Abstract
Background
To assess the impact of a continuous measure of adherence with infliximab maintenance treatment in Crohn’s disease (CD) during the first year of treatment on CD-related health care utilization, CD-related hospitalizations, inpatient costs, and length of hospital stay.
Patients and methods
A retrospective claims analysis using the IMS LifeLink Health Plan Claims Database (September 1, 2004, to June 30, 2009) was conducted. Continuous enrollment for 12 months before and 12 months after the index date was required. Patients were required to have at least two claims with an International Classification of Diseases, 9th Revision, Clinical Modification diagnosis code for CD (555.xx) pre-index and be aged ≥ 18 years at index. Patients with three infusions during the first 56 days post-index and at least one infusion following day 56 post-index were considered to have maintenance therapy. Adherence and nonadherence were defined as a medication possession ratio of ≥ 80% and < 80%, respectively.
Results
Four hundred forty-eight patients were included in the analysis (mean age, 42.6 years; 56% female; mean ± standard deviation [SD] and median number of infliximab infusions, 7.35 ± 1.60 and 8). The number of patients who met the definition of adherence was 344 (77%). CD-related health care utilization was not significantly impacted by adherence except for ancillary services and radiology. Fewer adherent patients were hospitalized compared with nonadherent patients (9% versus 16%; P = 0.03). Adherent patients had fewer mean ± SD and median days in the hospital (5.5 ± 3.4 and 5 days) compared with nonadherent patients (13.1 ± 14.2 and 8 days; P = 0.01). Mean ± SD and median hospital costs were significantly greater for nonadherent patients ($40,822 ± $49,238 and $28,864) compared with adherent patients ($13,704 ± $10,816 and $9938; P = 0.002).
Conclusion
Adherence with maintenance infliximab over 12 months was associated with lower rates of CD-related hospitalizations and inpatient costs and a shorter length of hospital stay.
Keywords: costs, Crohn’s disease, hospitalization, infliximab, length of stay, medication adherence
Introduction
Crohn’s disease (CD) is an idiopathic and chronic inflammatory bowel disease that affects approximately 144–198 per 100,000 people in North America.1 It is characterized by episodes of acute symptomatic inflammation of the gut (exacerbations) alternating with periods of reduced disease activity (remission). CD significantly impacts patients’ health-related quality of life, having deleterious effects on their physical, social, and emotional well-being.2–5 Because there is no known cure for the disease, patients with CD – especially younger patients with early-onset disease – may expect years of medical care, hospitalization, and surgery.3,4 Total CD-related treatment costs in the United States (US) using 2003–2004 estimates were $3.6 billion.6 Hospitalization and outpatient costs for this time period were 31.4% and 33.3% of total CD-related costs, respectively.6 Using more recent 2006 estimates, the total economic burden (direct medical and indirect costs) of CD in the US was $10.9–$15.5 billion.7
A variety of therapeutic agents, including 5-aminosalicylates, systemic corticosteroids, immunomodulators (eg, 6- mercaptopurine, azathioprine, and methotrexate), antibiotics, and biologic agents, have been used in the treatment of CD in an attempt to induce and maintain clinical remission.5 The advent of biologic agents in CD treatment has dramatically affected treatment expectations in these patients.
Infliximab is a chimeric immunoglobulin G1 monoclonal antibody biologic against tumor necrosis factor-α (TNF-α). TNF-α has been implicated in stimulating an inappropriate inflammatory gastrointestinal tract response, resulting in diarrhea, fever, abdominal pain, and weight loss associated with CD.8 Infliximab has proven to be efficacious in inducing and maintaining clinical remission and mucosal healing in adult and pediatric (aged ≥ 6 years) patients with moderate-to-severe CD and adult patients with fistulizing CD.9–12
One of the goals of CD treatment is to maintain remission in order to prevent exacerbations that require costly hospitalizations and surgeries.13 Infliximab has been shown to reduce hospitalizations, surgeries, and procedures, especially when the drug is prescribed as a scheduled maintenance versus periodic treatment regimen.14–19
Adherence to a treatment regimen in a chronic condition such as CD is critical for improving patient outcomes over the long term; however, few studies have evaluated the effect of a continuous measure of adherence with biologic agents on health care services utilization, outcomes, and costs in inflammatory bowel disease. The objective of the current study was to assess the observed impact of adherence with infliximab maintenance treatment in CD during the first year of treatment on CD-related health care utilization, hospitalizations, inpatient costs, and length of hospital stay.
