Abstract
Both the olfactory and the trigeminal systems are able to respond to intranasal presentations of chemical vapor. Accordingly, when the nose detects a volatile chemical, it is often unclear whether we smell it, feel it, or both. The distinction may often be unimportant in our everyday perception of fragrances or aromas, but it can matter in experiments that purport to isolate olfactory processes or study the interaction between olfaction and chemesthesis. Researchers turn to a small pool of compounds that are believed to be “pure olfactory” stimuli with little or no trigeminal impact. The current report reexamines one such commonly used compound, namely eugenol, a flavor and fragrance ingredient that has anesthetic properties under some conditions. Using a standard method involving many trials during an experimental session (Experiment 1), subjects were unable to reliably lateralize eugenol, consistent with claims that this compound is detected primarily through olfaction. However, with more limited exposure (Experiments 2 and 3), subjects were able to lateralize eugenol. We speculate that anesthetic properties of eugenol could blunt its trigeminal impact in some paradigms. Regardless, the current experiments suggest that eugenol can in fact stimulate the trigeminal nerve but in a complex concentration–dependent manner. Implications and strategies for selection of model odorants are discussed.
Keywords: chemesthesis, lateralization, pure odorant, trigeminal
Introduction
The human nose detects volatile compounds via at least 2 sensory systems. The olfactory system detects chemicals using specialized receptor neurons distributed on a limited dorsal area of the nasal mucosa and sends signals to the brain via the first cranial (olfactory) nerve. In the nose, mouth, eyes, and other facial areas, the trigeminal system detects chemicals using the more widely distributed free endings of the fifth cranial (trigeminal) nerve, though other systems could also be involved (Tizzano et al. 2010). This facial chemical feel is a subcomponent of body-wide chemesthesis. Most volatile compounds can stimulate both sensory systems, though higher concentrations are generally required to stimulate the trigeminal nerve. Thus, when the nose detects a chemical, we might smell it, feel it, or both. Both sensory systems contribute to our experience of fragrance and aroma, and in daily life, the distinction may not matter unless the odorant creates an irritating or painful experience.
In contrast, the distinction is critical in laboratory experiments that focus on olfaction or interactions between olfaction and chemesthesis (Cain and Murphy 1980; Hummel et al. 1996; Wysocki and Wise 2003). Several methods can determine whether people feel volatile compounds, thus enabling an experimental isolation of the olfactory system. One approach is to study intranasal perception in people who lack a functional sense of smell, that is, anosmics (Doty et al. 1978; Cometto-Muñiz and Cain 1990; Cometto-Muñiz et al. 2005). Another is nasal lateralization, a paradigm in which subjects simultaneously receive clean air in one nostril and chemical vapor in the other (Wysocki et al. 2003). Subjects then attempt to determine which nostril receives the chemical vapor. Published work suggests that people are unable to lateralize volatile chemicals based on olfaction but are able to do so when concentrations reach levels high enough to feel (Kobal et al. 1989; Lundström, Hummel, et al. 2003; Boyle et al. 2006; Frasnelli et al. 2009; but see Wysocki et al. 2003; Porter et al. 2005). Compounds that cannot be lateralized, or that anosmics cannot detect, have been used as “pure olfactory” or “nontrigeminal” stimuli in experiments. Common examples include vanillin, phenylethyl alcohol (PEA), hydrogen sulfide, and eugenol (though, as with any other volatile compound, concentration is an important consideration, a point to which we shall return in the General discussion).
