Abstract
Mice lacking both the P2X2 and the P2X3 purinergic receptors (P2X-dblKO) exhibit loss of responses to all taste qualities in the taste nerves innervating the tongue. Similarly, these mice exhibit a near total loss of taste-related behaviors in brief access tests except for a near-normal avoidance of acidic stimuli. This persistent avoidance of acids despite the loss of gustatory neural responses to sour was postulated to be due to continued responsiveness of the superior laryngeal (SL) nerve. However, chemoresponses of the larynx are attributable both to taste buds and to free nerve endings. In order to test whether the SL nerve of P2X-dblKO mice remains responsive to acids but not to other tastants, we recorded responses from the SL nerve in wild-type (WT) and P2X-dblKO mice. WT mice showed substantial SL responses to monosodium glutamate, sucrose, urea, and denatonium—all of which were essentially absent in P2X-dblKO animals. In contrast, the SL nerve of P2X-dblKO mice exhibited near-normal responses to citric acid (50 mM) although responsiveness of both the chorda tympani and the glossopharyngeal nerves to this stimulus were absent or greatly reduced. These results are consistent with the hypothesis that the residual avoidance of acidic solutions by P2X-dblKO mice may be attributable to the direct chemosensitivity of nerve fibers innervating the laryngeal epithelium and not to taste.
Keywords: ATP, cough, irritation, larynx, purinergic, taste
Introduction
The palatability of foods and liquids taken into the mouth depends on sensory qualities extracted by multiple chemosensory and mechanosensory modalities of the oral and nasal cavities. The vaporous components of foods reflux through the nasopharynx to activate olfactory neurons and free nerve endings of the nasal cavity. The solid and liquid components of foods can stimulate taste buds or free nerve endings in the oropharynx. The sensations arising from taste bud cells are limited to the 5 basic tastes: salt, sweet, sour, umami, and bitter. Other sensations of flavor, for example, spiciness or irritation, are attributable to other modalities, including free nerve endings arising from the trigeminal, glossopharyngeal (GL), and vagus nerves. Thus, a potential item of food might be rejected because of an aversive taste, for example, being too bitter, or because of pain or irritation produced by activation of free nerve endings, for example, a hot pepper might be too hot.
The sense of taste plays a major role in acceptance or rejection of potential food items. In general, sweet and umami tastes evoke positive responses (increased consumption), whereas bitter or sour tastes are aversive. Salty has a mixed hedonic according to concentration. We recently investigated a line of knockout mice that lack 2 purinergic receptors, P2X2 and P2X3 (P2X2/P2X3 double-KO mice; P2X-dblKO) that are expressed on taste nerves. In these P2X-dblKO mice, all taste functions are greatly reduced or absent (Finger et al. 2005). Specifically, the 2 taste nerves innervating taste buds on the tongue (chorda tympani [CT] and GL) in P2X-dblKO mice are almost completely unresponsive to all applied tastants, including sugars (sweet), monosodium glutamate (umami), denatonium benzoate (bitter), NaCl (salty), and citric acid and HCl (sour). In keeping with this lack of neural response, P2X-dblKO mice are also unable to recognize or respond to tastants in brief access tests and conditioned taste aversion paradigms (Eddy et al. 2009; Hallock et al. 2009). The singular exception to this is that P2X-dblKO animals retain a near-normal avoidance of acidic solutions despite the lack of neural response to acids in the taste nerves innervating the tongue (Finger et al. 2005). Because all taste buds in the oropharynx appear, on anatomical criteria, to depend similarly on the presence of purinergic signaling mechanisms, including P2X2 and/or P2X3 (Finger et al. 2005), we conjectured that avoidance of acidic solutions by the P2X-dblKO mice might be due to the ability of acid (sour) solutions to activate chemosensitive ion channels on the free nerve endings of the posterior oral cavity, larynx, and oropharynx.
The lingual face of the larynx and epiglottis is heavily invested with peptidergic, fibers displaying immunoreactivity for transient receptor potential vanilloid-1 (TrpV1) (Yoshida et al. 2000; Kitagawa et al. 2002; Uno et al. 2004), and other chemoresponsive Trp channels (Peyrot des Gachons et al. 2011). Sour (acidic) substances activate nerve fibers of the superior laryngeal (SL) nerve (Dickman and Smith, 1988) to evoke reflex swallowing in a concentration-dependent fashion (threshold about 5mM for citric acid; Kajii et al. 2002). Because TrpV1 channels respond to acids as well as capsaicin and other irritant chemicals (Arai et al., 2010), we reasoned that acidic solutions taken into the mouth might be detected by these laryngeal and epiglottal nerve fibers to trigger avoidance responses in the absence of a functional taste system in the P2X-dblKO mice. In order to test this possibility, we compared responses of the lingual taste nerves (CT and GL) and the SL nerve, which innervates both taste buds and free nerve endings in the larynx, in wild-type (WT) and P2X-dblKO mice. Because the larynx contains taste buds as well as free nerve endings, we predicted that the taste component of the neural response would be absent in the P2X-dblKO mice, whereas activity arising from the free nerve endings would be intact. Moreover, the GL, but not the CT, contains polymodal nociceptor fibers that innervate the oral mucosa. These mucosal fibers should be relatively unaffected by genetic deletion of P2X2 and P2X3. Thus, acids may also directly activate nerve fibers through the agency of acid-gated ion channels (Olson et al. 1998; Ichikawa and Sugimoto 2002; Fukuda et al. 2006). Accordingly, we also wanted to compare the responsiveness of the CT, a pure taste nerve, with that of the GL, which contains both taste and general mucosal afferents.
