Abstract
Medullary thyroid cancer (MTC) represents an aggressive form of thyroid malignancy. Some may occur spontaneously or can be associated with Multiple Endocrine Neoplasia syndromes, or Familial Medullary Thyroid Cancer syndrome. In these patients, the protooncogene RET (rearranged during transfection) is mutated. In patients who have unresectable or metastatic disease, the long term prognosis is poor. New treatments for this disease have focused on the use of targeted agents that inhibit the receptor tyrosine kinase of RET. One of these treatments, Vandetanib (Caprelsa, Astra Zeneca), recently has received approval from the Food and Drug Administration for the treatment of patients with progressive locally advanced and/or metastatic disease. This review highlights the studies that led to the drug’s approval, and discusses on the potential financial costs of treatment and side effects of this therapy. The main clinical studies evaluating Vandetanib for the treatment of other solid tumors will also be reviewed.
Keywords: Vandetanib, medullary thyroid cancer, RET
Introduction
Medullary thyroid cancer (MTC) account for 5% to 8% of thyroid carcinomas and arise from the calcitonin producing parafollicular cells (C cells).1 MTC is often asymptomatic when localized and as a result, 50% of patients have unresectable disease at the time of diagnosis. The hypercalcitonism typically seen with a larger burden of metastatic or locally advanced disease can result in systemic symptoms such as diarrhea, bone pain, or flushing. The prognosis for these patients remains poor, with only 40% of the patients alive after 10 years. Conversely, when the tumor is confined to the thyroid gland, the 10 year survival rate is approximately 95%.2–4 Conventional cytotoxic chemotherapy regimens as well as radiation therapy have been used in the treatment of unresectable MTC with limited success; unfortunately, they do not prolong survival.5–7 The gap in prognosis between localized and metastatic disease emphasizes the importance of early detection and the necessity of finding new therapeutic agents for advanced MTC.
Over the past decades, significant progress has been made in the understanding of MTC’s pathogenesis. Indeed, it has been shown that the proto-oncogene RET (Rearranged During Transfection), located on chromosome 10, is responsible for the development of both familial medullary thyroid cancer (FMTC) and sporadic MTC. RET mutations are observed in both sporadic MTC and FMTC. MTC can also be associated with other endocrine tumors, such as pheocromocytomas and primary hyperparathyroidism. These disorders, called the Multiple Endocrine Neoplasia Syndromes (MEN 2A and MEN 2B), explain the remaining 65% of hereditary MTC, with respectively, 55% and 10% of the cases.8
The understanding of MTC’s biology uncovered a possible role for targeted therapies in this malignancy. The recently approved small molecule Vandetanib, which targets the RET, Epidermal growth factor (EGF) and Vascular endothelial growth factor (VEGF) receptors, is a new therapeutic option for advanced MTC where classic chemotherapy regimens and radiation therapy are ineffective. This review will discuss recurrent molecular alterations observed in MTC and the means to target them. We will discuss the clinical studies that led to Vandetanib approval for MTC. Vandetanib’s effectiveness, side effects, and cost will be analyzed. The main clinical studies evaluating Vandetanib for the treatment other solid tumors will also be reviewed.
The RET, VEGF-R and EGF-R Pathways in MTC
The RET (rearranged during transfection) proto-oncogene
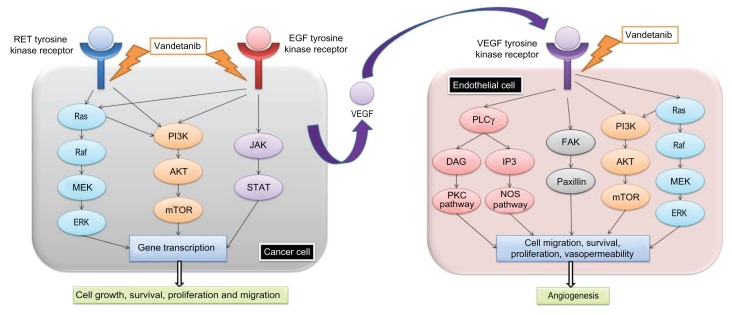
The RET proto-oncogene codes for a tyrosine-kinase receptor (TKR). These glycoproteins, including RET, receive extra-cellular signals and form homo- or hetero-dimers in response to binding of extracellular ligands. Their activation can lead to various cellular processes, such as differentiation, proliferation, apoptosis, or cell motility, depending on the ligand and receptor involved.9 The RET proto-oncogene encodes a receptor tyrosine kinase that is activated by the glial cell line-derived neurotrophic factor (GDNF) family. GDNF forms a complex with glycosylphosphatidylinositol (GPI)-anchored co-receptor, which links two RET proteins that initiate autophosphorylation of specific tyrosine residues within the tyrosine kinase domain of each RET molecule.10 Different pathways, including RAS-MAPK and PI3K-AKT, are activated downstream from the RET receptor and contribute to cell survival and proliferation (Fig. 1). RET proto-oncogene was first described in papillary thyroid cancer (PTC) where translocations involving the RET gene give rise to an aberrant fusion oncoprotein (RET/PTC), leading to constitutive activation of the RET receptor.11 Different point mutations increasing RET receptor TKR activity subsequently have been described. These gain of function mutations are observed in MTC, FMTC, MEN 2A, MEN 2B and a subset of sporadic pheocromocytomas.12,13 Conversely, RET loss of function mutations are associated with Hirschprung’s disease.14
Figure 1.
