Abstract
Mitochondrial division has emerged as a key mechanism for this essential organelle to maintain its structural integrity, intracellular distribution, and functional competence. An evolutionarily conserved dynamin-related GTPase, Dnm1p/Drp1, interacts with other proteins to form the core machinery involved in mitochondrial division. We summarize recent progress in understanding how the division machinery assembles onto mitochondria and how mitochondrial division contributes to cellular physiology and human diseases.
Introduction
Mitochondrial shape, size, and number are regulated by cycles of division and fusion [1–3]. These dynamic processes control mitochondrial distribution and function. In many cell types, these organelles form short tubules, continuously dividing and fusing to exchange their soluble and membrane components, which include DNA, proteins, and lipids (Fig. 1). Dynamic remodeling of mitochondrial structure in response to physiological and environmental cues is important to accommodate different demands on mitochondrial function in various cell types during growth, differentiation, and maintenance[4,5]. In the last decade, many proteins involved in mitochondrial division and fusion have been identified[6,7]. Current challenges in the field include understanding the mechanistic functions of each protein and deciphering the in vivo functions of mitochondrial dynamics in mammals. In this review, we aim to cover recent advances in these topics.
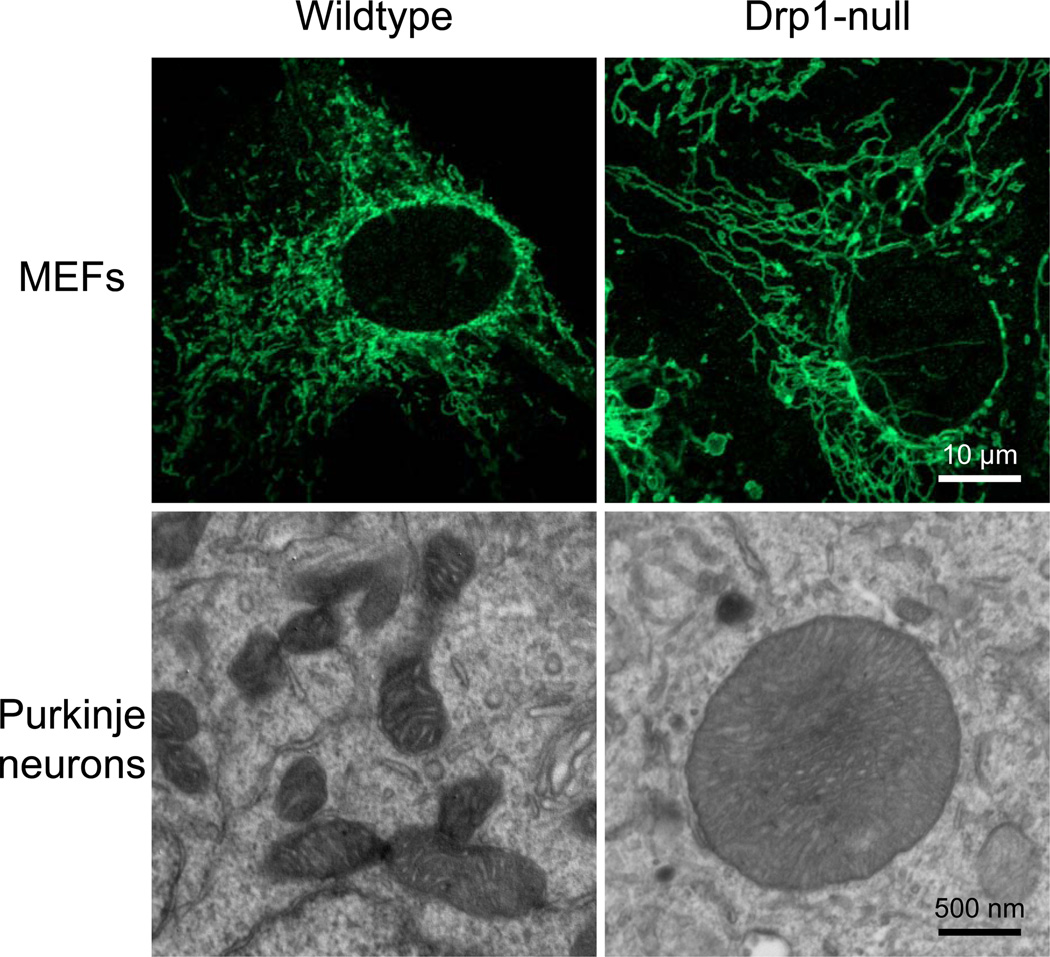
Figure 1.