Eugenol, a phenylpropene extracted from clove oil, nutmeg, cinnamon, basil, and bay leaf, is a particularly interesting stimulus. In a previous experiment, anosmics reported that this common flavor and fragrance ingredient did not produce noticeable irritation (Doty et al. 1978) and, in other experiments, normosmics failed to lateralize eugenol (Porter et al. 2005). Of course, to conclude that subjects cannot lateralize is to accept a null hypothesis, and the statistical power of the test will matter a great deal (we will return to this issue in the discussion, as described below). Nevertheless, these experiments would seem to suggest that eugenol is not a potent trigeminal stimulus and may be detected primarily via olfaction. Other work, however, has shown that eugenol can be lateralized when presented monorhinally (Hummel and Kobal 1994) or when presented roughly 3 times in a session, over multiple sessions, in which 12 odorants were evaluated for nasal lateralization (Cometto-Muñiz et al. 2005), that is, few well spaced trials with eugenol in a session.
The current experiments studied nasal lateralization of eugenol more thoroughly, using 3 different experimental methods. In Experiment 1, we attempted to measure both absolute detection and lateralization thresholds for eugenol using a modified staircase procedure (which entails a number of trials at various concentrations over the course of an experimental session) (Wysocki et al. 2003). In Experiment 2, subjects attempted to lateralize a moderate fixed concentration in a single trial. In Experiment 3, subjects attempted to lateralize neat (pure) eugenol in 11 trials.
Materials and methods
Experiment 1
The aim of this experiment was to determine sensitivity of intranasal chemesthesis to eugenol. It was conducted as a part of a much broader program designed to explore interactions between chemesthesis and olfaction.
Participants
Ten people (8 women) participated (8 Caucasians, 1 African American, and 1 Asian). Their ages ranged from 22 to 39 (mean ± standard deviation [SD] = 30 ± 10.4), and all signed informed consent approved by the University of Pennsylvania Institutional Review Board.
Chemosensory stimuli
Eugenol (ACROS Organics; 99% pure; CAS 97-53-0) was the stimulus of interest. We constructed a 3-fold dilution series in propylene glycol (which was slightly tinged to match the color of eugenol). Tinged propylene glycol diluted with clear diluent served as the series of matched blanks. The first step of eugenol was neat with a total of 19 steps. Each step had 10 mL of solution or the diluent. Stimuli were contained in clean 250 mL glass bottles with a screw-top cap having 2 holes. A short Teflon tube was inserted in 1 hole, over which a plastic fitting was applied. The fitting supported a Teflon nosepiece, which had been machined to have a hollow core and a convex tip to allow easy insertion of the proximal end into a nostril. The other hole in the cap was fitted with a longer Teflon tube, which ended just above the fluid in the bottle. This tube allowed external air to enter the bottle as the subject sniffed the headspace through the nosepiece.
Procedures
Initially, olfactory detection threshold (ODT) was obtained by using a 2-alternative forced-choice modified staircase method (Wetherill and Levitt 1965; Wysocki et al. 1997), a procedure that is commonly used by chemosensory researchers to measure olfactory thresholds (Wysocki and Wise 2003). On the first trial, the subject was given 2 sets of bottles; 1 pair consisted of 2 blanks and the other pair consisted of 1 blank and a dilution concentration step of eugenol previously determined to be slightly above average ODT, namely step 13, that is, 0.000565% v/v. The set of bottles that was presented first on each trial was determined randomly; however, all individuals received the same sequence. Upon receiving the pair of bottles, the subject inserted the Teflon nosepieces into each nostril and sniffed (the side of the nose receiving the odorant was randomized throughout testing). After both sets had been sampled, the subject indicated which set contained the odorant. If the odorant pair was correctly identified in 2 consecutive trials, the concentration was decreased by 1 dilution step (a potential reversal point). If an incorrect answer was provided, the concentration was increased by 1 dilution step (another potential reversal point). If the sequence in concentration exceeded 3 dilution steps, all previous reversals were ignored. The sequence terminated after at least 5 legitimate reversals had occurred. To calculate the threshold, the first legitimate reversal was ignored, and the mean of the concentrations of the next 4 reversals was defined as the detection threshold.
