Abstract
Cyclooxygenases (COX-1 and COX-2) are key enzymes in the conversion of arachidonic acid to prostaglandins and other lipid mediators. Because it can be induced by inflammatory stimuli, COX-2 has been classically considered as the most appropriate target for anti-inflammatory drugs. However, recent data indicate that COX-2 can mediate neuroprotection and that COX-1 is a major player in the neuroinflammatory process. We discuss the specific contributions of COX-1 and COX-2 in various neurodegenerative diseases and in models of neuroinflammation. We suggest that, owing to its predominant localization in microglia, COX-1 might be the major player in neuroinflammation, whereas COX-2, which is localized in neurons, might have a major role in models in which the neurons are directly challenged. Overall, the benefit of using COX-2 inhibitors should be carefully evaluated and COX-1 preferential inhibitors should be further investigated as a potential therapeutic approach in neurodegenerative diseases with an inflammatory component.
Introduction
Neuroinflammation is an important mechanism in the defense response to pathogenic events, traumatic injury and environmental toxins, but it is also recognized as a major contributor to various neurological and neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), traumatic brain injury (TBI), HIV dementia and prion diseases [1]. Brain-resident glial cells, mainly microglia (Box 1), mediate an innate immune response and contribute to the progression of the diseases [2–4]. Activated microglia produce several proinflammatory and neurotoxic mediators including complement, cytokines, chemokines, arachidonic acid (AA) and its lipid metabolites and reactive oxygen and nitrogen species (Figure 1), several of which contribute directly to neuronal injury [4–6].
Box 1. Microglia and their role in the CNS.
Microglia are resident immunocompetent and phagocytic cells of the CNS, and they are one of the three glia cell types with astrocytes and oligodendrocytes. Microglia are distributed in large non-overlapping regions throughout the mature CNS and they represent 10–15% of total brain cells. Under physiological conditions, microglia have long and highly branched (ramified) morphology with small cell bodies. Microglial processes are highly dynamic and undergo rapid extension and retraction and constantly carry out homeostatic surveillance to sense and respond to CNS abnormalities [74]. Microglia rapidly respond to various immunologic stimuli and abnormalities in the brain parenchyma and they depart from the surveillance mode to reactive phenotype to manage the altered homeostasis. Microglial activation is usually determined by the gradual changes of their morphology from the ramified state to the activated amoeboid state and by the upregulation of surface molecules such as the major histocompatibility complex [75]. Activated microglia have migratory and phagocytic properties to reach the injured site and remove cellular debris or foreign materials. Acute activation of microglia often results in secretion of neurotrophic factors that limit tissue injury by aiding in repair processes. However, activated microglia can also secrete a variety of proinflammatory mediators such as cytokines, chemokines, reactive oxygen and nitrogen species and PGs that are known to potentiate the inflammatory cascade and to cause neuronal damage by enhancing oxidative stress and activating cell-death pathways. Excessive and protracted microglial reaction can become deleterious and lead to chronic neuroinflammation and neurodegeneration.
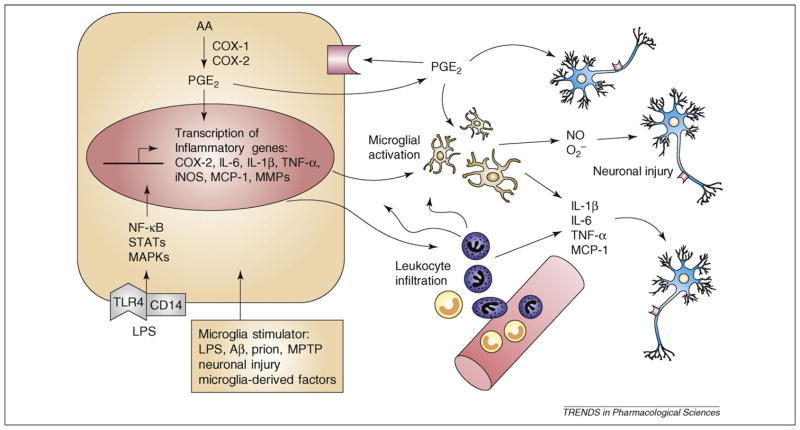
Figure 1.
Microglia-initiated inflammatory cascade and neuronal injury. Various stimuli can target microglia, including LPS, Aβ and prion. In particular, the interaction of LPS with TLR4–CD14 can trigger intracellular signaling cascades (e.g. NF-κB, MAPKs and JAK–STAT), leading to microglial activation and transcriptional induction of downstream proinflammatory mediators, including iNOS, NADPH oxidase and COX-2, and the subsequent release of cytokines (e.g. IL-1β, IL-6 and TNF-α), chemokines (e.g. MCP-1), nitric oxide and PGs, which are associated with neuroinflammation. Because COX-1 is constitutively expressed in microglia, it functions as the primary source of PGs in the early phase of inflammation. LPS and other inflammatory stimuli also activate MMPs, which regulate BBB permeability and, consequently, cause infiltration of peripheral leukocytes into the brain. Recruitment of peripheral leukocyte exacerbates the inflammatory response and results in neuronal damage. Abbreviations: BBB, blood–brain barrier; COX, cyclooxygenase; IL-1β, interleukin-1β; iNOS, inducible nitric oxidase synthase; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein 1; MMPs, matrix metalloproteinases; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor α.