Mitochondrial shape in MEFs and Purkinje neurons. Mitochondria in wildtype and Drp1-null MEFs were visualized by immunofluorescence microscopy using antibodies against Tom20. Mitochondria in Purkinje cells were visualized by electron microscopy.
Formation of mitochondrial division machinery
A key component of the mitochondrial division machinery is a dynamin-related GTPase called Dnm1p (for yeast)/Drp1 (for mammals)[8–13]. With the majority present in the cytosol, Dnm1p/Drp1 is recruited to the outer membrane for mitochondrial division. This protein has been shown to polymerize into highly ordered oligomers that most likely wrap around mitochondrial tubules with a diameter of approximately 500 nm[14,15]. In contrast to classical dynamin GTPases, which assemble onto the narrow neck of coated pits during endocytosis, Dnm1p/Drp1 convenes onto much wider mitochondrial tubules. A recent cryo-EM study by Mears et al. (2010)[16] showed that Dnm1p can form spirals with a diameter of approximately 100 nm, which is much larger than the classical dynamin GTPase which assembles at a diameter of approximately 20 nm. In addition, Dnm1p/Drp1 binds, constricts, and fragments membrane liposomes in vitro. Therefore, Dnm1p/Drp1 may work to pinch off wider tubular structures directly. However, Dnm1p/Drp1 helices were only observed when mitochondrial tubules are highly constricted at presumably later stages of mitochondrial division, suggesting the presence of other factors that may make the diameter of mitochondria shorter to help Dnm1p/Drp1 assemble in vivo. Nevertheless, the ability of Dnm1p/Drp1 to assemble is a key mechanism for mitochondrial division. Once assembled, GTPase activity is stimulated and leads to conformation changes necessary for severing membranes.
In cells, mitochondrial recruitment of Dnm1p/Drp1 requires other proteins present on the organelle surface. In yeast, the integral outer membrane protein Fis1p provides an anchoring site through two functionally overlapping WD40 domain-containing adaptor proteins, Mdv1p and Caf4p (Fig. 2)[17–29]. Lackner et al. (2009)[30] demonstrated that Mdv1p functions as a structural nucleation factor that promotes Dnm1p assembly on membranes and ultimately its GTPase activity. Mdv1p-dependent nucleation onto mitochondria could serve as a key regulatory step for mitochondrial division. Koirala et al. (2010)[31] solved the X-ray structure of the Mdv1p coiled-coil domain located in the middle of the protein, and showed that this domain forms intermolecular anti-parallel helices and mediates dimerization. This domain also affects interactions with Dnm1p, which are mediated by a WD-40 domain at the C-terminus of Mdv1p (Koirala et al., 2010). As Lanckner et al. (2009) suggested, Mdv1p further assembles into oligomers with Dnm1p, indicating that the coiled-coil domain of Mdv1p is also required for oligomerization and therefore regulation of its interactions with Dnm1p. Compared to Mdv1p, Caf4p plays a relatively minor role in Dnm1p assembly. Mammalian homologs of Mdv1p and Caf4p have not been identified.
Figure 2.
Mitochondrial division machinery in yeast and mammals.
In addition to the Fis1p-Mdv1p/Caf4p mechanism, a cortical protein, Num1p, also provides a separate binding site for Dnm1p on mitochondria and functions in mitochondrial division (Cerveny et al., 2007)[32,33]. Hammermeister et al. (2010)[34] showed that another outer membrane protein, Mdm36, facilitates stable association of Dnm1p and Num1p. In addition to division, this Num1p-Mdm36p-Dnm1p mechanism also links mitochondria to the cell cortex as, without this mechanism, mitochondria leave their usual location at the cell periphery and relocate to the cellular center. This cortical association likely participates in equal inheritance of mitochondria during cell division[34]. Mitochondria in cells lacking both Num1p and Dnm1p cannot stay in mother cells, and thus move to daughter cells during cell division under conditions such as high temperature[32]. Since mitochondria in yeast are transported along and anchored to actin filaments in the cortex[35], the Num1p-Mdm36p-Dnm1p mechanism may provide a connection to the actin cytoskeleton. This cytoskeletal connection may also promote efficient mitochondrial division by mechanically stretching and thinning mitochondrial tubules.