A schematic drawing of the interaction of RET, EGFR and VEGFR with growth factor pathways.
The VEGF-R pathway
Tumors require constant nutrient and oxygen supply. Consequently, they develop a neo-vasculature through a process called angiogenesis, particularly when they are growing rapidly. In contrast with physiologic situations such as wound repair, this process is deregulated in human malignancies in the form of constant activation. The most important angiogenesis stimulating pathway is VEGF signaling, where VEGF-A binds the VEGF tyrosine kinase receptors 1, 2 or 3 (VEGFR-1-3) leading to proliferation, survival, and migration of endothelial cells.15 A recent study showed that VEGF-A, VEGFR-1 and VEGFR-2 are overexpressed in more than 90% of MTC. No correlation between VEGF-A expression and extent of disease was found. The only prognostic factor related to vasculature identified by the investigators was microvessel density (MVD). Indeed, MVD was associated with poor prognosis, and all the patients with high MVD died.16 Another study showed that in MTC, the expression of VEGFR-2 is significantly higher in metastases compared to primary tumors.17
The EGFR (Epidermal Growth Factor Receptor) pathway
EGFR is a tyrosine kinase cell surface receptor activated by epidermal growth factor (EGF) and transforming growth factor alpha (TGFα). Upon stimulation by these ligands, the receptor forms a homodimer or a heterodimer with another member of the EGFR family, allowing auto-phosphorylation of several tyrosine residues of the receptor which in turns leads to activation of the downstream MAPK, AKT and JAK pathways involved in cell proliferation, migration, and adhesion (Fig. 1).18 Mutations and overexpression of EGFR are observed in numerous malignancies, including lung cancer,19 glioblastoma multiforme,20 anaplastic thyroid cancer,21 papillary thyroid cancer22 and MTC. Indeed, a recent study showed that 13% of MTC overexpress EGFR. Strikingly, 35% of the metastases tested displayed overexpression of EGFR, suggesting that this signaling pathway might play an important role in advanced disease.17
The small molecule Vandetanib targets the RET, EGF and VEGF receptors
RET mutations are believed to be the primary oncogenic event in a majority of MTC. Sustained angiogenesis is presumed to contribute to the pathogenesis of MTC. In addition, EGFR is specifically overexpressed in advanced MTC. Therefore, it seems reasonable to postulate that blocking these pathways might reverse cell growth and proliferation. The small molecule Vandetanib was shown to block MTC cell lines proliferation in vitro by blocking RET activity.23 Interestingly, upon RET inhibition, the proliferative capacity of MTC cell lines can be rescued, in part, by EGFR stimulation. This emphasizes the necessity to block these pathways simultaneously.24 Vandetanib competes with ATP binding in the catalytic domain of several tyrosine kinases. In vitro assays showed that Vandetanib is a potent inhibitor of VEGFR-2, VEGFR-3, EGFR, and RET kinases. This activity profile made it an optimal choice for evaluation in clinical trials.25
Pharmacokinetics and pharmacodynamics of Vandetanib
The two first phase I studies showed that absorption and elimination of Vandetanib after a single oral dose of 300 mg was slow: the time to maximum concentration ranged from 4 to 7.5 hours and the terminal half-life was approximately 90–120 hours. Steady-state plasma concentrations were obtained after 1 month of daily dosing.26,27 Other phase I studies were subsequently conducted with healthy volunteers to further characterize pharmacokinetic parameters such as metabolism and excretion. One study found a longer terminal half-life of approximately 10 days, compared with the 4 or 5 days of the previous studies. The absorption of Vandetanib was only slightly reduced by concomitant ingestion of food. Unchanged Vandetanib and three metabolites (N-desmethylvandetanib, vandetanib N-oxyde and glucuronide conjugate of vandetanib) were detected in plasma, urine and feces.28 In vitro studies showed that Vandetanib is converted into N- desmethylvandetanib by the CYP P450 3A4 and it has subsequently been demonstrated that Vandetanib exposure can be modified when administrated in combination with CYP 3A4 inducers or inhibitors.29 Approximately one half (44%) and one fourth (25%) of the Vandetanib dose was recovered over 21 days, in respectively, feces and urine, suggesting that these two routes might play an important role in Vandetanib elimination. Vandetanib exposure is increased in patients with renal impairment whereas exposure seems unchanged in patients with hepatic impairment.30 Recombinant enzyme assays showed that Vandetanib is a potent inhibitor of VEGFR-2 (IC(50) = 40 nM), VEGFR-3 (IC(50) = 110 nM), EGFR (IC(50) = 500 nM) and RET (IC(50) = 130 nM) and that the selectivity for these receptors was excellent.31
Phase I studies