Materials and methods
Animals
We utilized 3 lines of mice for these experiments. For the anatomical studies, we used a line expressing green fluorescent protein (GFP) under the control of the transient receptor potential melastin-5 (TrpM5) promoter. These mice, generated by R. Margolskee and S. Demak, are described in detail (Clapp et al. 2006) and have been utilized in many previous investigations on chemosensory systems. The TrpM5-GFP construct contained 5′–3′: 11 kb of mouse TrpM5 5′-flanking sequence, TrpM5 Exon 1 (untranslated), Intron 1, and the untranslated part of Exon 2, and eGFP. The TrpM5-driven GFP appears faithful to TrpM5 protein expression and is a convenient marker for taste buds as well as solitary chemosensory cells (Yoshida et al. 2000; Perez et al. 2002; Clapp et al. 2006; Kaske et al. 2007; Lin et al. 2008; Tizzano et al. 2011). For electrophysiological studies, we employed B6; 129-P2rx2tm1Ckn/P2rx3tm1Ckn (P2X-dblKO; n = 34) mice of either sex, along with the control WT line (n = 61) of similar background (mixed C57Bl6 and Ola) as in the previous studies (Finger et al. 2005; Eddy et al. 2009; Hallock et al. 2009; Stratford and Finger 2011). All housing and handling procedures were under the guidelines of either the Institutional Animal Care and Use Committees (IACUC) of University of Colorado Denver (Aurora, CO) or the committee for Laboratory Animal Care and Use at Kyushu University (Fukuoka, Japan).
Anatomical studies
In order to assess whether the degree of innervation of the larynx and pharynx were similar in WT and P2X-dblKO mice, animals of each genotype were prepared for immunohistochemistry for protein gene product 9.5 (ubiquitin-C-terminal hydrolase 1) to reveal total innervation or for calcitonin gene-related peptide (CGRP) to reveal peptidergic innervation, an indicator of capsaicin-sensitive (TrpV1-expressing) nerve fibers (Silver et al. 1991; Ishida et al. 2002; Gulbransen et al. 2008), largely polymodal nociceptors.
The mice were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally; Ovation Pharmaceuticals Inc.) and perfused transcardially first with 0.9% saline and then with 4% paraformaldehyde in 0.1 M phosphate buffer. The larynx and nearby pharynx and esophagus were removed and post-fixed for 2–6 h before being transferred to buffer containing 20% sucrose as a cryoprotectant. The tissue blocks were frozen onto a cryostat chuck with OCT (Optimal Cutting Temperature compound; Fisher Scientific) and sectioned onto slides at a thickness of 15–20 μm. After being allowed to air dry for 8 h or longer, the slides were washed in buffer and then treated for immunocytochemistry. Following treatment in 1% normal goat serum in 0.1 M phosphate buffer with 0.3% Triton X 100 detergent, the sections were incubated overnight in primary antisera directed against either PGP or CGRP (PGP9.5/rb, AbdSerotec, lot # 060810, code 7863-0504, 1:500; CGRP/rb, Bachem, lot A06256, code t-4032). Following 3 brief rinses, the tissue then was exposed to secondary antiserum (1:400; Alexa Fluor 568, goat anti-rabbit, Invitrogen, code A11036 lot 757107) for 1–3 h. Following buffer washes, the sections either were coverslipped directly with Fluoromount-G (Fisher Scientific) or were counterstained with 4′,6-diamino-2-phenylindole or NeuroTrace 640/660 deep-red fluorescent Nissl stain (Invitrogen cat # N21483) before coverslipping.
Images were acquired either on an Olympus Fluoview laser scanning confocal microscope using a ×20 oil immersion objective or with a monochrome Q-imaging camera on an Olympus BX41TF microscope with dry ×10, ×20, and ×40 objectives.
Recording from the CT, GL, and SL nerves
The detailed procedures for recording from the 3 nerves are given in Arai et al. (2010). In brief, under sodium pentobarbital anesthesia (40–50 mg/kg of body weight), the trachea of each animal was cannulated, and the mouse was then fixed in the supine position with a head holder to allow dissection of each of the nerves. The right CT nerve was exposed at its exit from the lingual nerve by removal of internal pterygoid muscle. Then, the CT nerve was dissected free from surrounding tissues and cut at the point of its entry to the bulla. Similarly, the right GL nerve was exposed by removal of the digastric muscle and posterior horn of the hyoid bone. The GL nerve then was dissected free from underlying tissues and cut near its entrance to the posterior lacerated foramen. To access the SL nerve, the sternohyoid and omohyoid muscles were retracted and tied. The right SL nerve was then dissected free from surrounding connective tissue and was cut close to its junction with the vagus nerve. For each nerve, the entire nerve was placed on a silver wire electrode and an indifferent electrode was positioned nearby in the wound. Neural responses induced by chemical stimulation of the tongue or throat was fed into an amplifier (Iyodenshikogaku K-1; total amplification of amplifier and integrator = ×100 000), monitored on an oscilloscope and an audio monitor. Whole-nerve responses were integrated with a time constant of 1.0 s and recorded on a computer for later analysis using PowerLab system (PowerLab/sp4; AD Instruments). The response magnitude was obtained by subtraction of the mean voltage prior to the stimulus (base line) from the mean voltage after taste stimulus.
Chemical stimulation
Chemical stimulation of the lingual taste fields was accomplished by flowing solutions across the anterior part of the tongue (for CT recording) or across the posterior part of the tongue (for GL recording) via an incision at the corner of the mouth. Test solutions flowed for ∼30 s (for CT recording) or ∼60 s (for GL recording). Between successive stimuli presentations, the tongue was rinsed with distilled water (DW) during the interval of ∼60 s (for CT recording) or ∼120 s (for GL recording). All test and rinse solutions flowed at a rate of ∼0.1 mL/s.
Chemical stimulation of the pharynx was accomplished after removal of much of the epiglottis and other ventral laryngeal cartilage allowing direct visualization of the upper part of the larynx. Furthermore, removal of much of the epiglottis minimized the mechanical responses of the organ allowing for more distinctive chemical responses. Stimulus solutions were then applied by pipette and allowed to stand for ∼30 s before being flushed away with a wash of 150 mM NaCl. The NaCl solution rinse was allowed to stand on the tissue during the interval of ∼60 s between stimulus applications because the SL nerve shows a robust response to DW as this tissue normally is bathed in saliva or mucus containing isotonic concentrations of NaCl (Dickman and Smith 1988; Smith and Hanamori 1991).