Fis1 was identified in mammals based on sequence homology to yeast Fis1p and has been suggested to recruit Drp1 to mitochondria[21,36–38]. However, surprisingly, Otera et al. (2010)[39] showed that mammalian Fis1 is dispensable for Drp1 recruitment. To demonstrate this, the authors generated Fis1-null cells and found no major mitochondrial division defects and normal Drp1 localization to mitochondria. This study also showed that Fis1 knockdown by siRNA used in previous studies has an offtarget effect, as it still elongates mitochondrial tubules in Fis1-null cells[39]. These findings are consistent with a previous study in which C. elegans lacking two Fis1 homologs does not exhibit mitochondrial division defects [40]. A recently identified integral outer membrane protein, mitochondria fission factor (Mff), was found to play a major role in Drp1 recruitment, presumably through direct interactions[39,41]. It appears that mitochondria have switched anchoring and assembly mechanisms for Dnm1p/Drp1 during evolution. Given the relatively weak interactions of Mff with Drp1, there may be additional proteins that facilitate their stable connection during mitochondrial division, such as yeast Mdv1p and Caf4p. Mitochondrial recruitment of Drp1 from the cytosol is regulated by a variety of post-translational modifications, including phosphorylation, sumoylation, and ubiquitination[42]. In light of these new findings, it would be interesting to determine whether these modifications regulate the interactions between Drp1 and Mff.
Physiological functions of mitochondrial division
Many studies using in vitro cell culture systems and relatively simple eukaryotic model organisms such as yeast, Drosophila, and C. elegans have demonstrated the involvement of mitochondrial division in such cellular functions as organelle shape, distribution, energy metabolism, apoptosis, and calcium signaling. However, until recently, its physiological function in mammals was unknown due to a lack of mouse models. Recent studies by Wakabayashi et al. (2009)[43] and Ishihara et al. (2009)[44] have generated and characterized complete and brain-specific Drp1 knockout mice. Drp1 was found to be required for embryonic development as complete knockouts die at E11.5, which is when the placenta develops. Embryonic fibroblasts derived from the knockout mice showed highly connected mitochondrial tubules and greatly decreased mitochondrial division rates. Despite dramatic changes in mitochondrial structure and dynamics, these knockout MEFs displayed normal respiratory activities and ATP production.
Analysis of Drp1-null MEFs also suggested that mitochondrial division regulates fusion. A balance between division and fusion is critical for the maintenance of mitochondria; therefore, it is logical to propose that these activities are synchronized. One possible explanation of this coordination is that the intrinsic properties of the fusion and division machineries determine their frequency independent of each other. Another possibility is that cells, upon sensing one activity, subsequently adjust the other to maintain a homeostatic balance between division and fusion. In support of the latter model, the study of Drp1-null MEFs, as well as other studies using Drp1-specific siRNA, revealed that loss of this protein leads to decreases in the steady state levels of Mfn1 and 2 [43–45]. These findings strongly suggest a model in which the abundance and/or activity of the mitochondrial division machinery regulates fusion. Further supporting this model, some factors that influence mitochondrial division, such as ubiquitin and Bcl-xL, also have the ability to modulate fusion[5,42,46–50].