In a first phase I study, 77 patients were enrolled in the USA and Australia. The patients all had solid tumors refractory to treatment, or had a cancer with no established treatment. They received a single dose of the drug, ranging from 50 mg to 600 mg, followed by 7 days of observation. After the observation period, they received the same dose once a day for 28 days until progression or dose-limiting toxicity. The most common drug related adverse events were diarrhea, rash, nausea, hypertension, and fatigue. Seven patients experienced an asymptomatic QTc interval prolongation. At 500 and 600 mg, three and seven out of eight patients respectively, experienced dose-limiting toxicities. Thus, 300 mg was the highest dose used during the cohort expansion phase of the study.26 A second phase I study was conducted in Japan with 18 patients. The dose-limiting toxicities were hypertension, diarrhea, headache, and toxic skin eruption. Again, 300 mg was identified to be the recommended dose. This study showed an objective response in some patients with non-small cell lung carcinoma.27 A phase I study was recently conducted in China and enrolled 36 patients with solid malignant tumors, mostly lung cancers. The patients received three different doses of Vandetanib (100 mg every other day, 100 mg daily or 300 mg daily) until disease progression or discontinuation of the study. Again, the most common drug-related side effects were rash and diarrhea, with respectively 42% and 39% of the patients. Three patients with MTC were included in this study. Interestingly, one of these patients, treated with 300 mg daily, was the only patient of the whole study who had an objective partial response. The investigators compared the pharmacokinetic data with the previous phase I studies and concluded that there was no marked difference in pharmacokinetics among Chinese, Japanese and Western patients.32
Phase II studies: Vandetanib in MTC
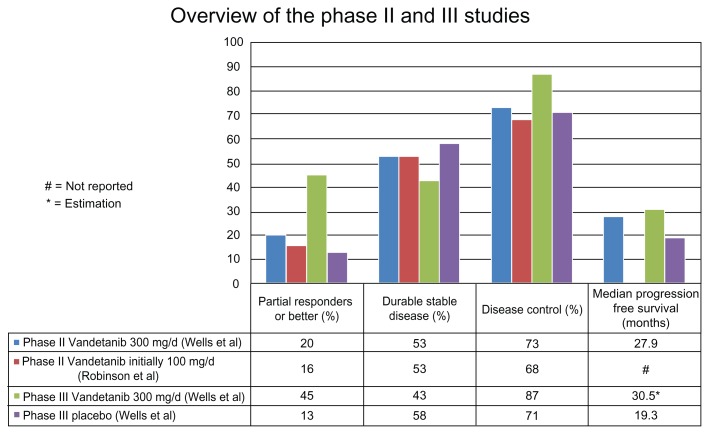
A first multicenter phase II study was initiated in November 2004 and enrolled patients with unresectable locally advanced or metastatic hereditary MTC until August 2006. Thirty patients received 300 mg Vandetanib a day and were assessed for objective tumor response following the Response Evaluation Criteria in Solid Tumors (RECIST). Secondary end points included progression-free survival (PFS), duration of response, disease control, safety and tolerability as well as changes in serum levels of MTC biomarkers. Determination of response was assessed by computed tomography (CT) and magnetic resonance imaging (MRI) obtained initially and after 12 week-intervals during treatment. Durable stable disease (>24 weeks) was observed in 16 out of 30 patients (53%). Partial responses were reported in six patients (20%). Thus, 22 patients (73%) had disease control on Vandetanib. Notably, 25 patients (83%) experienced some reduction in tumor size during treatment. The median duration of response (from first response to progression or death) was 10.2 months (range, 1.9–16.9). The median progression-free survival was 27.9 months. Six patients had stable disease for less than 24 weeks, one patient had progressive disease, and one patient was not evaluable because of impaired renal function. Serum tumor markers also were analyzed; the criteria for partial response was a decrease from baseline calcitonin and carcinoembryonic antigen (CEA) level. Decrease of baseline calcitonin and CEA was observed in 24 patients (80%) and 16 patients (53%), respectively. All patients with a partial response (n = 6) on imaging studies had a decrease (range, 73% to 99%) in serum calcitonin levels, and 4 partial responders had a marked decrease in CEA serum levels (range, 82% to 91%). At the time of data cutoff (February 22, 2008), 17 patients were still on treatment. Regarding safety and tolerability, 7 patients (23%) discontinued the treatment. Five out of 7 discontinuations were attributed to Vandetanib-related adverse events, including QTc prolongation, rash, nausea, and hemorrhagic diarrhea. In the whole cohort, the most common adverse events reported were diarrhea (70%), rash (20%), fatigue (19%), and nausea (19%).33
A second phase II study enrolled 19 patients with comparable eligibility criteria and the same primary endpoint (RECIST response criteria). However, a lower dose (100 mg) was used in this study, with escalation possibility to 300 mg upon disease progression. Objective partial responses were seen in 16% of patients, and stable disease in 53% of patients. Consequently, disease control was seen in 68% of all patients. The adverse effects were similar to the previous studies.34
Phase III studies: randomized placebo-controlled trial
