CTV coverage. Four different radiotherapy delivery techniques were tested in this study, as shown in Fig. 2: 2 opposing cranial fields (which is still commonly used for cranial irradiation during the CSI course), IMRT with 7 fields, IMAT with 3 arcs (2 360 degree arcs and 1 noncoplanar 180 degree arc), and spot-scanned IMPT with 3 incident fields.
Fig. 2.
Absorbed radiation dose shown in color-wash for (from left) opposing fields, IMRT, IMAT, and IMPT with the hippocampus segmented as the yellow contour. Treatment planning parameters corresponded to OAR setting 1 as given in Table 1.
For the opposing field technique, the OAR sparing was limited to partial shielding of the eyes and the oral cavity. To ensure that the results of the treatment planning in this study were as user-independent as possible, we defined a fixed set of dose-volume objectives for the 3 inversely-optimized techniques: IMRT, IMAT, and IMPT. The objectives were defined in relation to 4 different levels of OAR sparing priority as shown in Table 1, with the intent of finding how the CTV radiation dose homogeneity was affected by the different levels of OAR sparing. We derived a linear correlation between the mean dose received by the hippocampus and the SVZ, further referred to as neurocognitive OAR dose, and volume of the CTV receiving at least 95% of the prescribed dose, the V95. We then estimated what OAR sparing could be achieved, for each individual patient, with the different techniques if the V95 was set to be at least either 98% or 95%, with the mean target dose fixed at 100% of the prescribed dose for all techniques. By doing so, we attempted to obtain an objective measure of how much the different techniques were able to spare the neurocognitive OARs and how this was affected by the limit chosen as the acceptable target coverage.
Table 1.
Dose-volume objectives used in the inversely-optimized treatment planning. The same priority was applied for the eyes, hippocampus, and SVZ in each of the various OAR settings
| Structure | Volume (%) | Dose (Gy) | Priority |
|---|---|---|---|
| Body | 0 | 37.5 | 250 |
| CTV | 0 | 36.5 | 225 |
| 100 | 35.5 | 225 | |
| OAR setting 0 | – | – | – |
| OAR setting 1 | 0 | 5 | 65 |
| OAR setting 2 | 0 | 5 | 120 |
| OAR setting 3 | 0 | 5 | 160 |
Estimating the Risk of Neurocognitive Impairment
A dose-response relationship for neurocognitive outcome was, until recently, available from animal studies only. However, Armstrong et al. provided dose-response data based on long-term survivors of childhood CNS malignancies.27 The authors found a correlation between radiation dose to the temporal lobe, while controlling for dose to other parts of the brain, and the risk of reduced task efficiency, organization, and memory. The assumption in the present study is that sparing the hippocampus and SVZ would be as effective as sparing the whole temporal lobe in terms of reducing neurocognitive adverse effects. Stratifying their data into a separate MB/PNET group, Armstrong et al. published odds ratios (ORs) with 95% confidence intervals (CIs), per 10 Gy increase in temporal lobe dose, for developing various neurological sequelae. From these ORs and the baseline risk of impairment with no temporal lobe irradiation, we derived logistic dose-response functions as follows:
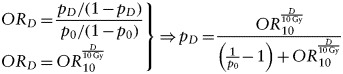 |
(1) |
where D is the dose in Gy, OR10 is the corresponding OR at 10 Gy, p0 is the baseline risk of impairment at zero dose, and pD is the risk of impairment at dose D. The baseline risk was estimated from patients from the whole cohort who had not received any temporal lobe irradiation, not only from the stratified group. Separate estimates were not given, possibly because there were only 5 patients in the MB/PNET group who did not receive any cranial irradiation,31 which was too few to obtain a reliable baseline estimate. Applying the neurocognitive OAR doses from the treatment planning to the derived dose-response relations, risks of neurocognitive impairment between radiotherapy techniques were estimated for 2 prescribed dose levels: 23.4 Gy and 36 Gy.