A series of standard tastants including 3 acids were used to assess function of the responsiveness of each nerve. Table 1 lists solutions and concentrations used in the experiment. In addition, the SL nerve response in some animals was tested with Urea (300 mM), l-menthol (10 mM), monopotassium glutamate (MPG; 1000 mM), denatonium benzoate (denatonium; 10 mM), and caffeine (100 mM) to examine their response characteristics in order to better understand the function of the nerve. Because P2X-dblKO mice are severely deficient in their responses to all taste stimuli, normalization to a chemical stimulus was impossible. Therefore, the response magnitudes for CT nerve in both WT and P2X-dblKO were normalized to electrical stimulation (ES); those for the GL nerve were normalized to the response to cold (∼4°C) 10 mM l-menthol; and those for the SL nerve were normalized to the response to DW. The procedures of ES were described previously (Yasumatsu et al. 2003, 2007).
Table 1.
List of solutions used in experiments
| Solutions | Concentrations (mM) | ||
| CT recordings | GL recordings | SL recordings | |
| Acetic acid | 1–50 | 1–50 | 50 |
| Citric acid | 1–50 | 1–50 | 50 (1–50 sa) |
| HCl | 1–10 | 1–10 | 10 |
| NaCl | 10–1000 | 10–1000 | 1000 |
| KCl | 10–1000 | 10–1000 | 1000 |
| NH4Cl | 10–1000 | 10–1000 | 1000 |
| MSG | 10–1000 | 10–1000 | 1000 |
| Sucrose | 10–1000 | 10–1000 | 1000 |
| QHCl | 0.1–20 | 0.1–20 | 10 |
| Urea | 300 | ||
| l-Menthol | 10 | ||
| MPG | 1000 | ||
| Denatonium | 10 | ||
| Caffeine | 100 | ||
All solutions were dissolved in water except for the citric acid concentration series used for SL nerve data which were dissolved in 150 mM saline.
Briefly, an Ag/AgCl electrode was placed on the inside wall of the flow chamber or was placed directly on the tongue when necessary. An Ag/AgCl indifferent electrode was positioned in nearby tissue. Anodal current was passed through the tongue from a ramp current generator (Denis-Sekkei). To ensure reliable electrical conductance, the bathing medium used during the current stimulation was 1 mM NaCl (Ninomiya and Funakoshi 1981). Previous studies demonstrated that anodal current with an intensity of 20 μA (rate of raise, 100 μA/s and duration, ∼20 s) provoked robust responses in the CT at 2 weeks after nerve crush, despite no significant neural responses to taste stimuli (Yasumatsu et al. 2003, 2007). Thus, this ES paradigm directly activates nerve fibers without the necessity for taste bud function. Therefore, we used the response to anodal current (20 μA, rate of raise, 100 μA/s and duration, ∼20 s) as the standard to calculate relative magnitudes of CT nerve response to each chemical stimulus (Finger et al. 2005).
The GL nerve of P2X-dblKO mice exhibits little or no response to taste stimuli but still retains robust responses to somatosensory stimuli (e.g., touch, low temperature and menthol solutions; Finger et al. 2005). Thus, we used the response to cold 10 mM l-menthol as the standard to calculate relative magnitudes of response to each chemical stimulus.
For the response measurements of the SL nerve, DW is used frequently as a standard stimulus because SL nerve fibers are highly responsive to DW (Shingai 1980; Shingai and Beidler 1985; Smith and Hanamori 1991). Furthermore, in our preliminary experiments, we found that the responses to DW did not differ significantly between the WT and P2X-dblKO mice. Thus, we used the response to DW as the standard to calculate relative magnitudes of response to each chemical stimulus.
Data analysis
For the analysis of whole-nerve responses to each stimulus, the magnitude of the integrated response at 5, 10, 15, 20, and 25 s (CT nerve), at 15, 20, 25, 30, 35, 40, and 45 s (GL nerve), or at 5, 10, 15, 20, and 25 s (SL nerve) after stimulus onset were measured and averaged. In case of ES, we measured 6, 9, 12, 15, and 18 s after stimulus onset to select at least 5 points because the stimulus was recorded for 20 s. With the use of these average values, relative response magnitude (averaged) for each test stimulus was calculated, with the response magnitude to ES (CT nerve), cold 10 mM l-menthol (GL nerve) or DW (SL nerve) defined as a unity (1.0). These relative response values were used for all statistical analysis.
Data are presented as group means ± the standard error and were analyzed using appropriate 2-way repeated measures analyses of variance (ANOVAs; Statistica; StatSoft). The concentrations of tastants tested were not entirely conserved between the CT and GL nerve recordings and different stimuli were used to normalize responses in these 2 nerves. Thus, statistical analysis of data from each of these nerves was made with separate 2-way repeated measure ANOVAs with strain (WT vs. P2X-dblKO) as the between-group factor and taste solution concentration as the within-group factor for responses to each stimulus (acetic acid, NaCl, etc.). Because not all stimulus concentrations were used in all preparations, statistical analyses (ANOVA and difference tests) utilized only a subset of the data, where complete or nearly complete series were available. However, means and standard errors were calculated for the full data set as shown in all figures. Because the SL nerve was largely unresponsive to lower concentrations of most taste stimuli (data not shown), data analysis of SL neural responses to only the highest concentration of each taste solution were made using a 2-way repeated measure ANOVA with taste solution as a within-group factor and strain as the between-group factor. Moreover, the SL nerve responds robustly to water, which served as a response standard to which all SL neural responses were normalized. Therefore, we also compared responses of the SL nerve with a range of concentrations of citric acid mixed in 150 mM NaCl using a 2-way repeated measures ANOVA with strain as a between subjects factor and concentration as a within-subjects factor. Tukey’s honest significant difference tests were used to assess statistically significant (P < 0.05) main effects or interactions. In addition to using post hoc analyses to compare differences between WT and P2X-dblKO mice, we also utilized these statistical analyses to calculate the threshold for CT and GL neural response to each taste, defined as the concentration of tastant whose response was significantly greater than the response to the lowest concentration for each taste in each nerve.