The promising results observed in phase II studies encouraged a large double-blind, randomized, phase III study. A total of 331 patients were enrolled between December 2006 and November 2007 and randomly assigned to receive 300 mg of Vandetanib or placebo (2:1 ratio). The eligibility criteria included metastatic or locally advanced unresectable disease, a performance status between 0 and 2 according to World Health Organization (WHO) classification, and serum calcitonin levels ≥500 pg/mL. Exclusion criteria were other severe medical conditions or recent chemotherapy or radiation-therapy (less than 4 weeks before randomization). The data cutoff occurred in July 2009 (median follow-up 24 months). The primary endpoint was progression-free survival (PFS) determined by the RECIST criteria. In this cohort, a large majority of patients had sporadic (90%) and metastatic (95%) MTC. 59% of the patients receiving Vandetanib had a confirmed RET mutation, and 40% had an unknown RET status. The primary endpoint was met: at 6 months, 83% of the patients on Vandetanib had progression-free survival versus 63% in the placebo group. The median progression-free survival was 19.3 in the placebo group and had not been reached at data cut-off in the Vandetanib group. Weibull models indicate a predicted median progression-free survival of 30.5 months on treatment. In the Vandetanib group, the secondary efficacy endpoints showed an objective response rate in 45% of patients, a disease control rate in 87% of patients, and calcitonin and CEA biochemical response rates of 69% and 52% of patients, respectively. The overall survival was immature at data cutoff. However, there was no significant survival difference between the two groups during the early follow-up. More precise data on survival will be available once 50% of the patients have died. The adverse events were similar to those observed in the phase II studies. However, 5 patients on Vandetanib had adverse events leading to death, including arrhythmia and acute heart failure. 35 Vandetanib was approved by the Food and Drug Administration (FDA) on April 6, 2011 and became the first systemic agent to be approved for locally advanced or metastatic MTC in the United States.36 It is commercialized by AstraZeneca under the Caprelsa® brand name in the United States. Figure 2 summarizes and compares the main results of the phase II and III studies.
Figure 2.
Analysis of clinical trials leading to the approval of Vandetanib.
Safety and tolerability of Vandetanib
The clinical studies leading to Vandetanib approval showed an acceptable safety and tolerability profile with a majority of the adverse events manageable with supportive therapy or dose reduction. However adverse events such as rash, diarrhea and nausea were commonly observed even at the lowest dosage. The Table 1 summarizes the most common adverse events observed in the clinical studies leading to Vandetanib approval. The phase III study uncovered potentially life threatening adverse events. Protocol defined QTc prolongation was observed in 8% of the patients on treatment but no torsades de pointes were reported. Five out of the 231 patients on Vandetanib experienced adverse events leading to death including aspiration pneumonia, respiratory arrest, respiratory failure, staphylococcal sepsis, arrhythmia and acute cardiac failure. Recently, a meta-analysis of 9 phase II or phase III studies evaluated the incidence of QTc prolongation in 2188 cancer patients treated with 300 mg Vandetanib a day. The overall incidence of all grade and high-grade QTc prolongation was respectively, 18% and 12% among thyroid cancer patients. The incidence was significantly higher in patients treated for thyroid cancer compared to other solid tumors, probably because of the longer treatment duration or as a result of abnormal thyroid function which can increase the susceptibly to acquire prolonged QTc intervals.37 Another meta-analysis evaluated the rash incidence in 2961 patients treated with 300 mg Vandetanib a day and found that the overall incidence of all-grade and high-grade rash were 46.1% and 3.5%, respectively.38 Other rare adverse events related to Vandetanib have been described in the literature such as Stevens-Johnson syndrome or ischemic cerebrovascular events.39,40 Due to the frequent and potentially life threatening adverse events a strategy called Vandetanib Risk Evaluation Mitigation Strategy (REMS) which allows prescription and distribution of the drug only by physicians and pharmacies certified by the REMS program, has been developed.41 Indeed, to prescribe Caprelsa® physicians need to complete a prescriber training, review the Caprelsa® education pamphlet and the full prescribing information and to be re-trained following substantive changes in the Caprelsa® REMS. The Caprelsa® enrollment form emphasizes that electrolytes should be closely monitored while on treatment, that drugs prolonging the QT interval should be avoided and that ECGs must be obtained periodically. If patients develop a QTc greater than 500 ms the treatment should be discontinued until the QTc returns to less than 450 ms and subsequently resumed at a lower dose. For additional safety, Caprelsa® is only dispensed by certified pharmacies which meet the Caprelsa® REMS requirements such as employee education on risks and provide medication guides to patients.42
Table 1.