Statistical Analysis
The largest source of uncertainty in this study was the OR estimates from Armstrong et al. on which we based our dose-response functions. To test whether the risks of impairment between treatment techniques were significantly different, a paired random number (Monte Carlo) test comparing the OR between 2 treatment techniques was performed. Samples were drawn randomly from log-normal distributions corresponding to the mean and 95% CI of the dose-response parameters. For each of the different neurocognitive end points, the OR between techniques with 95% CI was calculated by inverse variance weighting. To account, to some extent, for the possible underestimation of the variance resulting from the small number of patients, a bootstrapping procedure was applied. Ten million samples of the 6 patients were drawn with replacement. A mean point estimate OR with CI was then calculated for each of the 10 million samples and a normal distribution matched to each one. Finally, 1 sample was randomly drawn from each of the 10 million distributions, giving the final OR and 95% CI as the mean and 2.5–97.5 percentile of the randomly drawn samples.
Results
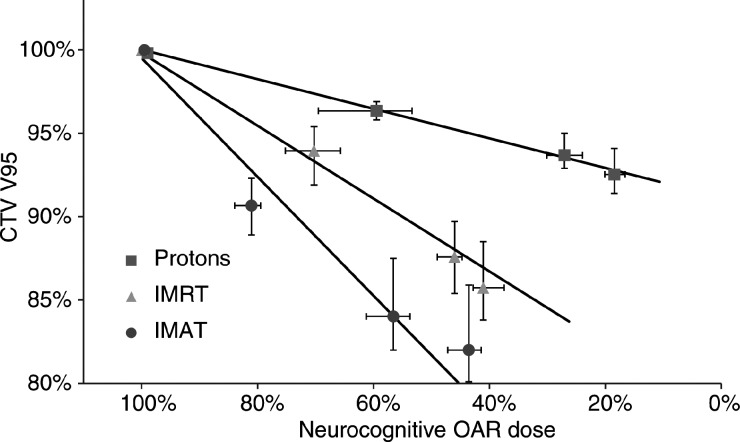
There was a clear effect on CTV coverage when tightening the OAR dose constraint in the treatment planning process, as shown in Fig. 3. The CTV coverage was least affected for the IMPT plans, suggesting that that the OARs can be spared to a greater extent with the proton technique. Of the highly conformal photon techniques, IMRT was slightly more effective than IMAT at sparing the neurocognitive OARs.
Fig. 3.
Mean values for the 6 patients showing how the coverage (V95) of the whole-brain target volume is affected by lowering the dose to the neurocognitive OARs. No priority settings were possible for the opposing-field technique. The solid lines represent linear regressions through the 4 data points and the uncertainty bars show the range of doses within the patient group.
On the basis of the data in Fig. 3, it was deemed reasonable to describe the correlation between CTV V95 and neurocognitive OAR dose by a simple linear relation. The neurocognitive OAR doses, corresponding to CTV V95 equal to either 98% or 95%, were calculated using the linear regressions in Fig. 3 and are given in Table 2. These doses thus represented the lowest neurocognitive OAR dose achievable, with the different techniques, for a CTV coverage of V95 at either 98% or 95%.
Table 2.
Mean doses (range) to neurocognitive organs at risk represented as percentage of the prescribed treatment dose.
| Technique | Hippocampus (%) | SVZ (%) | Neurocognitive OAR (%) |
|---|---|---|---|
| CTV V95 = 98% | |||
| Protons | 77.0 (71.0–80.6) | 79.2 (71.6–87.0) | 78.0 (71.3–81.1) |
| IMRT | 89.7 (87.0–92.7) | 90.5 (86.5–93.3) | 90.0 (86.9–93.0) |
| IMAT | 97.2 (95.9–98.4) | 98.7 (96.6–101.7) | 97.8 (96.2–99.8) |
| Opposing fields | 98.0 (97.2–99.3) | 100.4 (99.4–101.3) | 99.2 (98.9–100.0) |
| CTV V95 = 95% | |||
| Protons | 41.9 (25.9–50.3) | 42.7 (27.4–51.5) | 42.3 (26.6–51.2) |
| IMRT | 77.1 (72.5–81.1) | 77.2 (70.6–81.5) | 77.1 (71.5–81.3) |
| IMAT | 87.1 (82.9–89.4) | 89.6 (84.3–94.2) | 88.3 (83.6–91.0) |
| Opposing fields | 98.0 (97.2–99.3) | 100.4 (99.4–101.3) | 99.2 (98.9–100.0) |
Note: The neurocognitive OAR dose was taken as the average dose of the hippocampus and the SVZ.