Patients and methods
Data source
A retrospective, observational cohort was obtained for this analysis using medical and pharmacy claims data from the IMS LifeLink Health Plan Claims Database dated from September 1, 2004, to June 30, 2009. The IMS LifeLink Database comprises fully adjudicated medical and pharmaceutical claims for over 60 million patients from over 90 health plans across the US.20 The database content includes hospital admission and discharge dates, dates of service, procedure codes, patient demographics (eg, age, sex), length of hospital stay, medical services and drug costs, and drug information (eg, drug name, dose, strength, days’ supply and quantity dispensed, date of service).
Sample selection
Patients were included in this study if all of the following inclusion criteria were met: the patient had at least one medical claim for infliximab (the date of service on the first claim defined the index date); the patient had at least two diagnoses of CD as defined by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code of 555.xx during the 12-month pre-index period; there was no evidence of infliximab use during the 6-month preindex period; the patient was aged ≥ 18 years at the index date; and the patient was continuously enrolled in the health plan for at least 12 months before and 12 months after the index date.
Patients were excluded from the study if any of the following occurred: the patient was not continuously enrolled in the health plan for at least 12 months before and 12 months after the index date; the patient had at least one medical claim with an ICD-9-CM code for psoriasis (696.1), rheumatoid arthritis (714.xx), psoriatic arthritis (696.0), or ankylosing spondylitis (720.xx) during the pre-index period; or the patient had pharmacy National Drug Code claims for infliximab. Patients with a pharmacy National Drug Code claim for infliximab were excluded to focus results on the population of patients receiving all infliximab infusions from health care professionals submitting medical claims. Continuity in the type of claim used for infliximab billing purposes (ie, medical versus pharmacy) ensured that any observed gaps in therapy were not due to administrative errors consequential to changes in the type of claim used. Additionally, the date of service on an infliximab medical claim is representative of the date of actual infusion, whereas the date of service on an infliximab pharmacy claim is representative of when the drug claim was adjudicated by a pharmacy. The index date was defined as the first claim for infliximab received between September 1, 2005, and June 30, 2008.
Definitions and measures
The continuous measure of adherence was defined as a medication possession ratio (MPR) for patients receiving infliximab maintenance treatment. Patients were considered to have received infliximab maintenance treatment if they received three infusions during the first 56 days post-index and one or more infusions following day 56 during the 12-month post-index period. The MPR was calculated as the total days’ supply of infliximab administered during the 12-month post-index period divided by 360.
CD-related utilization of health care services, hospitalization rate, length of hospital stay (days), and inpatient costs were compared between maintenance infliximab-treated patients with adherence (MPR ≥ 80%) and patients without adherence (MPR < 80%).21,22 A claim was considered CD-related if an ICD-9-CM diagnosis code of CD was present in any position on the claim. A hospitalization was considered CD-related if an ICD-9-CM diagnosis of CD was present in any position on the discharge record. In the IMS LifeLink Database, temporal positioning of the ICD-9-CM diagnosis on the discharge record (eg, first, second, third) is not relevant for determination of reason for hospitalization. Presence of a diagnosis code in any position on the discharge record is indicative of a disease-related hospitalization.
Statistical analyses
Baseline characteristics between the adherent cohort and nonadherent cohort were compared. Chi-square and t-test/nonparametric Wilcoxon tests were used to test the statistical significance of nominal and continuous variables, respectively. Health care resource utilization and costs were analyzed and compared descriptively between the two adherence cohorts. Statistical significance was defined by a P value ≤ 0.05.
Results
The final sample consisted of 448 patients who met the inclusion criteria (Table 1), 344 (77%) of whom had an MPR ≥ 80% and were included in the adherence cohort, while the remaining 104 (23%) patients did not meet adherence (ie, MPR < 80%). The baseline characteristics were similar between the adherent and nonadherent cohorts (Table 2). The mean ± standard deviation (SD) age of the total population (n = 448) was 42.6 ± 14.8 years, and the majority (56%) of patients were female. The mean ± SD and median number of infliximab infusions during the 12-month post-index period in the total population was 7.35 ± 1.60 and 8, respectively.
Table 1.