Summary of adverse events observed in the main clinical studies leading to Vandetanib approval.
| Phase I, Holden et al 50–600 mg | Phase I, Tamura et al 100–400 mg | Phase II, Wells et al 300 mg | Phase III, Wells et al 300 mg | Phase III, Wells et al Placebo | |
|---|---|---|---|---|---|
| Diarrhea (%) | 38 | 61 | 70 | 56 | 26 |
| Rash (%) | 34 | 72 | 67 | 45 | 11 |
| Nausea (%) | 19 | 22 | 63 | 33 | 16 |
| Hypertension (%) | 18 | 39 | 33 | 32 | 5 |
| Fatigue (%) | 18 | 44 | 63 | 24 | 23 |
| ECG QTc prolongation (%) | 9 | 67 | 20 | 14 | 1 |
| Adverse events leading to death (n) | 0 | 0 | 0 | 5 | 0 |
Clinical utility of Vandetanib in the management of advanced MTC and cost considerations
All the previous studies evaluating classic chemotherapy regimens or radiation therapy failed to demonstrate significant objective responses in patients with advanced MTC. Accordingly, the results of the phase II and III studies are unique and encouraging. The disease can demonstrate an indolent course in many patients, some of whom may remain asymptomatic with stable disease for many years. The longterm survival rates for patients on Vandetanib versus placebo will need to be collected, and these will help to inform practice guidelines for locally advanced and metastatic medullary thyroid cancer going forward. The potentially serious cardiac side effects and cost of the treatment cannot be overlooked. A study conducted in the Netherlands analyzed the use and costs of oral anticancer agents between 2000 and 2008 and showed a 50-fold rise in costs of oral anticancer agents between 2000 and 2008; 67% of this rise was attributable to tyrosine kinase inhibitors (TKIs).43 One of the most effective tyrosine kinase inhibitors, imatinib, has been evaluated in many pharmacoeconomic studies. A summary of 6 such trials, found the medication to be cost effective in the treatment of gastro-intestinal stromal tumors (GIST) and also noted the cost effectiveness of second line treatment with sunitinib in resistant patients.44 MTC however is different from GIST as it is a much more indolent disease. The 1 year survival of patients with metastatic GIST, is increased from 32% to 95% with the addition of imatinib.44 MTC patients on the contrary often are asymptomatic and productive members of society. Since 1 month of Vandatinib treatment costs approximately $5–10,000, it will be crucial in future studies to identify the patients that are the most likely to benefit from the treatment as well as the correct time to begin therapy. It is probable that cost effective treatment for metastatic patients will only be seen in those who have a life expectancy of a limited number of months or years. However, it is also conceivable that Vandetanib can be used in a ‘neoadjuvant’ setting to convert patients with locally advanced disease to an operable status, or that it can be used in tandem with other agents under study in order to maximize clinical benefit, delaying the need for other supportive services and treatments and possibly reducing longer term costs. Moreover, a key issue will be to evaluate in detail whether Vandetanib contributes to enhanced control of pain and improvement of quality of life among patients with advanced MTC.
Vandetanib in the treatment of other malignancies
RET-PTC rearrangements are commonly found in patients with papillary thyroid cancer and Vandetanib has shown activity against this aberrant receptor on papillary cancer cell lines.45 Furthermore, differentiated thyroid tumors are highly vascular and might consequently respond to anti-angiogenic treatments. A phase II study was conducted in patients with locally advanced or metastatic differentiated (papillary or follicular) thyroid carcinoma. This study enrolled 145 subjects after radioiodine failure or radioiodine contraindication; 72 received Vandetanib and 73 received placebo. Remarkably, the PFS was significantly prolonged in the Vandetanib group versus placebo with 11 months and 5.8 months, respectively. However, there was no statistically difference in overall survival, disease control rate and overall response rate.46
Some objective responses were seen in patients with non-small cell lung carcinoma (NSCLC) in phase I studies. The EGFR pathway is commonly involved in NSCLC development and EGFR inhibitors such as Erlotinib and Gefitinib are used in selected patients with NSCLC. It has been proposed that resistance to these treatments might be a consequence of increased VEGF expression and signaling.47 Moreover, it has been shown in xenografts models of human NSCLC that resistance to EGFR TKIs can be reversed by Vandetanib.48 These results encouraged further clinical studies for previously treated NSCLC. A phase II study compared Vandetanib versus Gefitinib and showed that Vandetanib significantly prolonged PFS. However, overall survival was not significantly different.49 Another phase III study compared Vandetanib with Erlotinib and found no difference in PFS but Vandetanib was associated with a higher rate of adverse events.50 A recent study evaluated the effectiveness of Vandetanib versus placebo in patients with NSCLC after prior treatment with EGFR TKIs. No increase in overall survival was found in the Vandetanib group but the PFS and objective response rates were slightly more favorable in the treated group.51 The effectiveness of Vandetanib in association with Docetaxel52 or Pemetrexed53 has also been assessed in large phase III studies. The Docetaxel-Vandetanib association showed a modest prolongation of PFS whereas the Pemetrexed-Vandetanib combination failed to demonstrate any prolongation of PFS compared to Pemetrexed alone. Hence, this study showed a delay in worsening of lung cancer symptoms and significantly higher objective response rates in the patients treated with the bi-therapy.