Results
In both WT and P2X-dblKO animals, there was dense innervation of the laryngeal epithelium, especially including the oral face of the arytinoids (as reported previously for various species including rodents, Uno et al. 2004, and cats Yoshida et al. 2000). Fine caliber nerve terminals enter the epithelium to terminate, often as slight terminal varicosities at or within a few micra of the epithelial surface (white arrowheads, Figure 1).
Figure 1.
Fluorescence micrographs of longitudinal sections through the trachea of (A) WT and (B) P2X-dblKO mice showing similar dense epithelial innervation (arrowheads) of the oral face of the arytinoids as revealed by PGP-immunoreactivity (red). In panel A, a taste bud (tb) is identifiable by the presence of a taste cell labeled by TrpM5-driven GFP. Panel A: Nomarski image. B: Counterstained with Neurotrace Green which is pseudocolored blue.
Taste nerve responses
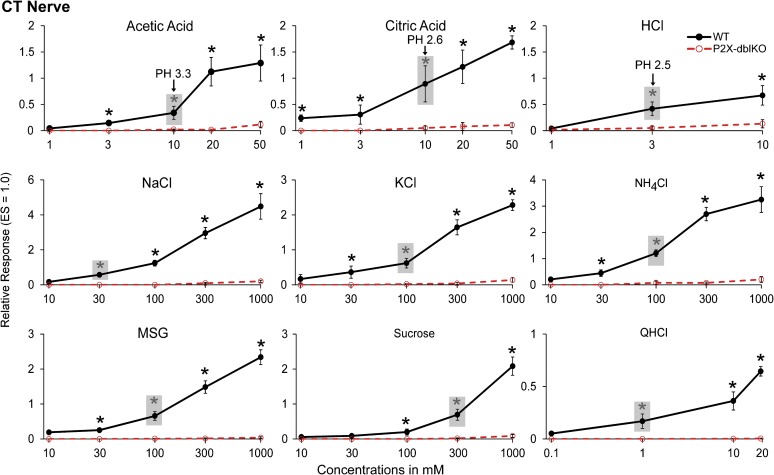
Recordings from the CT and GL nerves showed minimal responses to classical taste stimuli in the nerves of P2X-dblKO mice in contrast to the typical robust responses from the nerves of WT mice (Figures 2–4) as reported previously (Finger et al. 2005). The current study used a more extensive taste battery than the previous study but the findings are similar. None of the acids tested - acetic, citric, or HCl - evoked a significant taste nerve response in the CT nerve at low or moderate concentrations (≤10 mM for the weak acids and ≤3 mM for HCl) but did evoke noticeable activity at high concentrations although still significantly less than in WT animals (Figure 3; for acetic, citric, and HCl acids, respectively, effect of strain, F 1, 10 –20 = 2.1, 134.8, 9.4, all P’s < 0.05; effect of concentration, F 2 –4, 20 –40 = 24.9, 72.4, 16.5, all P’s < 0.05; strain × concentration interaction, F 2 –4, 20 –40 = 19.2, 56.7, 8.5, all P’s < 0.05 in all tastants). The acids did, however, evoke a low to moderate response in the GL nerve at mid concentrations, although still significantly less than in WT animals (Figure 4; for acetic, citric, and HCl acids, respectively, effect of strain, F 1, 6 –10 = 6.8, 354.1, 7.1, all P’s < 0.05; effect of concentration, F 2 –4, 20 –28 = 26.8, 31.9, 46.6, all P’s < 0.05; strain × concentration interaction, F 2 –4, 20 –28 = 5.6, 11.5, 18.2, all P’s < 0.05 in all tastants). For nonacidic tastants, responses in the CT and GL nerves of P2X-dblKO animals were absent or significantly reduced even at the highest concentrations tested. The difference in responsiveness of the CT and GL nerves to acids may be attributable to the mixed nature of the GL nerve (taste plus general epithelial innervation), whereas the CT is a pure taste nerve.
Figure 2.
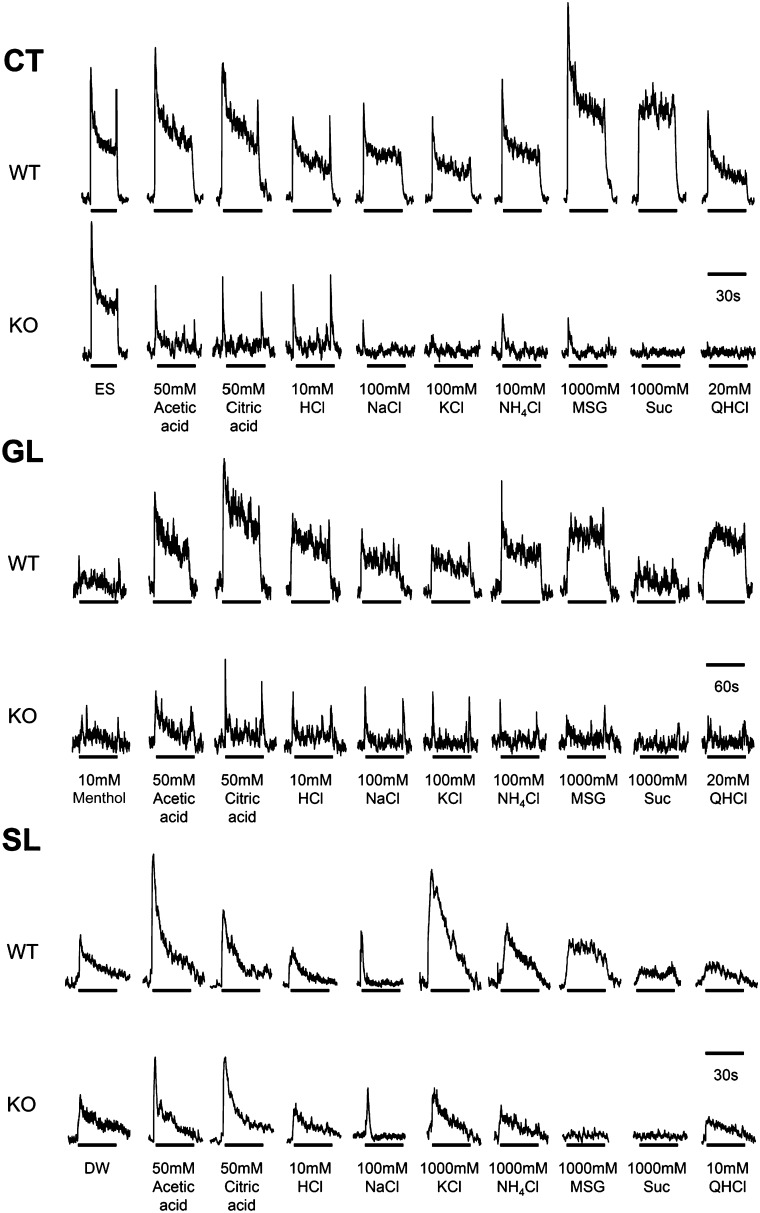
Representative traces of nerve recordings from the CT, GL, and SL nerves. Note that responses to most conventional tastants (MSG, sucrose, NaCl, and citric acid) are greatly reduced or absent in the CT and GL nerves of P2X-dblKO mice compared with WT animals. In the SL nerve, responses to acids, KCl and QHCl, remain robust, although responses to the classical tastants MSG and sucrose are greatly reduced compared with WT.