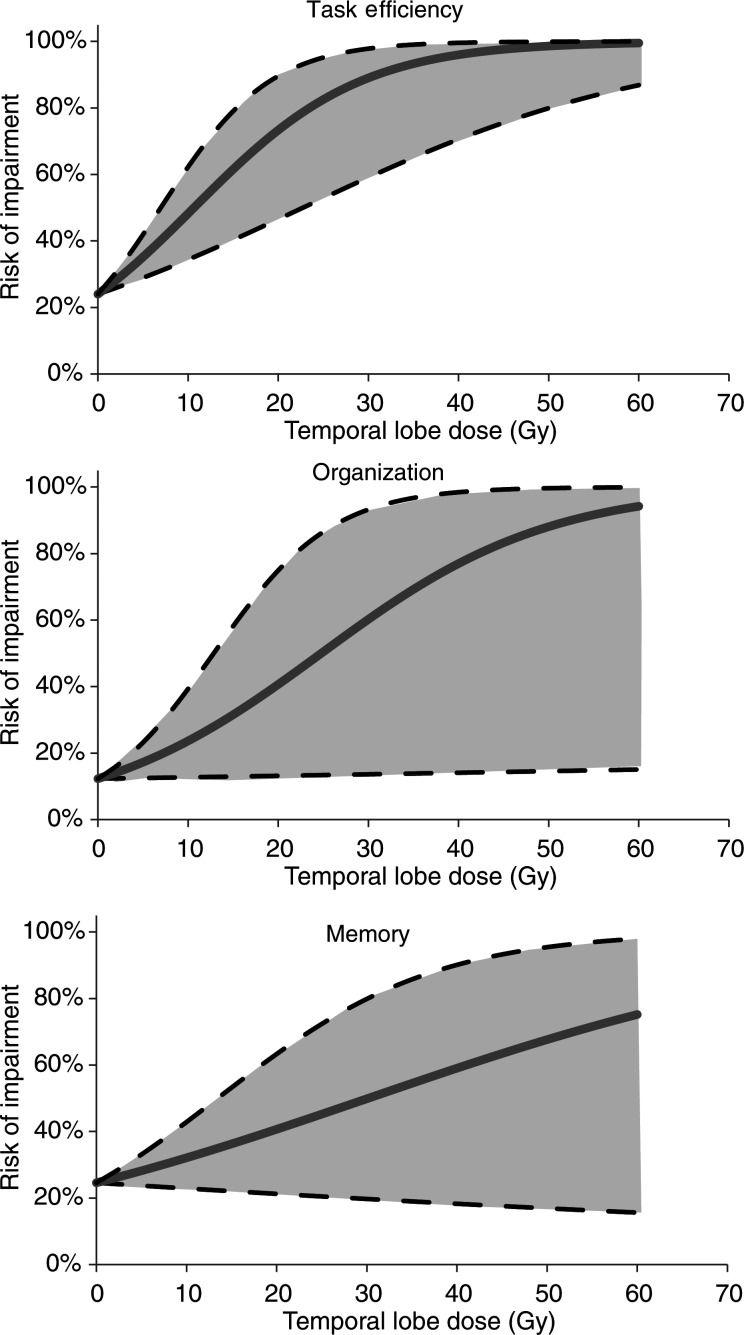
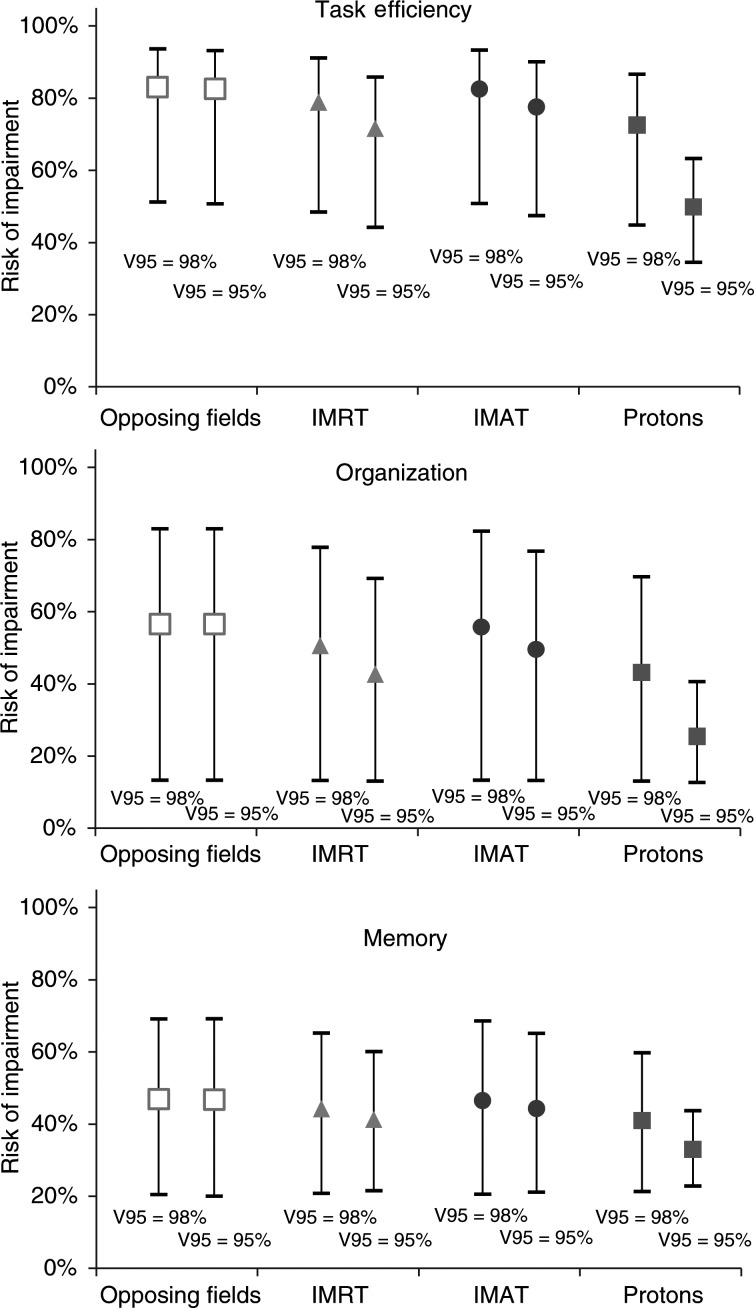
The logistic dose-response relationships shown in Fig. 4 were derived from the ORs and baseline risks in Armstrong et al. according to Equation 1. Figure 5 shows the estimated incidence of neurological sequelae based on the doses in Table 2 and the derived dose-response relationships.
Fig. 4.
Logistic dose-response functions relating to task efficiency, organization, and memory. The blue line shows the estimated dose-response relation and the shaded area the corresponding 95% confidence intervals (CI). Based on odds ratios (95% CI) of 2.95 (1.66–5.22), 2.21 (1.04–4.70) and 1.45 (0.91–2.30) for task efficiency, organization, and memory respectively. Baseline risks at zero Gy were taken from the group of patients not receiving any temporal lobe irradiation in Armstrong et al.