The hypothesis that neuroinflammation is a key component in the progression of neurodegenerative diseases, particularly AD, has been corroborated by several independent epidemiological studies. Early use of nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenase (COX) activity, reduces the risk of developing AD later in life [7,8]. Several epidemiological and experimental studies using parkinsonian models indicated that NSAIDs have neuroprotective properties also against dopaminergic neurotoxicity [9,10], although recent studies did not show any protective effect of NSAIDs in PD [11,12].
COX is the key and rate-limiting enzyme in the conversion of AA to prostaglandins (PGs) (Figure 2), which are lipid metabolites that are involved in several physiological and pathological processes, including inflammation [13]. Two distinct COX isoforms have been characterized, COX-1 and COX-2, that differ interms of regulatorymechanisms, tissue distribution and preferential coupling to upstream and downstream enzymes in the central nervous system (CNS) [14–16]. COX-1, which is constitutively expressed in most tissues, has been classically considered as the isoform primarily responsible for homeostatic PG synthesis [17]. By contrast, COX-2 is mainly induced in response to inflammatory stimuli, which led to the concept that selective inhibition of COX-2 can reduce inflammation without affecting the physiological functions of COX-1-derived PGs and to the massive development and marketing of NSAIDs that selectively inhibit COX-2 over COX-1. However, in the CNS COX-2 is also constitutively expressed, mainly in hippocampal and cortical glutamatergic neurons where it has a pivotal role in synaptic activity, long-term synaptic plasticity [18,19] and in the neurovascular coupling during functional hyperemia [18]. Thus, it is not surprising that in several experimental models COX-2 has been linked to anti-inflammatory and neuroprotective properties [20–22], raising some concern about the potential adverse effects of selective COX-2 inhibitors. Recent studies also have indicated a previously unrecognized proinflammatory role of COX-1 in the pathophysiology of acute and chronic neurological disorders [23–26].
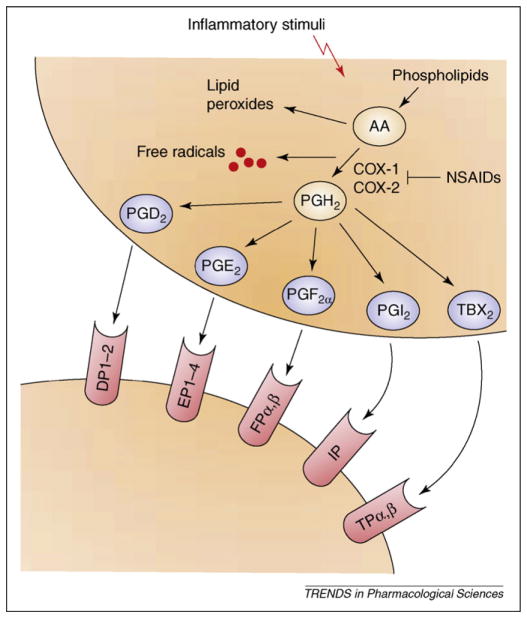
Figure 2.
The COX metabolic pathway of arachidonic acid (AA). In resting cells, AA is stored in an esterified form within the cell membrane phospholipids. Upon stimulation by neurotransmitters, neuromodulators or inflammatory stimuli, AA is released in its unesterified form from phospholipids and metabolized by different enzymes. Both COX-1 and COX-2 catalyze the stepwise conversion of AA into two short-lived intermediates, PGG2 and PGH2. PGH2 is then metabolized to a variety of PGs and related compounds (collectively referred to as prostanoids; PGD2, PGE2, PGF2α, PGI2 and TXA2) by the activity of specific terminal enzymes. These PGs can modulate physiological and pathological cellular functions by interacting with specific presynaptic or postsynaptic PG receptors expressed in the brain (i.e. DP1–2. EP1–4, FPα,β and IP). NSAIDs, a widely used class of anti-inflammatory drugs including ibuprofen, indomethacin and naproxen, can inhibit both COX-1 and COX-2 activity and alleviate pain and inflammation. Abbreviations: AA, arachidonic acid; COX, cyclooxygenase; PGH2, prostaglandin H2; PGI2, prostacyclin; TXB2, thromboxane B2; NSAIDs, nonsteroidal anti-inflammatory drugs.
Here, we propose to reconsider the prevailing hypothesis that, by being the isoform induced in response to inflammatory stimuli, COX-2 is the most appropriate pharmacological target for anti-inflammatory therapy, and we suggest that COX-1, owing to its predominant localization in microglia, is the major player in mediating the inflammatory response. We describe clinical and pre-clinical evidence on the specific involvement of COX-1 and COX-2 in experimental models of neuroinflammation and in neurological and neurodegenerative diseases with a marked inflammatory component.