Figure 3.
Relative responses of the CT nerve in WT. WT = solid lines and filled circles and P2X-dblKO = dotted lines and open circles. The CT is a pure taste nerve and shows virtually no responses to classical tastants except at the highest concentrations. Responses normalized to ES. The gray boxes highlight the lowest concentration of each stimulus that evokes a statistically significant response in WT mice; the corresponding pH values of the different acids are given for reference. Values indicated are expressed as mean ± SE. Number of subjects are 6–16 for WT mice and 7 for KO mice. *P < 0.05; Tukey’s post hoc.
Figure 4.
Relative responses of the GL nerve in WT (solid lines and filled circles) and P2X-dblKO (dotted lines and open circles) mice. The GL is a mixed nerve containing both taste and general epithelial fibers, including polymodal nociceptors. Like the CT, the GL shows greatly reduced responsiveness to nonirritant tastants, including MSG, sucrose, and QHCl. Ionic solutions, NaCl, KCl, and NH4Cl, and acids show some residual responses but are still significantly reduced compared with WT. Responses normalized to cold 10 mM l-menthol. The shaded boxes highlight the lowest concentration of each stimulus that evokes a statistically significant response in each strain; the corresponding pH values of the different acids are given for reference. Values indicated are expressed as mean ± SE. Number of subjects are 14–23 for WT mice and 5–9 for KO mice. *P < 0.05; Tukey’s post hoc.
SL nerve responses
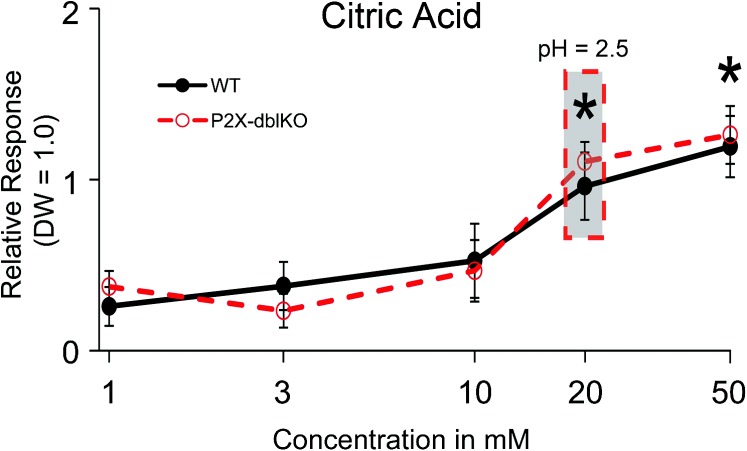
As reported by others (Hanamori and Smith 1986; Dickman and Smith 1988), the SL nerve of WT mice responded to concentrated solutions of most taste-related substances (Figures 2 and 5). Responses were particularly robust to acetic acid, citric acid, KCl, NH4Cl, and monosodium glutamate (MSG). In contrast to WT mice, P2X-dblKO mice showed diminished responses to KCl, NH4Cl, MSG, and sucrose (post hoc analysis of the significant strain × taste solution interaction, F (13, 148) = 2.3, P < 0.05) but relatively normal responses to acetic acid, citric acid, HCl, NaCl, and quinine hydrochloride (QHCl; all P’s = 0.7–0.9). The normally robust responses to MPG, urea, and denatonium were also significantly reduced or absent in the P2X-dblKO mice (all P’s < 0.05), but responses to l-menthol and caffeine were not different than WT controls (P’s = 0.7 and 0.9, respectively; Figure 5). Because the SL nerve is strongly responsive to water, which served as a response standard, we also compared responses of the SL nerve with a range of concentrations of citric acid mixed in 150 mM saline (Figure 6). The SL nerve showed a concentration-dependent response to citric acid (effect of concentration, F (4, 36) = 14.9, P < 0.05) that was not different between WT and P2X-dblKO mice (effect of strain, F, (1, 9) = 0.04, P = 0.8). In summary, in the P2X-dblKO mice, the SL nerve responses to acids were substantially intact, whereas responses to typical bitter, umami, or sweet tastants were eliminated or significantly reduced. The threshold for this residual responsiveness to acidic stimuli (20 mM) is near the avoidance threshold for both the WT and the P2X-dblKO mice. Thus, laryngeal sensitivity to acids may underlie much of the avoidance of acidic stimuli in brief access tests by the P2X-dblKO mice.
Figure 5.
Comparison of SL nerve responses with a variety of test solutions in WT (solid bar) and P2X-dblKO (hatched bar) mice. P2X-dblKO mice show markedly reduced responses to many tastants including MSG, sucrose, urea, and denatonium, suggesting a failure of taste buds to transmit this information to the taste fibers in the SL nerve. The SL nerve of P2X-dblKO mice does, however, show substantial responses to acids as well as other activators of general epithelial innervation, that is, menthol, KCl, and caffeine, suggesting that this component of the response may be attributable to nontaste fibers of the SL nerve. Responses are normalized to DW. Values indicated are expressed as mean ± SE. Number of subjects are 4–11 for WT mice and 4–13 for KO mice. *P < 0.05; Tukey’s post hoc.