Two lateralization threshold series were then conducted. Thresholds were obtained using a similar 2-alternative forced-choice modified staircase method. Unlike the ODT trials, each lateralization trial required that the subject sniff only from a single pair of bottles; one bottle was a blank and the other contained eugenol. The subject simultaneously placed the Teflon nosepieces into the nostrils and sniffed. After removing the nosepieces, the subject indicated whether the stimulus had been presented to the left or to the right nostril. In general, the sequence of events for determining lateralization thresholds was the same as those for determining detection thresholds but testing commenced at the subject's ODT. The lateralization threshold was then calculated as the mean of the concentration of irritant at which the 4 reversals occurred. After a 5-min rest, the procedure was repeated to obtain a second lateralization threshold.
Results and discussion
The average ODT was 3.76 × 10−5% v/v in solution (headspace concentration was not measured). ODTs ranged from 5.23 × 10−6% to 3.76 × 10−4% v/v across all 10 subjects. In the current context, this result is an important positive control. The subjects were able to detect eugenol (to discriminate it from a blank), and the staircase procedure was able to successfully characterize ODTs for the compound in all participants.
In contrast to the odor thresholds, the staircase method failed to yield valid lateralization thresholds. In each of the 20 attempts (2 attempts per subject), subjects failed to lateralize the highest concentration on some trials, and the staircase procedure called for a higher concentration than neat eugenol. Thus, according to the staircase procedure, the lateralization threshold for eugenol must be higher than that of headspace above neat eugenol at room temperature and pressure. Importantly, many of the subjects contributed lateralization thresholds in other experiments using other compounds, for example, acetic acid, acetone, butanol, and menthol. Lateralization thresholds were always obtained for each individual.
Thus far, the experiments are consistent with the idea that eugenol has little or no trigeminal impact. However, eugenol has long been used as local anesthetic in dentistry (Lee et al. 2005). Though large-scale clinical trials are lacking, eugenol is known to act on both voltage-gated sodium channels and the transient receptor potential channel TRPV1 (Park et al. 2009). Furthermore, oral administration in rats causes analgesia according to several pain models (Park et al. 2011). Of course, application of liquid to the oral mucosa or oral administration may differ from inhalation of vapor-phase eugenol. Regardless, if eugenol does desensitize the nasal mucosa over time, it could have complex effects, possibly even blunting its own trigeminal impact over the course of a test session so that it is detected by both the trigeminal and the olfactory systems on initial exposure but only by the olfactory system later.
Experiment 2
If eugenol does in fact stimulate the trigeminal nerve but desensitizes the mucosa over the course of an experimental session, then subjects should be able to lateralize in a single trial. Thus, we prepared and presented 11% (v/v) eugenol (diluted as above) versus a blank (diluent only) and asked 11 individuals (2 males) to take a single sniff from the Teflon nosepieces atop the bottles (the nostril that received eugenol was randomized). Of 11 subjects, 9 were able to correctly lateralize the eugenol-stimulated side, which was significantly greater than chance according to binomial statistics (P = 0.027). This brief experiment suggests that subjects can in fact feel, as well as smell, intranasal eugenol, though the very small number of experimental trials involved is a serious limitation.
Experiment 3
An additional group of subjects attempted to lateralize a higher fixed concentration of eugenol (neat) to provide additional evidence that intranasal eugenol can stimulate the trigeminal nerve. Each subject contributed multiple trials so that performance could be assessed within individuals using a high concentration of eugenol. Since previous studies showed that subjects are able to reliably lateralize a fixed concentration of menthol (Frasnelli, Albrecht, et al. 2011; Wise et al. 2011), subjects also attempted to lateralize menthol as a positive control.
Participants
Ten healthy adults (6 women) with an age range from 25 to 41 (mean ± SD = 32 ± 5.7) participated after providing informed signed consent. All aspects of the study were approved by the University of Pennsylvania Institutional Review Board.