Clinical and pre-clinical evidence of COX-1-mediated proinflammatory effects in neurodegenerative disorders and in models of neuroinflammation
In aged rats, COX-1 mRNA expression is selectively increased in the hippocampus, possibly causing an increased susceptibility to neuroinflammation [27], and COX-1-expressing microglia are found surrounding amyloid plaques in the AD brain [28], indicating a role of this isoform in the pathophysiology of the disease. Supporting this concept, a 6-month, double-blind, placebo-controlled study with indomethacin, a preferential COX-1 inhibitor, seemed to protect AD patients from cognitive decline [29] and reduced levels of β-amyloid (Aβ) in the hippocampus and cortex in a transgenic mouse model of AD [30]. The use of aspirin, a nonselective Aβ42-lowering agent that preferentially inhibits COX-1 by irreversibly acetylating its binding site, was also associated with a reduced risk of AD in humans [31].
In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, in which dopaminergic neurons in the substantia nigra are selectively injured, >65% of ventral midbrain PGE2 originated from COX-1 without any change in COX-1 expression; this indicates a predominant role of this isoform in PGE2 synthesis during MPTP-induced neurodegeneration [32]. Although COX-1 involvement in TBI has not been extensively studied, the evidence of accumulation of COX-1-expressing microglia and/or macrophages at peri-lesional areas and in the developing core in patients with TBI [23] indicates that further investigation of the potential beneficial effects of COX-1 inhibition is warranted. Coexpression of CD68 (phagocytic macrophages and microglial cells) in COX-1-expressing cells is found in areas of severe tissue damage and also around vacuoles in postmortem brains from patients with Creutzfeldt-Jacob disease (CJD) [33], a human encephalopathy belonging to the class of prion diseases, which are infectious, inherited or sporadic neurodegenerative disorders caused by the accumulation of the aberrantly folded prion protein. In sporadic CJD patients, high cerebrospinal fluid (CSF) levels of PGE2 were associated with short survival [34], indicating that the inflammatory response is correlated with the clinical outcome of the disease. Therefore, activated COX-1-expressing microglia in CJD patients could account for increased production of PGE2 and cytokines and might contribute to the complex process of neurodegeneration.
Elevated levels of PGE2, PGF2α and thromboxane B2 (TXB2) have been found in the CSF of patient with HIV-associated dementia and have been linked with the severity of cognitive impairment. Because COX-1 mRNA is upregulated approximately twofold in HIV-demented compared with the non-demented patients, whereas COX-2 mRNA expression is unchanged, the increase in the PG levels is likely to be selectively mediated by COX-1 [35]. Thus, the roles of microglia and COX-1 in the host defense system against viral pathogens deserve further investigation.
In spinal cords of a transgenic ALS mouse model (SOD1G93A mice), COX-1, and not COX-2, is the primary source of PGE2. However, deletion of COX-1 does not improve preservation of motor neurons and survival of transgenic mutant mice [36], indicating that although COX-1 can mediate neuroinflammation it does not contribute to functional deterioration in ALS.
Data on the role of COX-1 in ischemic brain injury are controversial and complicated by the use of different ischemia models. Furthermore, the ischemic model is complicated by the primary involvement of COX-2-expressing neurons and the secondary involvement of COX-1-expressing microglia in determining brain injury. Although mice lacking COX-1 are reported to be more vulnerable to focal cerebral ischemia, probably owing to a more severe cerebral blood-flow reduction in vulnerable regions [37], pharmacological inhibition of COX-1 potently reduced neuronal injury and oxidative stress in the hippocampus during transient global ischemia [38]. Interestingly, in human focal ischemic brains, COX-1-positive microglia accumulation was observed in peri-infarctional regions and in the developing necrotic core early after infarction [39]. Therefore, the potential therapeutic effects of COX-1 inhibition should be further investigated, particularly in the secondary post-ischemic neuroinflammatory phase.
One approach to understand the role of COX-1 in a pure model of neuroinflammation was to use the combination of pharmacological inhibition and gene disruption in mice after intracerebroventricular injection of lipopolysaccharide (LPS), a cell-wall component of Gram-negative bacteria widely used to activate innate immunity. LPS causes massive resident microglial activation and peripheral leukocyte infiltration into the CNS, accompanied by a robust and transient transcriptional activation of genes encoding proinflammatory cytokines, chemokines, PGs and free-radical-generating enzymes [26]. In response to LPS, COX-1-null mice have a decrease in glial activation, in inflammatory mediators such as PGE2, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and in protein oxidation, which are crucial factors contributing to the secondary progression of the inflammatory reaction and oxidative damage. Translocation and activation of nuclear factor-κB (NF-κB) and signal transducers and activators of transcription 3 (STAT3), which are important factors for signaling events during an inflammatory response, were also reduced in COX-1-null mice. Administration of SC-560, a specific COX-1 inhibitor, to wild-type mice before LPS injection recapitulates what is observed in the COX-1-null mice by decreasing brain levels of PGE2, PGD2, PGF2α and TXB2 and the expression of proinflammatory cytokines and chemokines [26]. COX-1 inhibition also seems to attenuate neuroinflammation-induced BBB disruption. Selective pharmacological inhibition of COX-1, but not of COX-2, reduced TNF-α-induced BBB disruption and free radical production [25], and indomethacin completely blocked LPS-induced permeability changes in cultured monolayers of brain microvessel endothelial cells [40].