Figure 6.
Relative responses of the SL nerve in WT (solid lines and filled circles) and P2X-dblKO (dotted lines and open circles) mice to different concentrations of citric acid dissolved in 150 mM saline. Responses are normalized to DW which evokes a substantial response in the SL nerve. The gray box highlights the lowest concentration that evokes a statistically significant response in both WT and P2X-dblKO mice; the corresponding pH values are given for reference. Values indicated are expressed as mean ± SE. Number of subjects are 6–16 for WT mice and 7 for KO mice. *P < 0.05; Tukey’s post hoc.
Discussion
The sense of taste guards the entrance to the alimentary canal and guides animals to reject foods that are perceived as too bitter or too sour. Detection of these taste qualities is usually attributed to taste buds which display specific receptor mechanisms for bitter, via the G protein-coupled receptor-coupled family of taste receptor type 2 (T2R) receptors, and for sour, probably involving an apical proton conductance (Chang et al. 2010).
Despite the diversity of receptors and transduction cascades, transmission of taste information from the taste cells to the taste nerves requires functional P2X purinergic receptors (Finger et al. 2005). In the CT nerve, a pure taste nerve, mice lacking P2X2 and P2X3 receptors show essentially no responses to all classes of tastants including acids (sour; current results and Finger et al. 2005). This study also extends this severe deficiency in taste responsiveness in P2X-dblKO mice to the SL nerve, suggesting that transmission of taste information from taste buds is severely compromised in all taste fields in these knockouts.
In keeping with the essential role for purinergic signaling in transmission of taste information to the nerve fibers, the SL nerve of P2X-dblKO mice is nonresponsive to the taste qualities of umami (MSG), sweet (sucrose), and bitter (denatonium and urea) although it shows strong responses to acids. In addition, the SL nerve of the P2X-dblKO mice continues to exhibit significant responses to a variety of substances, including KCl, QHCl, menthol, MPG, and caffeine. These residual responses suggest that nontaste bud–mediated mechanisms may underlie to responsiveness to these substances. For example, in the SL nerve of P2X-dblKO animals, MSG is relatively ineffective at evoking neural responses, whereas MPG is effective. Therefore, the potassium ion must be the key to the response of the SL—an inference supported by KCl being a relatively potent stimulus for that nerve. In fact, activation by MPG may be attributable to the K+ ion acting directly to activate free nerve endings in the epithelium rather than acting via a taste bud–mediated transduction.
For nearly all taste modalities, the lack of neural response in the P2X-dblKO mice is mirrored by a lack of behavioral responsiveness to potential tastants in short-term intake tests (Finger et al. 2005; Eddy et al. 2009; Hallock et al. 2009). The residual behavioral avoidance of acidic solutions seemed enigmatic given the nearly total absence of taste responses to these substances in the CT nerve. The current study suggests that this behavioral responsiveness may be attributable to persistent acid sensitivity of SL and GL nerves and likely acid sensitivity of the trigeminal nerve in the P2X-dblKO animals.
Neural responses to acids
Sensory nerve fibers themselves can be responsive to acids applied to an epithelial surface. Most peripheral nerves, including the trigeminal nerve, possess polymodal nociceptors, which respond to acidification as well as to tissue damage and extreme temperatures (Bryant and Silver 2000; Liu and Simon 2000). The GL and SL nerves, unlike the CT nerve, contain polymodal nociceptors that innervate the lingual and oropharyngeal epithelium (Terenghi et al. 1986; Miyazaki et al. 1999; Yoshida et al. 2000; Hayakawa et al. 2010). The peptidergic nociceptive fibers, which contain substance P and CGRP, are sensitive to capsaicin (Nagy et al. 1981) and express the TrpV1 capsaicin receptor (Ishida et al. 2002). Furthermore, a significant proportion of the GL and SL response to acids in WT mice is attributable to the acid sensitivity of the TrpV1 receptor (Arai et al. 2010). Similarly, the residual response of the GL nerve to acids in P2X-dblKO mice is likely to be due to activation of the Trp receptors on the polymodal nociceptors of the nerves. The fact that a significant neural response to citric and acetic acids occurs in the GL nerve of P2X-dblKO animals only at higher concentrations (100 mM citric acid) and not at taste thresholds (Scalera, 2004; 3–10 mM for CT and GL response to citric acid; this study and Stratford and Contreras 2009) suggests the residual response is largely due to acid-gated channels on the nerve fibers themselves rather than through taste cell–mediated transduction. In the circumvallate papillae, CGRP- (and by association, TrpV1-) expressing nerve fibers heavily innervate not only the perigemmal epithelium, as in anterior tongue, but also extend numerous branches within the taste bud proper (Finger 1986; Huang et al. 2003). Application of a weak acid, for example, citric acid, to the surface of the epithelium acidifies the full depth of the tissue nearly down to the basement membrane (Richter et al. 2003). Accordingly, intragemmal fibers with acid-gated ion channels are likely to respond directly to the acid without the need for taste cell transduction. We suggest that the residual GL response to weak acids in the P2X-dblKO mice is attributable to such acid-sensitive nerve fibers.
Even so, it seems unlikely that the near-normal behavioral avoidance of citric acid by P2X-dblKO mice (to 30 mM in 2-bottle tests; Finger et al. 2005) can be attributed to the small (<20%) residual GL response to high concentrations (>30 mM) of this substance. Rather, we suggest that the acid-responsiveness of the lingual trigeminal or SL nerves may largely underlie acid avoidance by P2X-dblKO animals. In support of this, the SL nerve response to citric acid is virtually identical to that of the WT over the critical concentration range (10–100 mM). Thus, the normal behavioral avoidance of citric acid by P2X-dblKO animals matches the near-normal response in the SL nerve.