Chemosensory stimuli
We used eugenol (CAS 97-53-0: Sigma Aldrich, >99% pure) and L-menthol (menthol; CAS 2216-51-5: Fisher Scientific, Acros Organics, labeled >99% pure). The purity of the eugenol was verified using gas chromatography/mass spectrometry to ensure that lateralization performance was unlikely to be influenced by trace contaminants. Neat eugenol (20 mL) was presented in amber glass sniff bottles (as described above for Experiment 1). Filtered light mineral oil served as a blank in lateralization trials but was not tinged because amber bottles were used. Menthol crystals (20 g) also were presented in identical amber glass sniff bottles. Sea salt (20 g) served as a blank.
Procedures
Methods of stimulus presentation matched those for Experiments 1 and 2 for the most part. However, because differences in stimulus appearance between menthol crystals and sea salt could not be completely eliminated with the use of amber bottles, subjects were blindfolded, and experimenters held the bottles for subjects while they held on to the nosepiece and sniffed. In 1 block of 11 trials, subjects attempted to lateralize eugenol. In another block of 11 trials, subjects attempted to lateralize menthol. The order of blocks (menthol first vs. eugenol first) was counterbalanced across subjects. A 30-s interval elapsed between successive trails for both stimuli. Two identical sets for each odorant were used to allow the headspace to regenerate between trials. In each block of trials, stimulation of the left or right nostril followed a pseudorandomized sequence, with each nostril stimulated either 5 or 6 times.
Results and discussion
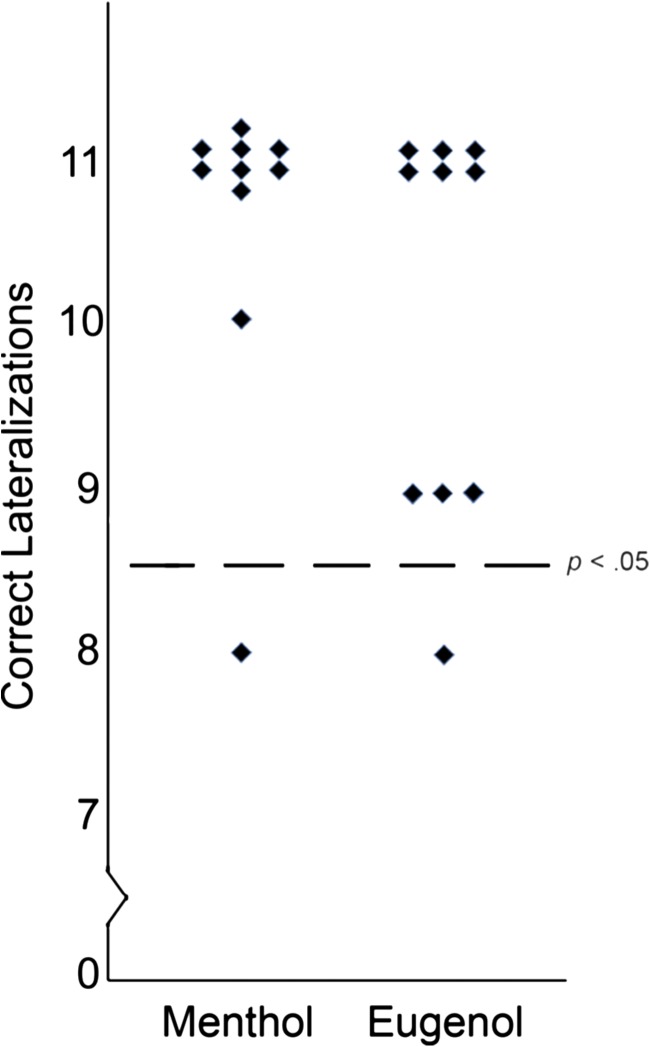
For eugenol, the mean number correct was 10.1 (SD ±1.2) (Figure 1). Relative to an expected value of 5.5 (chance level), lateralization scores for the group reached significance, χ2(9) = 40.82, P < 0.001. According to binomial statistics, an individual must achieve 9 correct to exceed chance (P = 0.033). Of 10 subjects, 9 met this criterion (binomial P = 0.0098). For menthol, the mean number correct was 10.6 (SD ±0.9). As with eugenol, lateralization scores for the group reached significance, χ2(9) = 48.82, P < 0.001, and lateralization performance exceeded chance for 9 of 10 individual subjects. The 1 participant that failed to reliably lateralize each compound (different individuals for the 2 compounds) obtained 8 of 11 correct. Thus, under the conditions of the current experiment, humans were able to lateralize both menthol and eugenol. These results support those of Experiment 2 in suggesting that intranasal eugenol in fact does stimulate the trigeminal nerve and agree with the findings of Hummel and Kobal (1994) and Cometto-Muñiz et al. (2005).