Fig. 5.
Mean values for the 6 patients of the estimated complication incidences, given with 95% confidence intervals, for a prescribed target dose of 23.4 Gy. Comparisons are made between radiotherapy techniques and the choice of acceptable target coverage. Estimates are based on the dose-response curves derived from Armstrong et al. and Monte Carlo sampled to yield the confidence limits.
As shown in Fig. 5, the risks of developing various neurological impairments were estimated to be lower with IMPT, compared with photon therapy. In addition, relaxing the CTV coverage constraint has a large impact on the possible neurocognitive function sparing with IMPT but little impact for the photon techniques.
Discussion
The clinical relevance of low radiation doses to the CNS remains controversial, with a few studies reporting measurable decline in mental functioning after doses as low as 2 Gy and below.32,33 However, there is no doubt that the high doses of radiation prescribed for treating MB lead to neurocognitive deficits and are likely to influence later academic achievements and social life.34–36 Sparing the entire temporal lobe from irradiation would considerably reduce the dose to a large part of the target volume in MB and, thereby, likely increase the risk of relapse. Here, we assume that sparing the hippocampus and SVZ provides the same cognitive function sparing, but with the ability of maintaining a CTV coverage of V95 at either 95% or 98%. We show that advanced radiation therapy techniques can reduce the dose to the hippocampus and SVZ without compromising the dose coverage of the whole-brain target volume.
To put our dosimetric findings into a clinical context, the risks of developing various neurocognitive sequelae were estimated, based on data from long-term follow-up of pediatric MB survivors.27 IMPT is superior to the conventional technique and also significantly better than IMRT and IMAT. When the CTV V95 constraint is relaxed from 98% to 95%, this gives a benefit mainly with the IMPT technique. In practice, however, this could partly be offset by the risk involved in lowering the target coverage constraints for IMPT because of the sharp dose fall-off at the distal field edge.
Slightly lower risk of impairment is estimated for IMRT than for IMAT but with wide CI, resulting from uncertainty in the dose-response parameters. However, the paired Monte Carlo test shows that ORs between techniques are all significantly different at the 95% level (Table 3). The high level of significance in the paired test reflects that, in each comparison, the estimates for all 6 patients favored the same technique, albeit to varying degrees. For a linear correlation between dose and the logarithm of the OR as assumed by Armstrong et al.,27 a lower dose will always give a lower OR as long as the slope is positive (OR, >1). Consequently, the paired comparison of techniques circumvents the effect of uncertainty in the magnitude of a positive slope of the dose-response curve. Indeed, any positive dose-response function will retain the relative ranking of the plans. However, systematic errors could affect the comparison, for example, if mean dose is a poor predictor of toxicity or if there is a negative dose-response over a range of dose. Such systematic effects are not accounted for in the CI in Table 3. Furthermore, the small number of patients could lower the generalizability of our findings to a larger patient cohort.
Table 3.
Estimates of odds ratios between the different treatment techniques and the 2 different dose prescriptions with corresponding 95% confidence intervals (CIs)
| Task efficiency |
Organization |
Memory |
||||
|---|---|---|---|---|---|---|
| 36 Gy | 23.4 Gy | 36 Gy | 23.4 Gy | 36 Gy | 23.4 Gy | |
| Protons/IMRT V95 = 98% | 0.64 (0.58–0.71) | 0.75 (0.70–0.80) | 0.73 (0.66–0.81) | 0.82 (0.76–0.88) | 0.87 (0.80–0.93) | 0.91 (0.87–0.95) |
| Protons/IMRT V95 = 95% | 0.28 (0.23–0.33) | 0.44 (0.38–0.49) | 0.43 (0.35–0.51) | 0.56 (0.49–0.65) | 0.67 (0.57–0.78) | 0.76 (0.68–0.86) |
| IMRT/IMAT V95 = 98% | 0.77 (0.70–0.84) | 0.84 (0.78–0.89) | 0.83 (0.75–0.90) | 0.88 (0.82–0.93) | 0.92 (0.87–0.96) | 0.94 (0.91–0.98) |
| IMRT/IMAT V95 = 95% | 0.68 (0.61–0.74) | 0.78 (0.72–0.83) | 0.76 (0.68–0.84) | 0.83 (0.77–0.89) | 0.88 (0.82–0.94) | 0.92 (0.88–0.96) |
Note: An upper CI limit below 1.00 represents significantly different odds ratios between techniques in the paired Monte Carlo test.