The SL nerve provides dense peptidergic epithelial innervation to the oral face of the larynx and outer faces of the arytinoids (Figure 1 and Yoshida et al. 2000; Koike et al. 2004). Most peripheral pain fibers, including those of the vagus and GL nerves (Fukuda et al. 2006), possess acid-sensing ion channels (ASICs) as well as pH-sensitive Trp channels (Leffler et al. 2006). The ASIC channels tend to open at a pH slightly lower than neutral, that is, in the range of pH 5.0–7.0, although ASIC 2A gates at a much lower pH (Wu et al. 2004; Blanchard and Kellenberger 2011). Because the GL and SL nerves only respond at much lower pH, for example, pH 2.5–3.5, we suggest that most of the neural response to acid is attributable to non-ASIC mechanisms, most likely pH-sensitive Trp channels. Many free nerve endings express the capsaicin receptor, TrpV1 (Tominaga et al. 1998; Okano et al. 2006), which is gated by low pH as well as temperature and capsaicin (Tominaga et al. 1998). Indeed, Arai et al. (2010) showed that the bulk of the response of the SL to acetic acid is blocked by the TrpV1 antagonist, iodo-resiniferatoxin. Similarly, more than half of the response of the GL nerve to acetic acid is blocked by this TrpV1 antagonist. In somatic pain nerves, TrpV1 is responsible for the bulk of pH sensitivity down to a pH of ∼5 (Leffler et al. 2006). However, the acid solutions we utilized have a pH range substantially lower than that (<3.3). Therefore, the presence of TrpV1 receptors on free nerve endings of these nerves does not fully explain the responsiveness to citric acid in the P2X-dblKO mice. Moreover, that the TrpV1 antagonist does not substantially reduce the response of the SL to citric acid or HCl, implies that these acids are likely acting via another mechanism. TrpA1 is another good candidate for neural responses to acids, in that, it is responsive to intracellular acidification by weak acids, such as acetic acid, but is nonresponsive to extracellular acidification as would occur from strong acids (Wang et al. 2011). Because TrpA1 is coexpressed in a subset of TrpV1-expressing ganglion cells, both channels may underlie the acid sensitivity of free nerve endings in the oropharyngeal mucosa.
In summary, our results show substantial responsiveness to weak acids in the SL nerve of P2X-dblKO mice. In the taste-specific CT nerve, these same acidic stimuli are ineffective at producing a response suggesting that the residual SL response may be attributable to the many free nerve endings in the laryngeal epithelium. Similarly, the GL nerve of P2X-dblKO mice shows significant albeit reduced responses to weak acids, that is also likely due to the presence of general mucosal nontaste fibers in that nerve. This residual sensitivity to acid stimuli in both GL and SL nerves likely accounts for the continued avoidance of acidic solutions by the P2X-dblKO mice even in the absence of a functional taste system.
Funding
This work was supported by grants from the National Institutes of Health, National Institute for Deafness and Communicative Disorders [R01 DC007495 to T.E.F., P30 DC04657 to T.E.F. and Diego Restrepo]; Japan Society for the Promotion of Science KAKENHI [18109013, 18077004, 23249081 to Y.N.].
Acknowledgments
The authors are grateful to Debra Cockayne and Roche Biosciences, Palo Alto, CA, for permitting us to use the P2X2/P2X3 double-KO mice and the matched WT line. We also thank Bob Margolskee of Monell Chemical Senses Center for allowing us to utilize the TrpM5-GFP line of mice to visualize taste buds and chemosensory cells.
References
- Arai T, Ohkuri T, Yasumatsu K, Kaga T, Ninomiya Y. The role of transient receptor potential vanilloid-1 on neural responses to acids by the chorda tympani, glossopharyngeal and superior laryngeal nerves in mice. Neuroscience. 2010;165:1476–1489. doi: 10.1016/j.neuroscience.2009.11.051. [DOI] [PubMed] [Google Scholar]
- Blanchard MG, Kellenberger S. Effect of a temperature increase in the non-noxious range on proton-evoked ASIC and TRPV1 activity. Pflugers Arch. 2011;461:123–139. doi: 10.1007/s00424-010-0884-3. [DOI] [PubMed] [Google Scholar]
- Bryant BP, Silver WL. Chemesthesis: the common chemical sense. In: Finger TE, Silver WL, Restrepo D, editors. The neurobiology of taste and smell. 2nd ed. New York: Wiley-Liss; 2000. pp. 73–100. [Google Scholar]
- Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci U S A. 2010;107:22320–22325. doi: 10.1073/pnas.1013664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman JD, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res. 1988;450:25–38. doi: 10.1016/0006-8993(88)91541-7. [DOI] [PubMed] [Google Scholar]
- Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses. 2009;34:789–797. doi: 10.1093/chemse/bjp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger T. Peptide immunocytochemistry demonstrates multiple classes of perigemmal nerve fibers in the circumvallate papilla of the rat. Chem Senses. 1986;11:135–144. [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ichikawa H, Terayama R, Yamaai T, Kuboki T, Sugimoto T. ASIC3-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia. Brain Res. 2006;1081:150–155. doi: 10.1016/j.brainres.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Gulbransen B, Silver W, Finger TE. Solitary chemoreceptor cell survival is independent of intact trigeminal innervation. J Comp Neurol. 2008;508:62–71. doi: 10.1002/cne.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock RM, Tatangelo M, Barrows J, Finger TE. Residual chemosensory capabilities in double P2X2/P2X3 purinergic receptor null mice: intraoral or postingestive detection? Chem Senses. 2009;34:799–808. doi: 10.1093/chemse/bjp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamori T, Smith DV. Central projections of the hamster superior laryngeal nerve. Brain Res Bull. 1986;16:271–279. doi: 10.1016/0361-9230(86)90042-0. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M. Calcitonin gene-related peptide immunoreactive neurons innervating the soft palate, the root of tongue, and the pharynx in the superior glossopharyngeal ganglion of the rat. J Chem Neuroanat. 2010;39:221–227. doi: 10.1016/j.jchemneu.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Wu YH, Lu KS. Immunoelectron microscopic studies on protein gene product 9.5 and calcitonin gene-related peptide in vallate taste cells and related nerves in the guinea pig. Microsc Res Tech. 2003;62:383–395. doi: 10.1002/jemt.10396. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. The co-expression of ASIC3 with calcitonin gene-related peptide and parvalbumin in the rat trigeminal ganglion. Brain Res. 2002;943:287–291. doi: 10.1016/s0006-8993(02)02831-7. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Ugawa S, Ueda T, Murakami S, Shimada S. Vanilloid receptor subtype-1. VR1. is specifically localized to taste papillae. Brain Res Mol Brain Res. 2002;107:17–22. doi: 10.1016/s0169-328x(02)00441-2. [DOI] [PubMed] [Google Scholar]