Figure 1.
Number of correct lateralization trails for each individual and each odor. Dashed line in graph indicates a statistical value of P < 0.05 according to the binominal distribution.
General discussion
A few publications infer or explicitly state that eugenol is a pure olfactory stimulus, having no irritant properties (Von Skramlik 1926; Allen 1929; Doty 1975; Doty et al. 1978; Porter et al. 2005). The results of Experiment 1 are consistent with this view. However, Experiments 2 and 3 provide clear evidence that eugenol also produces intranasal irritation via the trigeminal system. The results of Experiments 2 and 3 are consistent with at least 2 published reports which showed that eugenol vapor can be correctly lateralized under some circumstances (Hummel and Kobal 1994; Cometto-Muñiz et al. 2005) and can produce patterns of evoked potentials consistent with activation of the trigeminal nerve (Hummel and Kobal 1994).
Though the current experiments do not directly test the hypothesis that eugenol anesthetizes the nose over the course of an experimental session, this is a possible explanation given that eugenol can act as a local anesthetic (Park et al. 2009) and has for many years been used as such in dentistry clinics (though it has not been shown that sniffing eugenol vapor can anesthetize the nasal mucosa). Eugenol might also cause more rapid or profound self-adaptation than other nasal-trigeminal stimuli. Regardless, it is clear that eugenol is not strictly an olfactory stimulus and that the ability to lateralize will depend on both concentration and dynamics of stimulation.
Regarding concentration, in one study in which anosmics reported no intranasal irritation, subjects sampled the headspace above neat eugenol (Doty et al. 1978). However, because the wide-mouth odor-vessels were not sealed, subjects could have taken in room air along with eugenol vapor, thereby diluting the samples. In addition, the judgments were subjective, and the criterion by which irritation was judged may not have been totally clear. Finally, because anosmics can differ from normosmics with respect to irritation sensitivity, anosmics may not be a perfect model (Frasnelli et al. 2006, 2007). In a recent study in which normosmics were unable to lateralize eugenol, concentrations were not provided (Porter et al. 2005). Regardless, the extent to which eugenol stimulates the trigeminal nerve will almost certainly depend on concentration and method of presentation, which future studies can elucidate in more detail.
Eugenol has often been used as a stimulus in olfactory neuroimaging studies, sometimes with the explicit or implicit motivation that it is a good model to isolate olfactory processing (Yousem et al. 1999; Savic and Gulyas 2000; Savic et al. 2000, 2005; Bengtsson et al. 2001; Suzuki et al. 2001). However, as demonstrated above, eugenol is a complex stimulus that in certain concentrations most probably acts as an anesthetic on the trigeminal system and potentially also on the olfactory system, whereas in other concentrations, eugenol produces a clear irritation. Based on these data, we recommend that eugenol should not be used in chemosensory experiments unless the expressed interest is merely to produce the perceptual quality of cloves.