During brain development, neurons establish an elongated shape with long axons and branched dendrites. Proper distribution of mitochondria within these cells is crucial. Indeed, in vitro studies have shown that the frequency of mitochondrial division becomes greater than that of fusion with smaller mitochondria distributed to different neuronal regions, including synapses, during development[50–52]. Wakabayashi et al. (2010) deleted Drp1 in the developing cerebellum since this protein is expressed highly in Purkinje cells. This study showed that loss of Drp1 blocked normal cerebellar development. Interestingly, due to ongoing mitochondrial fusion without division, the Purkinje neurons contained giant round mitochondria, which are distinct from the highly interconnected, elongated tubules seen in Drp1-null fibroblasts (Fig. 1). Thus, mitochondrial division appears to regulate the overall shape of this organelle. Unlike Purkinje cells, granule cells have normal short tubular mitochondria in the absence of Drp1, suggesting cell type-specific requirements of this protein for mitochondrial division. Mitochondrial division may occur via a Drp1-independent mechanism in granule cells. Ishihara et al. (2010) also found similar developmental defects when Drp1 was deleted in the brain using nestin-Cre, which is expressed in a wider region of the central and peripheral nervous system. Culture of neurons from Drp1 knockouts in vitro showed defects in synapse formation and increased sensitivity to apoptotic stimuli. Together, these studies have demonstrated the physiological importance of mitochondrial division for mouse embryonic and brain development, as well as generated tools to further study mitochondrial division in more physiological contexts.
Mitochondrial division in human diseases
Further supporting its physiological importance, altered mitochondrial division has been associated with several diseases, especially those affecting neurons [53–55]. For example, a mutation (A395D) in Drp1 causes postnatal death due to brain developmental defects[56]. A recent biochemical study has shown that this disease mutation blocks higher order assembly of Drp1 and its mitochondrial localization[57]. In addition, Charcot-Marie-Tooth type 4A is caused by mutations in GDAP1, an outer membrane protein involved in mitochondrial division; however, its molecular mechanisms are less well understood[58–62].
A recent study described that the pathogenesis of Alzheimer's disease, one of the most common age-related diseases, may also involve changes in mitochondrial dynamics. In this disease, mitochondrial dysfunction is the earliest known event and fragmentation of this organelle and its altered distribution have been reported [63–65]. Cho et al. (2009) provided interesting evidence for the activation of Drp1-mediated mitochondrial division in Alzheimer's disease[66]. In this study, the authors found that S-nitrosylation of Drp1 was increased in Alzheimer's patients, leading to activation of Drp1 GTPase activity, excess mitochondrial division, and ultimately neural injury. How excess division leads to neural injury remains to be determined. However, Bossy et al. (2010) have challenged this model[67]. Further studies are required to resolve this important issue.
Similarly, Huntington’s disease has also been linked to mitochondrial dysfunction. In particular, mitochondrial division has been implicated as increased Drp1 levels were observed in the brain of these patients [68]. Activation of Drp1-dependent mitochondrial division by phosphorylation/dephosphorylation is also suggested to be involved in hypersensitivity to apoptosis in cellular models for Huntington’s disease [69]. Another study also showed that fragmentation of mitochondria due to excess division over fusion contributes to cell death in Huntington’s cellular models [70].
In addition, by using hypertensive rats with hypertension-induced brain injury, Qi et al. (2010)[71] showed that, upon oxidative stress, Drp1 becomes phosphorylated by delta protein kinase C and induces excess mitochondrial division, resulting in neuronal injury. These studies suggest a common theme in that hyperactivation of mitochondrial division, like its complete loss, leads to physiological abnormalities and disease. Therefore, well-balanced mitochondrial dynamics is critical for cellular health.
Mitochondrial DNA mutations and deletions have been implicated as underlying causes for normal aging and aging-related disease. Mitochondrial DNA is highly susceptible to oxidative stress and can accumulate mutations and deletions over time. A balance between division and fusion is critical for maintaining the integrity of mitochondrial DNA and therefore contributes to these processes. For example, excess fragmentation of mitochondria increases mutations and loss of mitochondrial DNA[72]. Also inhibition of mitochondrial division results in increased oxidative damage and loss of mitochondrial DNA[73]. Interestingly, separation of damaged mitochondria from healthy ones by division may help target them for degradation, which is a possible cellular quality control strategy[74]. Defects in this process have also been associated with Parkinson’s disease, another age-related neurodegenerative syndrome. This topic is discussed in another review within this issue.