- Kajii Y, Shingai T, Kitagawa J, Takahashi Y, Taguchi Y, Noda T, Yamada Y. Sour taste stimulation facilitates reflex swallowing from the pharynx and larynx in the rat. Physiol Behav. 2002;77:321–325. doi: 10.1016/s0031-9384(02)00854-5. [DOI] [PubMed] [Google Scholar]
- Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa J, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1342–R1347. doi: 10.1152/ajpregu.00556.2001. [DOI] [PubMed] [Google Scholar]
- Koike S, Uno T, Bamba H, Shibata T, Okano H, Hisa Y. Distribution of vanilloid receptors in the rat laryngeal innervation. Acta Otolaryngol. 2004;124:515–519. doi: 10.1080/00016480310000674. [DOI] [PubMed] [Google Scholar]
- Leffler A, Monter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels. ASICS. in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006;139:699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99:1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin, acid and heat-evoked currents in rat trigeminal ganglion neurons: relationship to functional VR1 receptors. Physiol Behav. 2000;69:363–378. doi: 10.1016/s0031-9384(00)00209-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Shin T, Murata Y, Masuko S. Pharyngeal branch of the vagus nerve carries intraepithelial afferent fibers in the cat pharynx: an elucidation of the origin and central and peripheral distribution of these components. Otolaryngol Head Neck Surg. 1999;120:905–913. doi: 10.1016/S0194-5998(99)70335-9. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Hunt SP, Iversen LL, Emson PC. Biochemical and anatomical observations on the degeneration of peptide-containing primary afferent neurons after neonatal capsaicin. Neuroscience. 1981;6:1923–1934. doi: 10.1016/0306-4522(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Role of ions in generation of taste nerve responses to electrical tongue stimulation in rats. Jpn J Physiol. 1981;31:891–902. doi: 10.2170/jjphysiol.31.891. [DOI] [PubMed] [Google Scholar]
- Okano H, Koike S, Bamba H, Toyoda K, Uno T, Hisa Y. Participation of TRPV1 and TRPV2 in the rat laryngeal sensory innervation. Neurosci Lett. 2006;400:35–38. doi: 10.1016/j.neulet.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel. ASIC. localizes to small primary afferent neurons in rats. Neuroreport. 1998;9:1109–1113. doi: 10.1097/00001756-199804200-00028. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Peyrot des Gachons C, Uchida K, Bryant B, Shima A, Sperry JB, Dankulich-Nagrudny L, Tominaga M, Smith AB, 3rd, Beauchamp GK, Breslin PA. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J Neurosci. 2011;31:999–1009. doi: 10.1523/JNEUROSCI.1374-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol. 2003;547(Pt 2):475–483. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalera G. Acid taste thresholds assessed by conditioned taste aversion and two-bottle preference in rats. Physiol Behav. 2004;82:411–423. doi: 10.1016/j.physbeh.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Shingai T. Water fibers in the superior laryngeal nerve of the rat. Jpn J Physiol. 1980;30:305–307. doi: 10.2170/jjphysiol.30.305. [DOI] [PubMed] [Google Scholar]
- Shingai T, Beidler LM. Response characteristics of three taste nerves in mice. Brain Res. 1985;335:245–249. doi: 10.1016/0006-8993(85)90476-7. [DOI] [PubMed] [Google Scholar]
- Silver WL, Farley LG, Finger TE. The effects of neonatal capsaicin administration on trigeminal nerve chemoreceptors in the rat nasal cavity. Brain Res. 1991;561:212–216. doi: 10.1016/0006-8993(91)91597-t. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hanamori T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J neurophysiol. 1991;65:1098–1114. doi: 10.1152/jn.1991.65.5.1098. [DOI] [PubMed] [Google Scholar]
- Stratford JM, Contreras RJ. Saliva and other taste stimuli are important for gustatory processing of linoleic acid. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1162–R1170. doi: 10.1152/ajpregu.00217.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford JM, Finger TE. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci. 2011;31:9101–9110. doi: 10.1523/JNEUROSCI.0404-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenghi G, Polak JM, Rodrigo J, Mulderry PK, Bloom SR. Calcitonin gene-related peptide-immunoreactive nerves in the tongue, epiglottis and pharynx of the rat: occurrence, distribution and origin. Brain Res. 1986;365:1–14. doi: 10.1016/0006-8993(86)90716-x. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in Solitary Chemosensory Cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Uno T, Koike S, Bamba H, Hirota R, Hisa Y. Capsaicin receptor expression in rat laryngeal innervation. Ann Otol Rhinol Laryngol. 2004;113:356–358. doi: 10.1177/000348940411300503. [DOI] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol. 2011;137:493–505. doi: 10.1085/jgp.201110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–43724. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Katsukawa H, Sasamoto K, Ninomiya Y. Recovery of amiloride-sensitive neural coding during regeneration of the gustatory nerve: behavioral-neural correlation of salt taste discrimination. J Neurosci. 2003;23:4362–4368. doi: 10.1523/JNEUROSCI.23-10-04362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu K, Kusuhara Y, Shigemura N, Ninomiya Y. Recovery of two independent sweet taste systems during regeneration of the mouse chorda tympani nerve after nerve crush. Eur J Neurosci. 2007;26:1521–1529. doi: 10.1111/j.1460-9568.2007.05761.x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Tanaka Y, Hirano M, Nakashima T. Sensory innervation of the pharynx and larynx. Am J Med. 2000;108(Suppl 4a):51S–61S. doi: 10.1016/s0002-9343(99)00342-3. [DOI] [PubMed] [Google Scholar]