Clinical and pre-clinical evidence of COX-2 involvement in neurodegenerative disorders and in models of neuroinflammation
There is a conflicting view about the role of COX-2 in models of neurotoxicity [41] and in neurodegenerative diseases. In AD, changes in COX-2 expression seem to depend on the stage of the disease, with an upregulation of COX-2 in early AD and a downregulation in advanced AD stages, which also correlate with PGE2 levels in the CSF [42,43]. However, after several large-scale randomized clinical trials with selective COX-2 inhibitors failed to show any beneficial effects in AD patients with mild to severe cognitive impairment [7,44,45], COX-2 inhibition does not seem to offer any therapeutic potential in AD. In support of the clinical data, the COX-2-selective inhibitor nimesulide did not reduce Aβ levels in the hippocampus and cortex of transgenic AD mice [30]. These data overall indicate that either preferential COX-1 inhibition is a better therapeutic approach than selectively targeting COX-2, that NSAIDs should be administered early before the onset of the symptoms, or that the beneficial effects are due to COX-independent effects of NSAIDs. To this end, some NSAIDs inactivate the transcription factors NF-κB and activator protein-1 (e.g. aspirin and COX-2-selective inhibitors) or activate the peroxisome proliferator-activated receptor-γ (PPAR-γ; e.g. indomethacin and ibuprofen). Moreover, a subset of NSAIDs, such as ibuprofen, flurbiprofen and diclofenac, have been shown to reduce serum levels of Aβ1–42, a primary component of senile plaques in AD [46,47]. However, a recent study using a pooled dataset from six prospective studies indicated that NSAIDs, which were categorized as selective or nonselective Aβ42-lowering agents, reduced the risk of AD without any apparent advantage for the subset of NSAIDs selectively lowering Aβ42, indicating that all conventional NSAIDs have a similar protective effect in humans [31]. Therefore, we suggest that the anti-inflammatory and neuroprotective effects of NSAIDs are specifically linked to the inhibition of COX-1 activity.
In postmortem PD specimens COX-2 expression is induced specifically within dopaminergic neurons of the substantia nigra and in the MPTP model of PD during the destruction of the nigrostriatal pathway [32,48]. Nevertheless, COX-2 deletion does not change the expression of inflammatory and oxidative markers after MPTP [32], and recent studies did not show any protective effect of NSAIDs in PD [12,49–51]. Because of the complexity of the disease, it is possible that combination of NSAIDs with other pharmacological agents with different mechanisms of action might enhance treatment effectiveness.
COX-2 expression has found to be increased in motor neurons, interneurons and glial cells in seven human sporadic ALS patients [52]. Levels of COX-2 mRNA and PGE2 are also increased in mutant SOD1 mice, and selective COX-2 inhibitors (e.g. celecoxib and rofecoxib) improve motor performance, extend survival and reduce CSF levels of PGE2 [53]. However, in a double-blinded, placebo-controlled clinical trial, celecoxib (800 mg/day) administered for 12 months to 300 ALS subjects did not slow the decline in muscle strength, vital capacity, motor unit number estimates, or affect survival [54]. Thus, COX-2 is unlikely to contribute to the progression of the disease in human ALS subjects.
In experimental TBI, COX-2 is expressed in both neurons and glia and has been implicated in brain injury [55]. However, a recent report indicates that COX-2 activity plays only a minor part in determining outcome after TBI, because COX-2 gene disruption did not change lesion volume 21 days after controlled cortical impact and did not alter spatial memory performance [56]. The ability of selective COX-2 inhibitors to limit brain injury remains controversial. Although the selective COX-2 inhibitor nimesulide reversed motor and cognitive abnormalities [57], another COX-2-selective inhibitor (celecoxib) worsened motor, but not cognitive, performance [58], and rofecoxib, which shows a higher selectivity for COX-2, failed to limit neuronal cell death in experimental TBI [59]. This might be due to the nonspecific effects of COX-2 inhibitors and differences in severity and location of TBI in the various animal models used. Another possibility is that AA, which is not undergoing COX-2 metabolism, might be metabolized to different products such as epoxyeicosatrienoic acids and lipoxins, which possess antiinflammatory and antithrombotic effects and, thus, could contribute to COX-2-independent protective effects [56].
Although COX-2-selective inhibition showed some promise in experimental autoimmune encephalomyelitis (a model of MS) [60], no correlation between PGE2, a major product of COX-2, and clinical scores of MS severity or biomarkers of axonal or astroglial injury has been found in MS patients [61].