Finally, Cassidy-Stone et al. (2008) identified a small molecule inhibitor of Dnm1p/Drp1, mdivi-1 [75]. Mdivi-1 has been used to suppress mitochondrial division in both cell culture and animal models [76]. Use of this inhibitor may have therapeutic potential to readjust mitochondrial dynamics in diseases in which division is overactivated or fusion is impaired.
Drp1 in Bax activation during apoptosis
Whether Drp1 is involved in apoptosis remains controversial. Since this topic has been reviewed extensively in many articles [77–82], we will only briefly summarize recent findings by focusing on Drp1 in apoptosis, especially on results obtained from in vivo models. Knockout studies have demonstrated that Drp1 is required for apoptosis during neural tube formation in vivo [43]. In support of this role, decreased Drp1 association with mitochondria, thereby leading to suppressed apoptosis, has been observed during myocardial infarction (Wang et al., 2010). Similarly, treatment of mice with a Drp1 inhibitor, mdivi-1, partially protected the heart from ischemia/reperfusion injury [83]. In contrast, the contribution of Drp1 to apoptosis in vitro cell culture systems using Drp1-null cells is little to none, suggesting that Drp1 involvement in apoptosis differs depending on cell type and physiological context [43,44]. Drp1 most likely promotes the efficiency of certain types of apoptosis, but its involvement is not essential.
As a function of Drp1 in apoptosis, Montesuit et al. (2010)[84] have shown that Drp1 activates Bax oligomerization, a key step in cytochrome c release from the mitochondrial intermembrane space. Using biochemical approaches, this study revealed that Drp1 induces Bax oligomerization in the mitochondrial outer membrane through hemifusion. Surprisingly, purified Drp1 binds to cardiolipin, a mitochondria-specific phospholipid, and promotes hemifusion of cardiolipin-containing liposomes in vitro. This study also showed that, while hemifusion is a key mechanism, mitochondrial division is not since mutations in the Drp1 GTPase domain, which block mitochondrial division, did not affect Bax activation. Furthermore, Drp1 can be replaced by cytochrome c, which can hemifuse liposomes under certain biochemical conditions. Separation of Drp1-mediated mitochondrial division and apoptosis (e.g., its GTPase activity is dispensable) has been also supported by other studies using BIK-induced cristae opening to release cytochrome c [85]. Also, cytochrome c release from isolated mitochondria without division has been shown to occur in a Drp1-dependent manner in vitro [75]. Further supporting a role for Drp1 in Bax oligomerization, reports indicate that both proteins colocalize on mitochondria after induction of apoptosis[86]. Upon activation, Bax promotes Drp1 sumoylation, which is proposed to stabilize Drp1 on the mitochondrial membrane, suggesting a positive feedback loop for Bax activation and Drp1 mitochondrial association [87].
Conclusions
Mitochondria face continuous oxidative damage due to respiratory activities in this organelle. As animals and humans age, such damage accumulates and mitochondria become dysfunctional. Recent research has made clearer the importance of control of mitochondrial dynamics in defense mechanisms against age-related dysfunction and environmental stress. While there has been tremendous progress in identifying the proteins involved in mitochondrial dynamics, current challenges lie in deciphering how these proteins work together biochemically and how this process functions physiologically. An in vitro reconstitution of mitochondrial division may be needed to reveal biochemical roles of each protein. In addition, future studies are needed to identify the physiological consequences of altered mitochondrial division in different tissues. A thorough understanding of mitochondrial division will provide valuable insight for designing strategies to improve health and well-being.
Acknowledgements
We apologize that we could not cite all of our colleagues’ relevant research due to space restrictions. We acknowledge M. Iijima for critical reading of the manuscript. This work was supported by grants from the NIH (GM089853), MDA (69361), and AHA (0730247N) to H.S.