The use of Vandetanib has also been evaluated in advanced breast cancer because the EGFR and VEGFR pathways play a role in breast cancer growth, progression and invasion.54 A phase II study evaluated Vandetanib 100 mg or 300 mg daily for patients with advanced metastatic breast cancer. No objective responses were seen, and only 1 patient out of the 46 enrolled had stable disease.55 It has been shown that Vandetanib can induce apoptosis in human breast cancer cell lines when used as a single agent or in combination with Paclitaxel.56 A phase II study compared the association Vandetanib-Docetaxel versus placebo-Docetaxel in pretreated patients with advanced breast cancer but the association provided no clinical benefit.57
Several phase I studies for metastatic colorectal cancer were recently conducted; in a first study Vandetanib was associated with Capacitabine-Oxaliplatine and Bevacizumab was added as a fourth agent in one subgroup. The association of the two anti-angiogenic drugs resulted in severe diarrhea requiring IV hydration in 3 out of 4 patients.58 Other phase I studies tested the FOLFIRI-Vandetanib59 or mFOLFOX6-Vandetanib60 associations which were generally well tolerated. Larger studies will help to determine whether the addition of Vandetanib might provide a clinical benefit in colorectal cancer.
Clinical studies evaluating Vandetanib alone or in combination have also been led in hepatocellular carcinoma,61 refractory prostate cancer,62 advanced urothelial cancer,63 and metastatic pancreatic adenocarcinoma.64 These studies showed globally a good tolerance to Vandetanib but failed to demonstrate any clinical benefit. Further studies are required to define whether Vandetanib might play a role in the management of these malignancies.
Footnotes
Author Contributions
Conceived and designed the experiments: HAD, ND. Analysed the data: HAD, ND, JAS, SR. Wrote the first draft of the manuscript: ND. Contributed to the writing of the manuscript: ND, HAD, SR, JAS. Agree with manuscript results and conclusions: HAD, ND, JAS, SR. Jointly developed the structure and arguments for the paper: HAD, ND, JAS, SR. Made critical revisions and approved final version: HAD, ND, JAS, SR. All authors reviewed and approved of the final manuscript.
Competing Interests
HAD was on advisory board for Astra Zeneca October 2010. JAS is a consultant to Amylin and has received speaker’s fees from Veracyte. All other authors declare no competing interests.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects.
References
- 1.Leboulleux S, Baudin E, Travagli JP, Schlumberger M. Medullary thyroid carcinoma. Clinical Endocrinology. 2004;61:299–310. doi: 10.1111/j.1365-2265.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- 2.Modigliani E, Cohen R, Crampos JM, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’étude des tumeurs à calcitonine. Clinical Endocrinology. 1998;48(3):265–73. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 3.Dottorini ME, Assi A, Sironi M, Sangalli G, Spreafico G, Colombo L. Multivariate analysis of patients with medullary thyroid carcinoma. Prognostic significance and impact on treatment of clinical and pathological variables. Cancer. 1996;77(8):1556–65. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1556::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 4.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107(9):2134–42. doi: 10.1002/cncr.22244. [DOI] [PubMed] [Google Scholar]
- 5.Matuszczyk A, Petersenn S, Bockisch A, et al. Chemotherapy with doxorubicin in progressive medullary and thyroid carcinoma of the follicular epithelium. Horm Metab Res. 2008;40(3):210–3. doi: 10.1055/s-2008-1046781. [DOI] [PubMed] [Google Scholar]
- 6.Nocera M, Baudin E, Pellegriti G, Cailleux AF, Mechelany-Corone C, Schlumberger M. Treatment of advanced medullary thyroid cancer with an alternating combination of doxorubicin-streptozocin and 5 FU dacarbazine. British Journal of Cancer. 2000;83(6):715–71. doi: 10.1054/bjoc.2000.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fersht N, Vini L, A’Hern R, Harmer C. The role of radiotherapy in the management of elevated calcitonin after surgery for medullary thyroid cancer. Thyroid. 2001;11(12):1161–8. doi: 10.1089/10507250152741019. [DOI] [PubMed] [Google Scholar]
- 8.Raue KF, Rondot S, Raue F. Molecular genetics and phenomics of RET mutations: Impact on prognosis of MTC. Molecular and Cellular Endocrinology. 2010;332:2–7. doi: 10.1016/j.mce.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Pitt SC, Chen H. The phosphatidylinositol 3-kinase/akt signaling pathway in medullary thyroid cancer. Surgery. 2008;144(5):721–4. doi: 10.1016/j.surg.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Runeberg-Roos P, Saarma M. Neurotrophic factor receptor RET: structure, cell biology, and inherited diseases. Annals of Medicine. 2007;39(8):572–80. doi: 10.1080/07853890701646256. [DOI] [PubMed] [Google Scholar]