In stroke, neurons, which express COX-2, are directly injured, whereas COX-1-expressing microglia only contribute to the secondary neuroinflammatory response. Several studies have shown that the infarct volume and neurological deficits produced by ischemia are improved by deletion of the COX-2 gene or selective COX-2 inhibition in the early phase after ischemia [37,38,62]. The role of COX-2 in focal ischemia seems to be related to the activation of the PGE2-dependent E-prostanoid 1 receptor, which contributes to neuronal calcium overload in the early phase of cerebral ischemia [63]. Therefore, although COX-2 seems to have an important role in the progression of ischemic brain injury, it could have little to do with the toxicity of post-ischemic inflammation. Supporting this concept, in a model of middle cerebral artery occlusion, the COX-2-selective inhibitor NS398 produced a reduction in infarct volume without attenuation in inflammatory gene expression [64]. Indeed, in experimental models of primary neuroinflammation, COX-2 inhibition or genetic deletion have been linked to detrimental effects. For instance, COX-2-null mice show increased glial activation and inflammatory markers after LPS injection compared with their wild-type mice, without any change in COX-1 expression [21]. Specifically, the expression of markers of inflammation and oxidative stress, such as cytokines and chemokines (e.g. IL-1β, TNF-α and macrophage inflammatory protein-1α), and subunits of NADPH oxidase were found to be increased in COX-2-null mice and in mice given chronic celecoxib, a selective COX-2 inhibitor, after centrally injected LPS [21]. COX-2 inhibition increases the gene expression of inflammatory factors, such as microsomal PGE2 synthase 1, toll-like receptor 2 (TLR2), CD14 and monocyte chemotactic protein 1 (MCP-1), in vascular-associated brain cells and parenchymal microglia also after systemic injection of LPS [65]. Inhibition of COX-2 activity also exacerbates endotoxin-induced ocular inflammation [66] and has been recently linked to exacerbated neuronal cell death also in thiamine-deficiency-induced Wernicke’s encephalopathy [67]. In neuroinflammation-induced BBB breakdown, COX-2-selective inhibition with rofecoxib does not prevent the disruption of tight junction proteins and BBB integrity [68], whereas selective COX-1 inhibition reduces TNF-α-induced BBB disruption [25], indicating that COX-1 is more directly involved than COX-2 in mediating BBB disruption and migration of peripheral leukocytes. Specific changes in COX-1 and COX-2 in various neurological and neurodegenerative disorders are summarized in Table 1.
Table 1.
Reported changes of COX-1 and COX-2 in neurodegenerative diseases
| Disorder | COX-1 | COX-2 |
|---|---|---|
| AD | Small trial with indomethacin (preferential COX-1 inhibitor) beneficial in protecting from cognitive decline. Use of aspirin (preferential COX-1 inhibitor) associated with reduced risk of AD. Increased COX-1-expressing microglia surrounding amyloid plaques. |
Upregulation of COX-2 in early AD, and downregulation in advanced AD stages. Randomized trials with COX-2-selective inhibitors not effective. |
| PD | COX-1 expression unchanged by MPTP, but >65% of ventral midbrain PGE2 originated from COX-1. Aspirin (preferential COX-1 inhibitor) and mixed COX inhibitors neuroprotective in parkinsonian models. |
COX-2 expressed within dopaminergic neurons of substantia nigra in the PD postmortem brain. COX-2 induced in the MPTP model of PD during destruction of nigrostriatal pathway. COX-2-selective inhibitors neuroprotective in parkinsonian models. |
| TBI | Accumulation of COX-1 expressing microglia at peri-lesional areas and in the developing core in patients with TBI. | Conflicting reports on COX-2-selective inhibitors in experimental TBI: nimesulide reversed motor and cognitive abnormalities; celecoxib worsened motor but not cognitive performance; and rofecoxib did not limit neuronal cell death. No difference in lesion volume 3 weeks after TBI and no changes in Morris water maze performance in COX-2-null versus wild-type mice. |
| Prion diseases | Accumulation of COX-1-expressing microglia in areas of severe tissue damage and around vacuoles in CJD patients. | Not determined. |
| HIV dementia | Twofold increase in COX-1 mRNA expression in the demented versus non-demented patients. | COX-2 mRNA expression is unchanged. |
| Stroke |
COX-1 deletion increases vulnerability to focal cerebral ischemia. COX-1 inhibition reduced neuronal injury and oxidative stress in hippocampus during transient global ischemia. |
COX-2 expression upregulated in neurons in early ischemia. COX-2 inhibition or genetic deletion reduces infarct volume and neurological deficits. |
| ALS | COX-1, and not COX-2, is the primary source of PGE2 in spinal cords of SOD1G93A mice. However, deletion of COX-1 does not improve preservation of motor neurons and survival of transgenic mutant mice. | Levels of COX-2 mRNA and PGE2 increased in mutant SOD1 mice. Selective COX-2 inhibitors (celecoxib and rofecoxib) improve motor performance, extend survival and reduce CSF levels of PGE2. COX-2 expression increased in motor neurons, interneurons and glial cells in seven human sporadic ALS patients. Double-blinded, placebo-controlled clinical trial with celecoxib (800 mg/day) administered for 12 months in ALS subjects did not show any beneficial effect. |
| MS | Not determined. | No correlation between PGE2 and clinical scores of MS severity or biomarkers of axonal or astroglial injury. |
Concluding remarks
Several recent studies have emphasized the unique and complex roles of COX-1 and COX-2 in the mechanism of CNS inflammation. Accumulating evidence indicates that COX-1 plays a previously unrecognized part in several neurodegenerative diseases including AD, PD, HIV-associated dementia, TBI and ischemic stroke. Although the actual mechanisms by which COX-1 is involved in neuroinflammation and neurodegeneration remain to be elucidated, the fact that the pharmacological inhibition or genetic ablation of COX-1 activity attenuate the inflammatory response and neuronal loss indicates that NSAIDs with higher selectivity for COX-1 rather than COX-2 are more likely to reduce neuroinflammation and should be further investigated as a potential therapeutic approach in neurodegenerative diseases with a marked inflammatory component.