If moderate levels of eugenol produce even perithreshold (not clearly recognized but detected above chance) irritation, what could this mean for interpretation of experiments? To take one example, Chen et al. (2011) conducted an experiment in which nominally pure odors of different qualities were presented to opposite nostrils to determine whether subjects could lateralize odors of different quality. The 1 pair of odors that was lateralized above chance level included eugenol. In light of the current results, even some mild trigeminal stimulation on a few trials could have produced the result, which provides an alternative interpretation to the conclusion that subjects can lateralize odors of different quality.
In other studies, eugenol has been used to mask the presence of other compounds. In studies of human chemical communication, the odors of chemicals produced by the human body have been masked with eugenol (Lundström, Goncalves, et al. 2003). Because eugenol was presented both with and without putative chemosignals, use of eugenol does not invalidate the conclusions of these studies. However, the complex physiological effects mean that eugenol might not be an ideal choice if the goal is to simply mask odor. This consideration might be particularly important if dependent measures include autonomic nervous system response because even very low levels of trigeminally active compounds can effect sympathetic response (Jacquot et al. 2004).
What volatiles should be used when the aim is to experimentally manipulate the olfactory system in isolation? PEA is widely used as a pure olfactory stimulus. Yet, ethmoid nerve recordings in rats show that PEA vapor can stimulate the trigeminal nerve in at least some individual animals (Silver and Moulton 1982), and one human study suggests that some people can lateralize PEA vapor at above chance levels (Frasnelli, Hummel, et al. 2011). To date, vanillin vapor has not been reported to elicit a trigeminal response (Doty et al. 1978; Cometto-Muñiz et al. 2005; Frasnelli, Hummel, et al. 2011). Unfortunately, vanillin is often an impractical model stimulus because it may not render a strong percept and tends to leave a residual odor in olfactometers. Regardless, one should keep in mind that compounds that produce no clear irritation at room temperature may do so when vapor-phase concentration is increased by heating, although this does not occur for vanillin (Cometto-Muñiz et al. 2007). In the extreme case of applying neat liquid to the mucosa, even PEA and vanillin can elicit a clear sensation (Prah and Benignus 1984). Furthermore, hydrogen sulfide, another compound sometimes used as a pure olfactory stimulus, can actually cause neurogenic inflammation of the airways under some conditions (Trevisani et al. 2005). Thus, any interpretation of experimental findings that depends on stimuli being detected only by olfaction should be received with caution. In addition to asking what volatiles should be used to avoid trigeminal stimulation, one must also consider concentration.
Rather than searching for a “pure odor,” a more direct solution is to present a lower concentration that is able to produce a clear odorous sensation but without rendering a measurable trigeminal response. A lateralization task similar to that described above will probably offer the most useful information regarding trigeminal stimulation. Of course, to conclude that an odor cannot be lateralized is to accept a null hypothesis. One should consider how even a weak perithreshold trigeminal response might bias results and determine whether the lateralization task used is powerful enough to identify such effects. Furthermore, it may not be possible to depend on published results, unless the method of stimulation is exactly the same.
Regarding isolation of the trigeminal system, to the best of our knowledge, no nontoxic compound exists that activates the trigeminal system in isolation. Carbon dioxide (CO2) is often used in studies exploring trigeminal processing, and few subjects report an odor sensation when stimulated with CO2. However, although it produces little or no conscious odor perception, CO2 does activate olfactory neurons at low concentrations in some nonhuman species (Coates and Ballam 1990; Hu et al. 2007).
In conclusion, most previous research in which eugenol served as a stimulus, either as an odorant per se or as a masker of another odorant, should be reevaluated. In the future, investigators should carefully consider concentration and dynamics of stimulation if eugenol is selected as a pure olfactory stimulus.
Funding
This work was supported by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders [5P50 DC00214].
Acknowledgments
We wish to thank Lydia Milbury for help in acquiring data for Experiment 3.