References
- 1.Okamoto K, Shaw JM. Mitochondrial Morphology and Dynamics in Yeast and Multicellular Eukaryotes. Annu Rev Genet. 2005 doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Chan DC. Mitochondrial dynamics in mammals. Curr Top Dev Biol. 2004;59:119–144. doi: 10.1016/S0070-2153(04)59005-1. [DOI] [PubMed] [Google Scholar]
- 3.Jensen RE, Aiken Hobbs AE, Cerveny KL, Sesaki H. Yeast mitochondrial dynamics: fusion, division, segregation, and shape. Microscopy Research and Technique. 2000;51:573–583. doi: 10.1002/1097-0029(20001215)51:6<573::AID-JEMT7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta. 2009;1793:154–170. doi: 10.1016/j.bbamcr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17:563–569. doi: 10.1016/j.tcb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 7.Westermann B. Molecular machinery of mitochondrial fusion and fission. J Biol Chem. 2008;283:13501–13505. doi: 10.1074/jbc.R800011200. [DOI] [PubMed] [Google Scholar]
- 8.Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 11.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legesse-Miller A, Massol RH, Kirchhausen T. Constriction and Dnm1p recruitment are distinct processes in mitochondrial fission. Mol Biol Cell. 2003;14:1953–1963. doi: 10.1091/mbc.E02-10-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otsuga D, Keegan BR, Brisch E, Thatcher JW, Hermann GJ, Bleazard W, Shaw JM. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 18:20–26. doi: 10.1038/nsmb.1949. Together with their previous work (Ingels et al, 2005), the authors revealed by cryo-EM that Dnm1p forms spiral structures around liposomes with a diameter of approximately 100 nm. The authors further compared the structure of Dnm1p and classical dynamin and showed interesting differences between these two related GTPases. While dynamin associates directly with membranes through its PH-domain, Dnm1p did not show clear direct contact with membranes. Also, unlike dynamin, Dnm1p assembles as a two-start helix and shows no axial compression upon addition of GTP.
- 17.Tieu Q, Okreglak V, Naylor K, Nunnari J. The WD repeat protein, Mdv1p, functions as a molecular adaptor by interacting with Dnm1p and Fis1p during mitochondrial fission. J Cell Biol. 2002;158:445–452. doi: 10.1083/jcb.200205031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated Mitochondrial Fission Is a Multi-step Process Requiring the Novel Integral Membrane Component Fis1p. J Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerveny KL, Jensen RE. The WD-repeats of Net2p interact with Dnm1p and Fis1p to regulate division of mitochondria. Mol Biol Cell. 2003;14:4126–4139. doi: 10.1091/mbc.E03-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karren MA, Coonrod EM, Anderson TK, Shaw JM. The role of Fis1p-Mdv1p interactions in mitochondrial fission complex assembly. J Cell Biol. 2005;171:291–301. doi: 10.1083/jcb.200506158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- 22.Wells RC, Picton LK, Williams SC, Tan FJ, Hill RB. Direct binding of the dynamin-like GTPase, Dnm1, to mitochondrial dynamics protein Fis1 is negatively regulated by the Fis1 N-terminal arm. J Biol Chem. 2007;282:33769–33775. doi: 10.1074/jbc.M700807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Chan DC. Structural basis for recruitment of mitochondrial fission complexes by Fis1. Proc Natl Acad Sci U S A. 2007;104:18526–18530. doi: 10.1073/pnas.0706441104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin EE, Graumann J, Chan DC. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakobs S, Martini N, Schauss AC, Egner A, Westermann B, Hell SW. Spatial and temporal dynamics of budding yeast mitochondria lacking the division component Fis1p. J Cell Sci. 2003;116:2005–2014. doi: 10.1242/jcs.00423. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Neutzner A, Tjandra N, Youle RJ. Novel structure of the N terminus in yeast Fis1 correlates with a specialized function in mitochondrial fission. J Biol Chem. 2005;280:21444–21452. doi: 10.1074/jbc.M414092200. [DOI] [PubMed] [Google Scholar]
- 27.Naylor K, Ingerman E, Okreglak V, Marino M, Hinshaw JE, Nunnari J. Mdv1 interacts with assembled dnm1 to promote mitochondrial division. J Biol Chem. 2006;281:2177–2183. doi: 10.1074/jbc.M507943200. [DOI] [PubMed] [Google Scholar]
- 28.Schauss AC, Bewersdorf J, Jakobs S. Fis1p and Caf4p, but not Mdv1p, determine the polar localization of Dnm1p clusters on the mitochondrial surface. J Cell Sci. 2006;119:3098–3106. doi: 10.1242/jcs.03026. [DOI] [PubMed] [Google Scholar]
- 29.Cerveny KL, McCaffery JM, Jensen RE. Division of mitochondria requires a novel DMN1-interacting protein, Net2p. Mol Biol Cell. 2001;12:309–321. doi: 10.1091/mbc.12.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lackner LL, Horner JS, Nunnari J. Mechanistic analysis of a dynamin effector. Science. 2009;325:874–877. doi: 10.1126/science.1176921. With purified proteins and synthetic liposomes, this study shows that Mdv1p interacts specifically with GTP-bound Dnm1p and promotes Dnm1p assembly onto membranes. These new data suggest a role for Mdv1p in GTP-regulated formation of division machinery on the surface of mitochondria.