It would be anticipated that, by being predominantly localized in microglia and, thus, being able to immediately secrete PGs in response to microglial activation even before COX-2 induction, COX-1 could be the major player in neuroinflammation. By contrast, COX-2, which is mainly localized in pyramidal neurons, is expected to predominantly contribute to increased PG synthesis in response to insults that directly challenge neurons, such as ischemia and excitotoxicity. Under these circumstances, COX-2 inhibition seems to afford protection without altering the inflammatory response. Thus, the type of insult, the cellular target of the stimulus and whether neuroinflammation is a primary or a secondary response could determine whether COX-2 activity mediates neurotoxicity or neuroprotection (Figure 3). Although COX-2 activity could mediate neurotoxicity in models of direct neuronal injury, its role in neuroinflammation might be dramatically different and be linked to neuroprotection via production of specific neuroprotective lipid mediators or via selective activation of specific receptors. Recently, novel COX-2-derived endogenous eicosanoids (such as lipoxin A4) and E- and D-series resolvins and protectins, with anti-inflammatory and pro-resolving actions, have been characterized [69] and unraveled the traditional view of COX-2 products as proinflammatory only. Moreover, COX-2, but not COX-1, can metabolize endogenous endocannabinoids (i.e. anandamide and 2-arachidonoylglycerol) [70], which have been suggested to control immune response and influence neurogenesis [71]. Another possibility is that the distinct anti-inflammatory versus proinflammatory effects are mediated via distinct and functionally antagonistic EP receptors that are expressed on multiple cell types in the CNS [72,73].
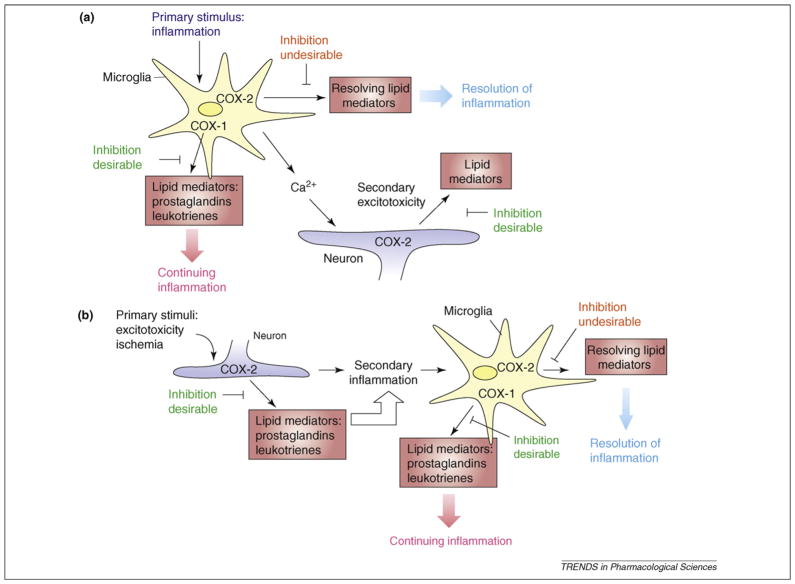
Figure 3.
COX-mediated neurotoxicity or neuroprotection and downstream cellular events depend on whether the neuroinflammatory response is a primary (a) or a secondary response (b). COX-1 is predominantly localized in microglia and, thus, can immediately secrete PGs in response to inflammatory stimuli. Thus, COX-1 inhibition in primary neuroinflammation can attenuate the neuroinflammatory response. By contrast, COX-2, which is mainly localized in pyramidal neurons, contributes predominantly to increased PG synthesis in response to insults that directly challenge neurons, such as in ischemic and excitotoxic conditions. Under these circumstances, COX-2 inhibition can be beneficial in the early phase, whereas in the secondary neuroinflammatory response COX-1 inhibition might also be beneficial.
Clearly, further studies on COX-1- and COX-2-derived specific products during inflammation and the resolution of inflammation are warranted to better understand the unique role of each isoform in these processes. The potential beneficial effects of COX-2 in the resolution of inflammation and in the return to tissue homeostasis need to be considered when developing treatment strategies aimed at reducing neuroinflammation.