Attrition of infliximab study population, by reason
| Patients excluded | Patients remaining | |||
|---|---|---|---|---|
|
|
|
|||
| n | % | n | % | |
| Total number of patients before any attrition | 18,500 | 100.0 | ||
| Less than 18 years of age | 609 | 3.3 | 17,891 | 96.7 |
| Not enrolled for 360 days pre-index | 7631 | 41.2 | 10,260 | 55.5 |
| Not enrolled for 360 days post-index | 2115 | 11.4 | 8145 | 44.0 |
| No evidence (≥ 2 claim days) of pre-index CD | 5971 | 32.3 | 2174 | 11.8 |
| Has claim for an exclusionary comorbidity in 360 days pre-index | 208 | 1.1 | 1966 | 10.6 |
| Has infliximab claim in 180 days pre-index | 782 | 4.2 | 1184 | 6.4 |
| Has colectomy within 84 days post-index | 27 | 0.1 | 1157 | 6.3 |
| Has infliximab NDC claim post-index | 66 | 0.4 | 1091 | 5.9 |
| Has non-index biologic post-index | 78 | 0.4 | 1013 | 5.5 |
| Data quality issue | 16 | 0.1 | 997 | 5.4 |
| Does not have three induction infusions | 513 | 2.8 | 484 | 2.6 |
| Does not have maintenance infusions | 36 | 0.2 | 448 | 2.4 |
| Patients available for analysis | 448 | 2.4 | ||
Abbreviations: CD, Crohn’s disease; NDC, National Drug Code.
Table 2.
Demographic characteristics by cohort
| Characteristic | Total, n = 448 | Adherent cohort (MPR ≥ 80%), n = 344 | Nonadherent cohort (MPR < 80%), n = 104 | P value |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 42.6 (14.8) | 42.4 (14.9) | 43.3 (14.2) | |
| Median | 43 | 42 | 45 | 0.48 |
| Sex, n (%) | ||||
| Female | 251 (56) | 199 (58) | 52 (50) | |
| Male | 197 (44) | 145 (42) | 52 (50) | 0.16 |
| Payer type, n (%) | N/A | |||
| Commercial plan | 424 (95) | 325 (94) | 99 (95) | |
| Medicaid | 2 (<1) | 1 (<1) | 1 (1) | |
| Medicare risk | 10 (2) | 10 (3) | 0 (0) | |
| Self-insured | 12 (3) | 8 (2) | 4 (4) | |
| Region, n (%) | 0.33 | |||
| Northeast | 69 (15) | 48 (14) | 21 (20) | |
| Midwest | 187 (42) | 149 (43) | 38 (37) | |
| South | 132 (29) | 103 (30) | 29 (28) | |
| West | 60 (13) | 44 (13) | 16 (15) | |
| First infliximab infusion place of service, n (%) | N/A | |||
| Outpatient office | 295 (66) | 234 (68) | 61 (59) | |
| Outpatient hospital | 60 (13) | 45 (13) | 15 (14) | |
| Inpatient hospital | 48 (11) | 34 (10) | 14 (13) | |
| Unknown | 30 (7) | 22 (6) | 8 (8) | |
| All other POS codes | 15 (3) | 9 (3) | 6 (6) | |
Abbreviations: MPR, medication possession ratio; N/A, not applicable; POS, place of service; SD, standard deviation.
Pre-infliximab CD-related utilization
When looking at the 12 months prior to the first infliximab infusion, use of pharmacy (immunomodulators, 5-aminosalicylates, corticosteroids, other biologics, and other pharmacy services) and outpatient services was similar between the adherent and nonadherent cohorts, except for emergency room visits and ancillary/other outpatient services (Table 3). A statistically significant difference in the percentage of patients with at least one emergency room visit claim was observed between the adherent and nonadherent cohorts (12% versus 21%; P = 0.02) for the 12 months pre-index. The median number of claims for ancillary/other outpatient services among patients with at least one claim was significantly higher in the adherent cohort than in the nonadherent cohort (4 versus 3, P = 0.02) prior to infliximab exposure. No significant differences were found in the proportion of patients with a hospitalization, length of hospital stay, or inpatient costs (Table 3).
Table 3.