- 11.Pierotti MA, Santoro M, Jenkins RB, et al. Characterization of an inversion on the long arm of chromosome 10 juxtaposing D10S170 and RET and creating the oncogenic sequence RET/PTC. Proc Natl Acad Sci U S A. 1992;89(5):1616–20. doi: 10.1073/pnas.89.5.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodish MB, Stratakis CA. RET oncogene in MEN2, MEN2B, MTC and other forms of thyroid cancer. Expert Rev Anticancer Ther. 2008;4:625–32. doi: 10.1586/14737140.8.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnichon N, Vescovo L, Amar L, et al. Gimenez-Roqueplo AP. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974–85. doi: 10.1093/hmg/ddr324. [DOI] [PubMed] [Google Scholar]
- 14.Angrist M, Bolk S, Halushka M, Lapchak PA, Chakravarti A. Germline mutations in glial cell line-derived neurotrophic factor (GDNF) and RET in a Hirschsprung disease patient. Nat Genet. 1996;14(3):341–4. doi: 10.1038/ng1196-341. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid. 2010;20(8):863–71. doi: 10.1089/thy.2009.0417. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Antona C, Pallares J, Montero-Conde C, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr Relat Cancer. 2010;17(1):7–16. doi: 10.1677/ERC-08-0304. [DOI] [PubMed] [Google Scholar]
- 18.Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology. Curr Opin Oncol. 2001;13(6):506–13. doi: 10.1097/00001622-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 20.Ohgaki H. Genetic pathways to glioblastomas. Neuropathology. 2005;25(1):1–7. doi: 10.1111/j.1440-1789.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Lee GK, Kong SY, et al. Epidermal growth factor receptor status in anaplastic thyroid carcinoma. J Clin Pathol. 2007;60(8):881–4. doi: 10.1136/jcp.2006.041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen BK, Ohtsuki Y, Furihata M, et al. Co-overexpression of p53 protein and epidermal growth factor receptor in human papillary thyroid carcinomas correlated with lymph node metastasis, tumor size and clinicopathologic stage. Int J Oncol. 1999;15(5):893–8. doi: 10.3892/ijo.15.5.893. [DOI] [PubMed] [Google Scholar]
- 23.Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62(24):7284–90. [PubMed] [Google Scholar]
- 24.Vitagliano D, De Falco V, Tamburrino A, et al. The tyrosine kinase inhibitor ZD6474 blocks proliferation of RET mutant medullary thyroid carcinoma cells. Endocr Relat Cancer. 2010;18(1):1–11. doi: 10.1677/ERC-09-0292. [DOI] [PubMed] [Google Scholar]
- 25.Ciardiello F, Caputo R, Damiano V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9(4):1546–56. [PubMed] [Google Scholar]
- 26.Holden SN, Eckhardt SG, Basser R, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005;16(8):1391–7. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 27.Tamura T, Minami H, Yamada Y, et al. A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006;1(9):1002–9. [PubMed] [Google Scholar]
- 28.Martin P, Oliver S, Kennedy SJ, et al. Pharmacokinetics of vandetanib: three phase I studies in healthy subjects. Clin Ther. 2012;34(1):221–37. doi: 10.1016/j.clinthera.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Martin P, Oliver S, Robertson J, Kennedy SJ, Read J, Duvauchelle T. Pharmacokinetic drug interactions with vandetanib during coadministration with rifampicin or itraconazole. Drugs R D. 2011;11(1):37–51. doi: 10.2165/11586980-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weil A, Martin P, Smith R, et al. Pharmacokinetics of vandetanib in subjects with renal or hepatic impairment. Clin Pharmacokinet. 2010;49(9):607–18. doi: 10.2165/11534330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62(16):4645–55. [PubMed] [Google Scholar]
- 32.Zhang L, Li S, Zhang Y, et al. Pharmacokinetics and tolerability of vandetanib in Chinese patients with solid, malignant tumors: an open-label, phase I, rising multiple-dose study. Clin Ther. 2011;33(3):315–27. doi: 10.1016/j.clinthera.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Wells SA, Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28(5):767–72. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2010 Jun;95(6):2664–71. doi: 10.1210/jc.2009-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FDA. FDA Approves New Treatment for Rare Form of Thyroid Cancer. FDA; News and Events. [Apr 6, 2011]. Web [Feb 27, 2012]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm250168.htm. [Google Scholar]
- 37.Zang J, Wu S, Tang L, et al. Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: a systematic review and meta-analysis. PLoS One. 2012;7(2):e30353. doi: 10.1371/journal.pone.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME. Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. J Clin Endocrinol Metab. 2012 Feb 29; doi: 10.1210/jc.2011-2677. [DOI] [PubMed] [Google Scholar]
- 39.Yoon J, Oh CW, Kim CY. Stevens-johnson syndrome induced by vandetanib. Ann Dermatol. 2011;23(Suppl 3):S343–5. doi: 10.5021/ad.2011.23.S3.S343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duplomb S, Benoit A, Mechtouff-Cimarelli L, et al. Unusual adverse event with vandetanib in metastatic medullary thyroid cancer. J Clin Oncol. 2012;30(2):e21–3. doi: 10.1200/JCO.2011.38.2796. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson SC, Peterson J, Yektashenas B. Risk evaluation and mitigation strategies (REMS): educating the prescriber. Drug Saf. 2012;35(2):91–104. doi: 10.2165/11597840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.FDA. Approved Risk Evaluation and Mitigation Strategies (REMS) FDA; [Jun 22, 2011]. Web [Apr 5, 2012]. http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM253441.pdf. [Google Scholar]