References
- Allen WF. Effect of various inhaled vapors on respiration and blood pressure in anesthetized, unanesthetized, sleeping, and anosmic subjects. Am J Physiol. 1929;88:620–632. [Google Scholar]
- Bengtsson S, Berglund H, Gulyas B, Cohen E, Savic I. Brain activation during odor perception in males and females. Neuroreport. 2001;12:2027–2033. doi: 10.1097/00001756-200107030-00048. [DOI] [PubMed] [Google Scholar]
- Boyle JA, Lundström JN, Knecht M, Jones-Gotman M, Schaal B, Hummel T. On the trigeminal percept of androstenone and its implications on the rate of specific anosmia. J Neurobiol. 2006;66:1501–1510. doi: 10.1002/neu.20294. [DOI] [PubMed] [Google Scholar]
- Cain WS, Murphy CL. Interaction between chemoreceptive modalities of odour and irritation. Nature. 1980;284:255–257. doi: 10.1038/284255a0. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou W, Chen D. A graded olfactory contrast between nasal passages enables stereo human olfaction. P#127. St Pete Beach (FL): Association of Chemoreception Sciences; 2011. p. 69. [Google Scholar]
- Coates EL, Ballam GO. Olfactory receptor response to CO2 in bullfrogs. Am J Physiol. 1990;258:R1207–R1212. doi: 10.1152/ajpregu.1990.258.5.R1207. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS. Thresholds for odor and nasal pungency. Physiol Behav. 1990;48:719–725. doi: 10.1016/0031-9384(90)90217-r. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH. Determinants for nasal trigeminal detection of volatile organic compounds. Chem Senses. 2005;30:627–642. doi: 10.1093/chemse/bji056. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH, Sanchez-Moreno R. Cutoff in detection of eye irritation from vapors of homologous carboxylic acids and aliphatic aldehydes. Neuroscience. 2007;145:1130–1137. doi: 10.1016/j.neuroscience.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Intranasal trigeminal detection of chemical vapors by humans. Physiol Behav. 1975;14:855–859. doi: 10.1016/0031-9384(75)90081-5. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Albrecht J, Bryant B, Lundström JN. Perception of specific trigeminal chemosensory agonists. Neuroscience. 2011;189:377–383. doi: 10.1016/j.neuroscience.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli J, Charbonneau G, Collignon O, Lepore F. Odor localization and sniffing. Chem Senses. 2009;34:139–144. doi: 10.1093/chemse/bjn068. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Hummel T, Berg J, Huang G, Doty RL. Intranasal localizability of odorants: influence of stimulus volume. Chem Senses. 2011;36:405–410. doi: 10.1093/chemse/bjr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli J, Schuster B, Hummel T. Subjects with congenital anosmia have larger peripheral but similar central trigeminal responses. Cereb Cortex. 2007;17:370–377. doi: 10.1093/cercor/bhj154. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Schuster B, Zahnert T, Hummel T. Chemosensory specific reduction of trigeminal sensitivity in subjects with olfactory dysfunction. Neuroscience. 2006;142:541–546. doi: 10.1016/j.neuroscience.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Hummel T, Barz S, Lotsch J, Roscher S, Kettenmann B, Kobal G. Loss of olfactory function leads to a decrease of trigeminal sensitivity. Chem Senses. 1996;21:75–79. doi: 10.1093/chemse/21.1.75. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G. Chemosensory event related potentials: effects of dichotomous stimulation with eugenol and dipyridyl. In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and taste XI. Berlin (Germany): Springer; 1994. pp. 659–663. [Google Scholar]
- Jacquot L, Monnin J, Brand G. Unconscious odor detection could not be due to odor itself. Brain Res. 2004;1002:51–54. doi: 10.1016/j.brainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Kobal G, Van Toller S, Hummel T. Is there directional smelling? Experientia. 1989;45:130–132. doi: 10.1007/BF01954845. [DOI] [PubMed] [Google Scholar]