References
- 1.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann N Y Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 2.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 4.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block ML, et al. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 6.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 7.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Vlad SC, et al. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernan MA, et al. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology. 2006;66:1097–1099. doi: 10.1212/01.wnl.0000204446.82823.28. [DOI] [PubMed] [Google Scholar]
- 10.Wahner AD, et al. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007;69:1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- 11.Esposito E, et al. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Etminan M, et al. Non-steroidal anti-inflammatory drug use and the risk of Parkinson disease: a retrospective cohort study. J Clin Neurosci. 2008;15:576–577. doi: 10.1016/j.jocn.2007.02.095. [DOI] [PubMed] [Google Scholar]
- 13.Farooqui AA, et al. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 14.Bosetti F, et al. Prostaglandin E2 and microsomal prostaglandin E synthase-2 expression are decreased in the cyclooxygenase-2-deficient mouse brain despite compensatory induction of cyclooxygenase-1 and Ca2+-dependent phospholipase A2. J Neurochem. 2004;91:1389–1397. doi: 10.1111/j.1471-4159.2004.02829.x. [DOI] [PubMed] [Google Scholar]
- 15.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 16.Choi SH, et al. Cyclooxygenase-1 and -2 enzymes differentially regulate the brain upstream NF-κB pathway and downstream enzymes involved in prostaglandin biosynthesis. J Neurochem. 2006;98:801–811. doi: 10.1111/j.1471-4159.2006.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillis JW, et al. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Niwa K, et al. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des. 2008;14:1443–1451. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertolini A, et al. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks. Curr Med Chem. 2002;9:1033–1043. doi: 10.2174/0929867024606650. [DOI] [PubMed] [Google Scholar]
- 21.Aid S, et al. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation. 2008;5:17. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toscano CD, et al. Altered GABAergic neurotransmission is associated with increased kainate-induced seizure in prostaglandin-endoperoxide synthase-2 deficient mice. Brain Res Bull. 2008;75:598–609. doi: 10.1016/j.brainresbull.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwab JM, et al. Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J Neurosurg. 2002;96:892–899. doi: 10.3171/jns.2002.96.5.0892. [DOI] [PubMed] [Google Scholar]
- 24.Pepicelli O, et al. Cyclo-oxygenase-1 and -2 differently contribute to prostaglandin E2 synthesis and lipid peroxidation after in vivo activation of N-methyl-D-aspartate receptors in rat hippocampus. J Neurochem. 2005;93:1561–1567. doi: 10.1111/j.1471-4159.2005.03150.x. [DOI] [PubMed] [Google Scholar]
- 25.Candelario-Jalil E, et al. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J Neuroinflammation. 2007;4:25. doi: 10.1186/1742-2094-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi SH, et al. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aid S, Bosetti F. Gene expression of cyclooxygenase-1 and Ca2+-independent phospholipase A2 is altered in rat hippocampus during normal aging. Brain Res Bull. 2007;73:108–113. doi: 10.1016/j.brainresbull.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yermakova AV, et al. Cyclooxygenase-1 in human Alzheimer and control brain: quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol. 1999;58:1135–1146. doi: 10.1097/00005072-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Rogers J, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 30.Sung S, et al. Modulation of nuclear factor-κB activity by indomethacin influences Aβ levels but not Aβ precursor protein metabolism in a model of Alzheimer’s disease. Am J Pathol. 2004;165:2197–2206. doi: 10.1016/s0002-9440(10)63269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szekely CA, et al. No advantage of A β42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008;70:2291–2298. doi: 10.1212/01.wnl.0000313933.17796.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teismann P, et al. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deininger MH, et al. Cyclooxygenase-1 and -2 in brains of patients who died with sporadic Creutzfeldt-Jakob disease. J Mol Neurosci. 2003;20:25–30. doi: 10.1385/JMN:20:1:25. [DOI] [PubMed] [Google Scholar]
- 34.Minghetti L, Pocchiari M. Cyclooxygenase-2, prostaglandin E2, and microglial activation in prion diseases. Int Rev Neurobiol. 2007;82:265–275. doi: 10.1016/S0074-7742(07)82014-9. [DOI] [PubMed] [Google Scholar]
- 35.Griffin DE, et al. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- 36.Almer G, et al. Is prostaglandin E2 a pathogenic factor in amyotrophic lateral sclerosis? Ann Neurol. 2006;59:980–983. doi: 10.1002/ana.20847. [DOI] [PubMed] [Google Scholar]
- 37.Iadecola C, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Candelario-Jalil E, et al. Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem. 2003;86:545–555. doi: 10.1046/j.1471-4159.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwab JM, et al. Selective accumulation of cyclooxygenase-1-expressing microglial cells/macrophages in lesions of human focal cerebral ischemia. Acta Neuropathol. 2000;99:609–614. doi: 10.1007/s004010051170. [DOI] [PubMed] [Google Scholar]
- 40.de Vries HE, et al. Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. J Pharmacol Exp Ther. 1996;277:1418–1423. [PubMed] [Google Scholar]