- 31. Koirala S, Bui HT, Schubert HL, Eckert DM, Hill CP, Kay MS, Shaw JM. Molecular architecture of a dynamin adaptor: implications for assembly of mitochondrial fission complexes. J Cell Biol. 191:1127–1139. doi: 10.1083/jcb.201005046. The authors studied the coiled-coil domain of Mdv1p by solving its X-ray structure and its in vivo function. This domain exists as an unusually long, antiparallel structure for dimerization. By introducing a mutation in this domain, the authors further suggest that this domain is also important for its mitochondrial recruitment and co-assembly with Dnm1p on mitochondria.
- 32.Cerveny KL, Studer SL, Jensen RE, Sesaki H. Yeast mitochondrial division and distribution require the cortical num1 protein. Dev Cell. 2007;12:363–375. doi: 10.1016/j.devcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Schauss AC, McBride HM. Mitochondrial Fission: A Non-Nuclear Role for Num1p. Curr Biol. 2007;17:R467–R470. doi: 10.1016/j.cub.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 34. Hammermeister M, Schodel K, Westermann B. Mdm36 is a mitochondrial fission-promoting protein in Saccharomyces cerevisiae. Mol Biol Cell. 21:2443–2452. doi: 10.1091/mbc.E10-02-0096. This group previously identified many genes through a genome-wide screen for yeast mutants defective in mitochondrial morphology. One of these genes, Mdm36p, was demonstrated in this new study to facilitate Dnm1p-Num1p interaction for mitochondrial division. This study also demonstrated that the Num1p-Mdm36p-Dnm1p mechanism is important for anchoring mitochondria to the cell cortex.
- 35.Boldogh IR, Pon LA. Interactions of mitochondria with the actin cytoskeleton. Biochim Biophys Acta. 2006;1763:450–462. doi: 10.1016/j.bbamcr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 36.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 37.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 191:1141–1158. doi: 10.1083/jcb.201007152. This study provides surprising and intriguing data that mammalian Fis1 is dispensable for mitochondrial division and does not function in anchoring Drp1 to the mitochondrial outer membrane. Instead, Mff plays a direct role in Drp1- mitochondria association.
- 40.Kanazawa T, Zappaterra MD, Hasegawa A, Wright AP, Newman-Smith ED, Buttle KF, McDonald K, Mannella CA, van der Bliek AM. The C. elegans Opa1 homologue EAT-3 is essential for resistance to free radicals. PLoS Genet. 2008;4:e1000022. doi: 10.1371/journal.pgen.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. These studies (43 and 44) describe the first Drp1 knockout mice and demonstrate the physiological function of mitochondrial division in mammalian embryonic and brain development.
- 44.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 45.Mopert K, Hajek P, Frank S, Chen C, Kaufmann J, Santel A. Loss of Drp1 function alters OPA1 processing and changes mitochondrial membrane organization. Exp Cell Res. 2009;315:2165–2180. doi: 10.1016/j.yexcr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci. 123:619–626. doi: 10.1242/jcs.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, et al. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. Embo J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB, 3rd, Goebbels S, Nave KA, Arnold BA, Jonas EA, Pineda FJ, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Chang DT, Reynolds IJ. Differences in mitochondrial movement and morphology in young and mature primary cortical neurons in culture. Neuroscience. 2006;141:727–736. doi: 10.1016/j.neuroscience.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 53.Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 57. Chang CR, Manlandro CM, Arnoult D, Stadler J, Posey AE, Hill RB, Blackstone C. A lethal de novo mutation in the middle domain of the dynamin-related GTPase Drp1 impairs higher order assembly and mitochondrial division. J Biol Chem. 285:32494–32503. doi: 10.1074/jbc.M110.142430. The authors biochemically characterize a human Drp1 mutant (A395D) and demonstrate that it is defective in its assembly and mitochondrial localization.