Our estimated risks of neurological impairment are based on translating results from a study by Armstrong et al.27 based on long-term survivors of pediatric CNS malignancies. Our estimates are, thus, subject to the limitations of that study, such as the collection of data through questionnaires designed for neurocognitive function estimation. There are likely also some uncertainties in the radiation dosimetry, because this was based on retrospective evaluation of individual radiotherapy records and the fact that the majority of these patients were treated with older radiation delivery techniques. Despite these caveats, the data in this study are based on a large patient material followed up for a long time and with complete records of cranial radiotherapy for the 818 patients included. They also stratified patients specifically into a MB/PNET group, making the application in our study suitable.
The dose-response relationship between temporal lobe irradiation and neurocognitive impairment shown by Armstrong et al. was not limited to the MB/PNET group. For the whole patient cohort, the risk of task efficiency impairment was 24.0%, 34.7%, 48.3%, and 47.3% for temporal lobe doses of 0 Gy, 0–30 Gy, 30–50 Gy, and >50 Gy, respectively. The corresponding risks of organizational impairment were 12.3%, 12.2%, 17.0%, and 22.6% and 24.6%, 33.3%, 45.1%, and 51.4% for impaired memory function. In health-related quality of life estimates they saw a correlation between temporal lobe irradiation and social functioning, physical limitations, and general health difficulties. Armstrong et al. also showed that patients in the MB/PNET group have a steeper temporal lobe dose-response, compared with survivors of other CNS tumors. ORs per 10 Gy–dose increase were 2.95 (95% CI, 1.66–5.22), 2.21 (95% CI, 1.04–4.70), and 1.45 (95% CI, 0.91–2.30) for task efficiency, organization, and memory, respectively, in the MB/PNET group and 1.10 (95% CI, 1.00–1.21), 1.12 (95% CI, 0.99–1.26), and 1.14 (95% CI, 1.03–1.25) for all other CNS tumors. The authors stipulate that a possible contributor to the substantial neurocognitive impairment in MB survivors could be the high-dose posterior fossa boost, the hypothesis being that this could cause loss of supratentorial connections between the cerebellum and the frontal region of the brain, which might affect executive functioning. This could possibly bias the baseline estimates of neurocognitive function at low temporal lobe doses but will probably not affect the steepness of the dose-response relationships. The practice change in many centers towards boosting only the tumor bed with a margin, rather than the whole posterior fossa, means that neurocognitive decline attributable to cerebellar irradiation would depend on the size and location of the tumor. Unfortunately, despite extensive research, the cerebellar contribution to cognitive and affective regulation remains poorly understood.37
The potential risk of tumor relapse from hippocampal-sparing radiotherapy needs to be defined. However, the hippocampus and SVZ made up only 1.3% of the whole-brain volume on average for the patients in our study. Thus, only a small portion of the target is underdosed.