Pre-index utilization of CD-related health care services
| Services | Adherent cohort (MPR ≥ 80%), n = 344 | Nonadherent cohort (MPR < 80%), n = 104 | P value |
|---|---|---|---|
| Pharmacy services | |||
| Immunomodulators | |||
| Patients with at least one claim, n (%) | 179 (52) | 53 (51) | 0.85 |
| Claims | |||
| Mean (SD) | 4.87 (3.61) | 4.32 (3.46) | |
| Median | 4 | 3 | 0.32 |
| 5-aminosalicylates | |||
| Patients with at least one claim, n (%) | 184 (53) | 50 (48) | 0.33 |
| Claims | |||
| Mean (SD) | 5.22 (3.61) | 4.82 (3.06) | |
| Median | 4 | 4 | 0.70 |
| Corticosteroids | |||
| Patients with at least one claim, n (%) | 214 (62) | 57 (55) | 0.18 |
| Claims | |||
| Mean (SD) | 3.63 (2.73) | 3.77 (2.52) | |
| Median | 3 | 4 | 0.54 |
| Other pharmacy services | |||
| Patients with at least one claim, n (%) | 68 (20) | 13 (13) | 0.09 |
| Claims | |||
| Mean (SD) | 2.76 (3.22) | 3.31 (2.75) | |
| Median | 2 | 3 | 0.20 |
| Outpatient services | |||
| Emergency room visits | |||
| Patients with at least one claim, n (%) | 41 (12) | 22 (21) | 0.02 |
| Claims | |||
| Mean (SD) | 1.44 (0.95) | 1.41 (0.67) | |
| Median | 1 | 1 | 0.63 |
| Laboratory and pathology | |||
| Patients with at least one claim, n (%) | 287 (83) | 93 (89) | 0.14 |
| Claims | |||
| Mean (SD) | 12.22 (13.65) | 11.90 (10.60) | |
| Median | 9 | 10 | 0.67 |
| Radiology | |||
| Patients with at least one claim, n (%) | 173 (50) | 53 (51) | 0.91 |
| Claims | |||
| Mean (SD) | 2.64 (1.94) | 2.30 (1.56) | |
| Median | 2 | 2 | 0.36 |
| Physician office visits | |||
| Patients with at least one claim, n (%) | 309 (90) | 92 (88) | 0.69 |
| Claims | |||
| Mean (SD) | 4.75 (3.45) | 5.57 (4.38) | |
| Median | 4 | 5 | 0.07 |
| Surgical services | |||
| Patients with at least one claim, n (%) | 158 (46) | 48 (46) | 0.97 |
| Claims | |||
| Mean (SD) | 1.51 (0.94) | 1.33 (0.91) | |
| Median | 1 | 1 | 0.06 |
| Ancillary/all other outpatient services | |||
| Patients with at least one claim, n (%) | 263 (76) | 77 (74) | 0.61 |
| Claims | |||
| Mean (SD) | 6.33 (6.88) | 5.22 (7.63) | |
| Median | 4 | 3 | 0.02 |
| Inpatient services | |||
| Patients with at least one hospitalization, n (%) | 74 (22) | 21 (20) | 0.77 |
| Number of hospitalizations | |||
| Mean (SD) | 1.22 (0.50) | 1.14 (0.48) | |
| Median | 1 | 1 | 0.41 |
| Number of hospital days | |||
| Mean (SD) | 7.80 (7.26) | 6.38 (4.04) | |
| Median | 6 | 5 | 0.48 |
| Hospitalization costs, $ | |||
| Mean (SD) | 16,966 (18,286) | 12,046 (8617) | |
| Median | 10,178 | 8462 | 0.32 |
Abbreviations: CD, Crohn’s disease; MPR, medication possession ratio; SD, standard deviation.
Post-infliximab CD-related utilization
Utilization of CD-related health care services during the 12 months post-index is presented in Table 4. The mean ± SD number of infliximab pharmacy claims was significantly greater in patients with adherence than those without (8.1 ± 0.9 versus 5.0 ± 1.0; P < 0.001). There were no statistically significant differences in utilization of specific CD-related pharmacy services (ie, immunomodulators, 5-aminosalicylates, corticosteroids, and other pharmacy services) during the post-index period. The median number of ancillary and other outpatient services claims among patients with at least one claim was significantly greater in the adherent cohort compared with the nonadherent cohort (22 versus 18; P < 0.001); however, adherent patients with at least one radiology claim had fewer median radiology claims compared with nonadherent patients (2 versus 3; P = 0.004).
Table 4.