- 58.Cuesta A, Pedrola L, Sevilla T, Garcia-Planells J, Chumillas MJ, Mayordomo F, LeGuern E, Marin I, Vilchez JJ, Palau F. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 59.Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–242. doi: 10.1385/nmm:8:1-2:217. [DOI] [PubMed] [Google Scholar]
- 61.Niemann A, Wagner KM, Ruegg M, Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis. 2009;36:509–520. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Palau F, Estela A, Pla-Martin D, Sanchez-Piris M. The role of mitochondrial network dynamics in the pathogenesis of Charcot-Marie-Tooth disease. Adv Exp Med Biol. 2009;652:129–137. doi: 10.1007/978-90-481-2813-6_9. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis. 20(Suppl 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. These studies (66 and 67) examined the role of Drp1 S-nitrosylation in Alzheimer's disease and draw opposing conclusions. Cho et al, 2009 show increased levels of Drp1 S-nitrosylation in Alzheimer’s and that this modification stimulates its activity, leading to neuronal cell death. In contrast, Bossy et al, 2009 report that Drp1 S-nitrosylation did not specifically increase in this disease nor undergo nitrosylation-induced activation. Further studies will be necessary to resolve this interesting and conflicting issue.
- 67.Bossy B, Petrilli A, Klinglmayr E, Chen J, Lutz-Meindl U, Knott AB, Masliah E, Schwarzenbacher R, Bossy-Wetzel E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer's disease. J Alzheimers Dis. 20(Suppl 2):S513–S526. doi: 10.3233/JAD-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J, Moody JP, Edgerly CK, Bordiuk OL, Cormier K, Smith K, Beal MF, Ferrante RJ. Mitochondrial loss, dysfunction and altered dynamics in Huntington's disease. Hum Mol Genet. 19:3919–3935. doi: 10.1093/hmg/ddq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, Malorni W, Davies KJ, Carafoli E, Scorrano L. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington's disease to apoptotic stimuli. EMBO Mol Med. 2:490–503. doi: 10.1002/emmm.201000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Lim PJ, Karbowski M, Monteiro MJ. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet. 2009;18:737–752. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by delta protein kinase C under oxidative stress conditions, in vivo. Mol Biol Cell. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 141:280–289. doi: 10.1016/j.cell.2010.02.026. Using skeletal muscle-specific mouse knockouts for Mfn1 and 2, the authors showed that mitochondrial fusion helps maintain the integrity of mitochondrial DNA and suppresses the accumulation of mutations in mitochondrial DNA.
- 73.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: tools that translate basic biological research into medicine. Chem Biol. 17:578–583. doi: 10.1016/j.chembiol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perkins G, Bossy-Wetzel E, Ellisman MH. New insights into mitochondrial structure during cell death. Exp Neurol. 2009;218:183–192. doi: 10.1016/j.expneurol.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 80.James DI, Martinou JC. Mitochondrial dynamics and apoptosis: a painful separation. Dev Cell. 2008;15:341–343. doi: 10.1016/j.devcel.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Cheng WC, Leach KM, Hardwick JM. Mitochondrial death pathways in yeast and mammalian cells. Biochim Biophys Acta. 2008;1783:1272–1279. doi: 10.1016/j.bbamcr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cereghetti GM, Scorrano L. The many shapes of mitochondrial death. Oncogene. 2006;25:4717–4724. doi: 10.1038/sj.onc.1209605. [DOI] [PubMed] [Google Scholar]
- 83.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 84. Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 142:889–901. doi: 10.1016/j.cell.2010.08.017. Using biochemical fractionation for Bax activation, this study identified Drp1. Drp1 binds cardiolipin in vitro and promotes hemifusion of cardiolipincontaining liposomes. This activity was shown to be critical for Bax activation and cytochrome c release in cells.
- 85.Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. Embo J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]