Our study extends the recent study by Redmond et al., in that we compare not only IMRT with standard opposing fields but also IMAT and IMPT.38 IMRT is generally used with caution in children because of concerns about secondary malignancies when exposing large areas to low doses of radiation. In proton therapy, the risk of developing radiation-induced cancers due to secondary neutron irradiation is of special concern in children.39 The IMPT plans in this study used spot-scanned delivery, which exposes the patient to considerably lower secondary neutron doses than passive scattering techniques. Although beyond the scope of this study, the risk of secondary malignancies needs to be considered in the choice of treatment modality, especially when addressing the spinal part of a CSI treatment course. Furthermore, Merchant et al. have stated that a reduction in low- and medium-dose volumes in the supratentorial brain benefits long-term cognitive outcome, which again favors the IMPT technique.40
In summary, we demonstrate the dosimetric feasibility of sparing the hippocampus and SVZ during cranial irradiation, along with estimates of the potential clinical benefit. Our estimates show that the frequency of neurological adverse effects of radiotherapy could be considerably reduced, especially with intensity-modulated proton therapy. Validation of this strategy should come from large prospective clinical trials. Hopefully, our study can inspire such a trial, preferably with IMPT, because this technique is predicted to offer the greatest patient benefit.
Conflict of interest statement. P.M.R. has a research agreement with Varian Medical Systems. All other authors: None declared.
Funding
This work was supported by the Danish Child Cancer Foundation (to N.P.B.), Wilhelm and Martina Lundgren's scientific fund 2 (grant vet2-43/2010 ref 256 to M.B.), Sahlgrenska University Hospital's foundations (grant 5889 act 8181 to M.B.), Jubileumskliniken's anti-cancer research fund (grant 2006:10 to M.B.), and National Cancer Institute (grant 2P30 CA 014520-34 to S.M.B.).
Acknowledgments
Malin Blomstrand and N. Patrik Brodin are co-first authors.
References
- 1.Fossati P, Ricardi U, Orecchia R. Pediatric medulloblastoma: toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev. 2009;35(1):79–96. doi: 10.1016/j.ctrv.2008.09.002. doi:10.1016/j.ctrv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Danoff BF, Cowchock FS, Marquette C, Mulgrew L, Kramer S. Assessment of the long-term effects of primary radiation therapy for brain tumors in children. Cancer. 1982;49(8):1580–1586. doi: 10.1002/1097-0142(19820415)49:8<1580::aid-cncr2820490810>3.0.co;2-7. doi:10.1002/1097-0142(19820415)49:8<1580::AID-CNCR2820490810>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. doi: 10.1200/JCO.2005.00.703. doi:10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 4.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35(6):659–663. doi: 10.3109/02841869609083995. doi:10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 5.Khong PL, Leung LH, Fung AS, et al. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24(6):884–890. doi: 10.1200/JCO.2005.02.4505. doi:10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SL, Reddick WE, Glass JO, Gajjar A, Goloubeva O, Mulhern RK. Decline in corpus callosum volume among pediatric patients with medulloblastoma: longitudinal MR imaging study. AJNR Am J Neuroradiol. 2002;23(7):1088–1094. [PMC free article] [PubMed] [Google Scholar]
- 7.Nagel BJ, Palmer SL, Reddick WE, et al. Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. AJNR Am J Neuroradiol. 2004;25(9):1575–1582. [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. doi:10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. doi:10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 10.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–189. doi: 10.1016/0896-6273(93)90281-u. doi:10.1016/0896-6273(93)90281-U. [DOI] [PubMed] [Google Scholar]
- 11.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellstrom NA, Bjork-Eriksson T, Blomgren K, Kuhn HG. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27(3):634–641. doi: 10.1634/stemcells.2008-0732. doi:10.1634/stemcells.2008-0732. [DOI] [PubMed] [Google Scholar]
- 13.Andres-Mach M, Rola R, Fike JR. Radiation effects on neural precursor cells in the dentate gyrus. Cell Tissue Res. 2008;331(1):251–262. doi: 10.1007/s00441-007-0480-9. doi:10.1007/s00441-007-0480-9. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda A, Fukuda H, Swanpalmer J, et al. Age-dependent sensitivity of the developing brain to irradiation is correlated with the number and vulnerability of progenitor cells. J Neurochem. 2005;92(3):569–584. doi: 10.1111/j.1471-4159.2004.02894.x. doi:10.1111/j.1471-4159.2004.02894.x. [DOI] [PubMed] [Google Scholar]