- 41.Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007;102:577–586. doi: 10.1111/j.1471-4159.2007.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 43.Combrinck M, et al. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:85–88. doi: 10.1136/jnnp.2005.063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reines SA, et al. Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- 45.Thal LJ, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 46.Morihara T, et al. Ibuprofen suppresses interleukin-1β induction of pro-amyloidogenic α1-antichymotrypsin to ameliorate β-amyloid (Aβ) pathology in Alzheimer’s models. Neuropsychopharmacology. 2005;30:1111–1120. doi: 10.1038/sj.npp.1300668. [DOI] [PubMed] [Google Scholar]
- 47.Peretto I, et al. Synthesis and biological activity of flurbiprofen analogues as selective inhibitors of β-amyloid(1)(−)(42) secretion. J Med Chem. 2005;48:5705–5720. doi: 10.1021/jm0502541. [DOI] [PubMed] [Google Scholar]
- 48.Vijitruth R, et al. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J Neuroinflammation. 2006;3:6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ton TG, et al. Nonsteroidal anti-inflammatory drugs and risk of Parkinson’s disease. Mov Disord. 2006;21:964–969. doi: 10.1002/mds.20856. [DOI] [PubMed] [Google Scholar]
- 50.Bornebroek M, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology. 2007;28:193–196. doi: 10.1159/000108110. [DOI] [PubMed] [Google Scholar]
- 51.Hancock DB, et al. Smoking, caffeine, and nonsteroidal anti-inflammatory drugs in families with Parkinson disease. Arch Neurol. 2007;64:576–580. doi: 10.1001/archneur.64.4.576. [DOI] [PubMed] [Google Scholar]
- 52.Maihofner C, et al. Expression and localization of cyclooxygenase-1 and -2 in human sporadic amyotrophic lateral sclerosis. Eur J Neurosci. 2003;18:1527–1534. doi: 10.1046/j.1460-9568.2003.02879.x. [DOI] [PubMed] [Google Scholar]
- 53.Klivenyi P, et al. Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2004;88:576–582. doi: 10.1046/j.1471-4159.2003.02160.x. [DOI] [PubMed] [Google Scholar]
- 54.Cudkowicz ME, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 55.Strauss KI, et al. Prolonged cyclooxygenase-2 induction in neurons and glia following traumatic brain injury in the rat. J Neurotrauma. 2000;17:695–711. doi: 10.1089/089771500415436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmad M, et al. Genetic disruption of cyclooxygenase-2 does not improve histological or behavioral outcome after traumatic brain injury in mice. J Neurosci Res. 2008;86:3605–3612. doi: 10.1002/jnr.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cernak I, et al. Inhibition of cyclooxygenase 2 by nimesulide improves cognitive outcome more than motor outcome following diffuse traumatic brain injury in rats. Exp Brain Res. 2002;147:193–199. doi: 10.1007/s00221-002-1245-z. [DOI] [PubMed] [Google Scholar]
- 58.Dash PK, et al. Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J Neurotrauma. 2000;17:69–81. doi: 10.1089/neu.2000.17.69. [DOI] [PubMed] [Google Scholar]
- 59.Kunz T, et al. Cyclooxygenase-2, prostaglandin synthases, and prostaglandin H2 metabolism in traumatic brain injury in the rat. J Neurotrauma. 2002;19:1051–1064. doi: 10.1089/089771502760341965. [DOI] [PubMed] [Google Scholar]
- 60.Miyamoto K, et al. Selective COX-2 inhibitor celecoxib prevents experimental autoimmune encephalomyelitis through COX-2-independent pathway. Brain. 2006;129:1984–1992. doi: 10.1093/brain/awl170. [DOI] [PubMed] [Google Scholar]
- 61.Mattsson N, et al. Elevated cerebrospinal fluid levels of prostaglandin E2 and 15-(S)-hydroxyeicosatetraenoic acid in multiple sclerosis. J Intern Med. 2008 doi: 10.1111/j.1365-2796.2008.02035. ( http://www.interscience.wiley.com) [DOI] [PubMed]
- 62.Sasaki T, et al. Amelioration of hippocampal neuronal damage after transient forebrain ischemia in cyclooxygenase-2-deficient mice. J Cereb Blood Flow Metab. 2004;24:107–113. doi: 10.1097/01.WCB.0000100065.36077.4A. [DOI] [PubMed] [Google Scholar]
- 63.Kawano T, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 64.Kunz A, et al. Nuclear factor-κB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J Neurosci. 2008;28:1649–1658. doi: 10.1523/JNEUROSCI.5205-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blais V, et al. Cyclooxygenase 2 (COX-2) inhibition increases the inflammatory response in the brain during systemic immune stimuli. J Neurochem. 2005;95:1563–1574. doi: 10.1111/j.1471-4159.2005.03480.x. [DOI] [PubMed] [Google Scholar]
- 66.Tuo J, et al. Endotoxin-induced uveitis in cyclooxygenase-2-deficient mice. Invest Ophthalmol Vis Sci. 2004;45:2306–2313. doi: 10.1167/iovs.03-0756. [DOI] [PubMed] [Google Scholar]
- 67.Gu B, et al. Selective increase of neuronal cyclooxygenase-2 (COX-2) expression in vulnerable brain regions of rats with experimental Wernicke’s encephalopathy: effect of nimesulide. Metab Brain Dis. 2008;23:175–187. doi: 10.1007/s11011-008-9089-2. [DOI] [PubMed] [Google Scholar]
- 68.Pu H, et al. Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood–brain barrier. Brain Res. 2007;1184:333–344. doi: 10.1016/j.brainres.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 69.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153 (Suppl 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kozak KR, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 71.Wolf SA, Ullrich O. Endocannabinoids and the brain immune system: new neurones at the horizon? J Neuroendocrinol. 2008;20 (Suppl 1):15–19. doi: 10.1111/j.1365-2826.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- 72.Shie FS, et al. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- 73.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 74.Nimmerjahn A, et al. Resting microglial cells are highly dynamic surveillants of brain parenchyma in ivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 75.Stence N, et al. Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]