- 43.Timmers L, Beckeringh JJ, Herk-Sukel MP, Boven E, Hugtenburg JG. Use and costs of oral anticancer agents in the Netherlands in the period 2000–2008. Pharmacoepidemiol Drug Saf. 2011 doi: 10.1002/pds.2225. [DOI] [PubMed] [Google Scholar]
- 44.Blanke CD, Huse D. Cost effectiveness of tyrosine kinase inhibitor therapy in metastatic gastrointestinal stromal tumors. Journal of Medical Economics. 2010;13(4):681–90. doi: 10.3111/13696998.2010.534670. [DOI] [PubMed] [Google Scholar]
- 45.Verbeek HH, Alves MM, de Groot JW, et al. The effects of four different tyrosine kinase inhibitors on medullary and papillary thyroid cancer cells. J Clin Endocrinol Metab. 2011;96(6):E991–5. doi: 10.1210/jc.2010-2381. [DOI] [PubMed] [Google Scholar]
- 46.Leboulleux S, Bastholt L, Krause TM, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomized, double-blind phase II trial. Ann Oncol. 2010;21(Suppl 8):viii 315. doi: 10.1016/S1470-2045(12)70335-2. (abstr 1008PD) [DOI] [PubMed] [Google Scholar]
- 47.Ciardiello F, Bianco R, Caputo R, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. 2004;10(2):784–93. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 48.Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15(10):3484–94. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Natale RB, Bodkin D, Govindan R, et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase II study. J Clin Oncol. 2009;27(15):2523–9. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 50.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(8):1059–66. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 51.Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR) J Clin Oncol. 2012;30(10):1114–21. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 52.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619–26. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Boer RH, Arrieta Ó, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29(8):1067–74. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 54.Atalay G, Cardoso F, Awada A, Piccart MJ. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann Oncol. 2003;14(9):1346–63. doi: 10.1093/annonc/mdg365. [DOI] [PubMed] [Google Scholar]
- 55.Miller KD, Trigo JM, Wheeler C, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2005 May 1;11(9):3369–76. doi: 10.1158/1078-0432.CCR-04-1923. [DOI] [PubMed] [Google Scholar]
- 56.Sarkar S, Mazumdar A, Dash R, Sarkar D, Fisher PB, Mandal M. ZD6474 enhances paclitaxel antiproliferative and apoptotic effects in breast carcinoma cells. J Cell Physiol. 2011;226(2):375–84. doi: 10.1002/jcp.22343. [DOI] [PubMed] [Google Scholar]
- 57.Boér K, Láng I, Llombart-Cussac A, et al. Vandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo- controlled, randomized phase II study. Invest New Drugs. 2012;30(2):681–7. doi: 10.1007/s10637-010-9538-8. [DOI] [PubMed] [Google Scholar]
- 58.Cabebe EC, Fisher GA, Sikic BI. A phase I trial of vandetanib combined with capecitabine, oxaliplatin and bevacizumab for the first-line treatment of metastatic colorectal cancer. Invest New Drugs. 2011 Mar 15; doi: 10.1007/s10637-011-9656-y. [DOI] [PubMed] [Google Scholar]
- 59.Saunders MP, Wilson R, Peeters M, et al. Vandetanib with FOLFIRI in patients with advanced colorectal adenocarcinoma: results from an open-label, multicentre phase I study. Cancer Chemother Pharmacol. 2009;64(4):665–72. doi: 10.1007/s00280-008-0914-4. [DOI] [PubMed] [Google Scholar]
- 60.Michael M, Gibbs P, Smith R, Godwood A, Oliver S, Tebbutt N. Open-label phase I trial of vandetanib in combination with mFOLFOX6 in patients with advanced colorectal cancer. Invest New Drugs. 2009;27(3):253–61. doi: 10.1007/s10637-008-9182-8. [DOI] [PubMed] [Google Scholar]
- 61.Hsu C, Yang TS, Huo TI, et al. Vandetanib in patients with inoperable hepatocellular carcinoma: A phase II, randomized, double-blind, placebo-controlled study. J Hepatol. 2012 Jan 13; doi: 10.1016/j.jhep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Horti J, Widmark A, Stenzl A, et al. A randomized, double-blind, placebo-controlled phase II study of vandetanib plus docetaxel/prednisolone in patients with hormone-refractory prostate cancer. Cancer Biother Radiopharm. 2009;24(2):175–80. doi: 10.1089/cbr.2008.0588. [DOI] [PubMed] [Google Scholar]
- 63.Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30(5):507–12. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saletti P, Sessa C, De Dosso S, Cerny T, Renggli V, Koeberle D. Phase I dose-finding study of vandetanib in combination with gemcitabine in locally advanced unresectable or metastatic pancreatic adenocarcinoma. Oncology. 2011;81(1):50–4. doi: 10.1159/000330769. [DOI] [PubMed] [Google Scholar]