- 15.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 16.Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99(1):33–41. doi: 10.1016/s0306-4522(00)00151-2. doi:10.1016/S0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 17.Naylor AS, Bull C, Nilsson MK, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci USA. 2008;105(38):14632–14637. doi: 10.1073/pnas.0711128105. doi:10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georg Kuhn H, Blomgren K. Developmental dysregulation of adult neurogenesis. Eur J Neurosci. 2011;33(6):1115–1122. doi: 10.1111/j.1460-9568.2011.07610.x. doi:10.1111/j.1460-9568.2011.07610.x. [DOI] [PubMed] [Google Scholar]
- 19.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. doi:10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 20.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. doi:10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 21.Acharya MM, Christie LA, Lan ML, et al. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci USA. 2009;106(45):19150–19155. doi: 10.1073/pnas.0909293106. doi:10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10(9):1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- 23.Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- 24.Kieffer-Renaux V, Viguier D, Raquin MA, et al. Therapeutic schedules influence the pattern of intellectual decline after irradiation of posterior fossa tumors. Pediatr Blood Cancer. 2005;45(6):814–819. doi: 10.1002/pbc.20329. doi:10.1002/pbc.20329. [DOI] [PubMed] [Google Scholar]
- 25.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45(1):137–145. doi: 10.1016/s0360-3016(99)00177-7. doi:10.1016/S0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 26.Jalali R, Mallick I, Dutta D, et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77(4):974–979. doi: 10.1016/j.ijrobp.2009.06.025. doi:10.1016/j.ijrobp.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12(11):1173–1186. doi: 10.1093/neuonc/noq104. doi:10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406(4):449–460. doi:10.1002/(SICI)1096-9861(19990419)406:4<449::AID-CNE3>3.0.CO;2-I. [PubMed] [Google Scholar]
- 29.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. doi:10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 30.Lannering B, Marky I, Lundberg A, Olsson E. Long-term sequelae after pediatric brain tumors: their effect on disability and quality of life. Med Pediatr Oncol. 1990;18(4):304–310. doi: 10.1002/mpo.2950180410. doi:10.1002/mpo.2950180410. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. doi: 10.1093/jnci/djp148. doi:10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall P, Adami HO, Trichopoulos D, et al. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ. 2004;328(7430):19. doi: 10.1136/bmj.328.7430.19. doi:10.1136/bmj.328.7430.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ron E, Modan B, Floro S, Harkedar I, Gurewitz R. Mental function following scalp irradiation during childhood. Am J Epidemiol. 1982;116(1):149–160. doi: 10.1093/oxfordjournals.aje.a113389. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14(4):298–303. doi: 10.1016/j.ejpn.2009.12.006. doi:10.1016/j.ejpn.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. doi: 10.1037/a0016674. doi:10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boman KK, Lindblad F, Hjern A. Long-term outcomes of childhood cancer survivors in Sweden: a population-based study of education, employment, and income. Cancer. 2010;116(5):1385–1391. doi: 10.1002/cncr.24840. doi:10.1002/cncr.24840. [DOI] [PubMed] [Google Scholar]
- 37.Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Marien P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110(8):763–773. doi: 10.1016/j.clineuro.2008.05.013. doi:10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Redmond KJ, Achanta P, Grossman SA, et al. A radiotherapy technique to limit dose to neural progenitor cell niches without compromising tumor coverage. J Neurooncol. 2011;104(2):579–587. doi: 10.1007/s11060-011-0530-8. doi:10.1007/s11060-011-0530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newhauser WD, Fontenot JD, Mahajan A, et al. The risk of developing a second cancer after receiving craniospinal proton irradiation. Phys Med Biol. 2009;54(8):2277–2291. doi: 10.1088/0031-9155/54/8/002. doi:10.1088/0031-9155/54/8/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51(1):110–117. doi: 10.1002/pbc.21530. doi:10.1002/pbc.21530. [DOI] [PubMed] [Google Scholar]