- Lee MH, Yeon KY, Park CK, Li HY, Fang Z, Kim MS, Choi SY, Lee SJ, Lee S, Park K, et al. Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res. 2005;84:848–851. doi: 10.1177/154405910508400913. [DOI] [PubMed] [Google Scholar]
- Lundström JN, Goncalves M, Esteves F, Olsson MJ. Psychological effects of subthreshold exposure to the putative human pheromone 4,16-androstadien-3-one. Horm Behav. 2003;44:395–401. doi: 10.1016/j.yhbeh.2003.06.004. [DOI] [PubMed] [Google Scholar]
- Lundström JN, Hummel T, Olsson MJ. Individual differences in sensitivity to the odor of 4,16-androstadien-3-one. Chem Senses. 2003;28:643–650. doi: 10.1093/chemse/bjg057. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009;144:84–94. doi: 10.1016/j.pain.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Park SH, Sim YB, Lee JK, Kim SM, Kang YJ, Jung JS, Suh HW. The analgesic effects and mechanisms of orally administered eugenol. Arch Pharm Res. 2011;34:501–507. doi: 10.1007/s12272-011-0320-z. [DOI] [PubMed] [Google Scholar]
- Porter J, Anand T, Johnson B, Khan RM, Sobel N. Brain mechanisms for extracting spatial information from smell. Neuron. 2005;47:581–592. doi: 10.1016/j.neuron.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Prah JD, Benignus VA. Trigeminal sensitivity to contact chemical stimulation: a new method and some results. Percept Psychophys. 1984;35:65–68. doi: 10.3758/bf03205925. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H, Lindström P. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci U S A. 2005;102:7356–7361. doi: 10.1073/pnas.0407998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Gulyas B. PET shows that odors are processed both ipsilaterally and contralaterally to the stimulated nostril. Neuroreport. 2000;11:2861–2866. doi: 10.1097/00001756-200009110-00007. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Silver WL, Moulton DG. Chemosensitivity of rat nasal trigeminal receptors. Physiol Behav. 1982;28:927–931. doi: 10.1016/0031-9384(82)90216-5. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Suckling J, Fukuda R, Williams SC, Andrew C, Howard R, Ouldred E, Bryant C, Swift CG, et al. Functional magnetic resonance imaging of odor identification: the effect of aging. J Gerontol A Biol Sci Med Sci. 2001;56:M756–M760. doi: 10.1093/gerona/56.12.m756. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Creminon C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Skramlik E. Handbuch der Physiologie der niederen Sinne. Leipzig (Germany): Georg Thieme; 1926. [Google Scholar]
- Wetherill GB, Levitt H. Sequential estimation of points on a psychometric function. Br J Math Stat Psychol. 1965;18:1–10. doi: 10.1111/j.2044-8317.1965.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Wise PM, Preti G, Eades J, Wysocki CJ. The effect of menthol vapor on nasal sensitivity to chemical irritation. Nicotine Tob Res. 2011;13:989–997. doi: 10.1093/ntr/ntr107. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Cowart BJ, Radil T. Nasal trigeminal chemosensitivity across the adult life span. Percept Psychophys. 2003;65:115–122. doi: 10.3758/bf03194788. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Dalton P, Brody MJ, Lawley HJ. Acetone odor and irritation thresholds obtained from acetone-exposed factory workers and from control occupationally unexposed subjects. Am Ind Hyg Assoc J. 1997;58:704–712. doi: 10.1080/15428119791012342. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wise P. Methods, approaches, and caveats for functionally evaluating olfaction and chemesthesis. In: Deibler K, Delwiche JF, editors. Handbook of flavor characterization: sensory, chemical and psychophysiological. New York: Marcel Dekker; 2003. pp. 1–40. [Google Scholar]
- Yousem DM, Maldjian JA, Siddiqi F, Hummel T, Alsop DC, Geckle RJ, Bilker WB, Doty RL. Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res. 1999;818:480–487. doi: 10.1016/s0006-8993(98)01276-1. [DOI] [PubMed] [Google Scholar]