Post-index utilization of CD-related health care services
| Services | Adherent cohort (MPR ≥ 80%), n = 344 | Nonadherent cohort (MPR < 80%), n = 104 | P value |
|---|---|---|---|
| Pharmacy services | |||
| Infliximab | |||
| Patients with at least one claim, n (%) | 344 (100) | 104 (100) | |
| Claims | |||
| Mean (SD) | 8.06 (0.93) | 4.99 (1.00) | |
| Median | 8 | 5 | <0.001 |
| Immunomodulators | |||
| Patients with at least one claim, n (%) | 158 (46) | 46 (44) | 0.76 |
| Claims | |||
| Mean (SD) | 6.87 (3.85) | 5.80 (3.76) | |
| Median | 7 | 5 | 0.08 |
| 5-aminosalicylates | |||
| Patients with at least one claim, n (%) | 112 (33) | 33 (32) | 0.87 |
| Claims | |||
| Mean (SD) | 5.26 (3.60) | 4.45 (2.87) | |
| Median | 4 | 4 | 0.35 |
| Corticosteroids | |||
| Patients with at least one claim, n (%) | 135 (39) | 45 (43) | 0.46 |
| Claims | |||
| Mean (SD) | 3.02 (2.63) | 4.07 (3.58) | |
| Median | 2 | 2 | 0.12 |
| Other pharmacy services | |||
| Patients with at least one claim, n (%) | 254 (74) | 79 (76) | 0.66 |
| Claims | |||
| Mean (SD) | 9.08 (6.09) | 8.25 (6.60) | |
| Median | 8 | 6 | 0.10 |
| Outpatient services | |||
| Emergency room visits | |||
| Patients with at least one claim, n (%) | 42 (12) | 19 (18) | 0.11 |
| Claims | |||
| Mean (SD) | 1.52 (0.83) | 1.26 (0.56) | |
| Median | 1 | 1 | 0.24 |
| Laboratory and pathology | |||
| Patients with at least one claim, n (%) | 277 (81) | 87 (84) | 0.47 |
| Claims | |||
| Mean (SD) | 14.86 (18.10) | 15.53 (17.97) | |
| Median | 10 | 11 | 0.42 |
| Radiology | |||
| Patients with at least one claim, n (%) | 99 (29) | 35 (34) | 0.34 |
| Claims | |||
| Mean (SD) | 2.21 (1.66) | 3.51 (2.64) | |
| Median | 2 | 3 | 0.004 |
| Physician office visits | |||
| Patients with at least one claim, n (%) | 314 (91) | 98 (94) | 0.33 |
| Claims | |||
| Mean (SD) | 6.49 (4.77) | 6.40 (5.87) | |
| Median | 6 | 5 | 0.69 |
| Surgical services | |||
| Patients with at least one claim, n (%) | 96 (28) | 37 (36) | 0.13 |
| Claims | |||
| Mean (SD) | 2.05 (2.02) | 2.03 (1.46) | |
| Median | 1 | 1 | 0.38 |
| Ancillary/all other outpatient services | |||
| Patients with at least one claim, n (%) | 336 (98) | 102 (98) | 0.81 |
| Claims | |||
| Mean (SD) | 25.38 (14.30) | 19.98 (13.91) | |
| Median | 22 | 18 | <0.001 |
Abbreviations: CD, Crohn’s disease; MPR, medication possession ratio; SD, standard deviation.
Post-index CD-related hospitalizations, length of hospital stay, and inpatient costs are reported in Table 5. A lower proportion of patients in the adherent cohort was hospitalized than in the nonadherent cohort (9% versus 16%, P = 0.03). Among all patients, the mean ± SD number of hospitalizations was lower in those with adherence than in those without (0.10 ± 0.36 versus 0.21 ± 0.52), as were inpatient costs ($1235 ± 5067 versus $6673 ± 24,631).
Table 5.
Post-index CD-related hospitalizations, lengths of hospital stay, and inpatient costs by cohort
| All patients, n = 448 | Adherent cohort (MPR ≥ 80%), n = 344 | Nonadherent cohort (MPR < 80%), n = 104 | P value | |
|---|---|---|---|---|
| Hospitalizations among all patients, mean (SD) | 0.13 (0.40) | 0.10 (0.36) | 0.21 (0.52) | |
| Hospitalization costs among all patients, mean (SD) | $2497 ($12,837) | $1235 ($5067) | $6673 ($24,631) | |
| Patients with at least one hospitalization, n (%) | 48 (11) | 31 (9) | 17 (16) | 0.03 |
| Number of hospitalizations | ||||
| Mean (SD) | 1.2 (0.5) | 1.2 (0.5) | 1.3 (0.5) | |
| Median | 1 | 1 | 1 | 0.20 |
| N umber of hospital days | ||||
| Mean (SD) | 8.2 (9.5) | 5.5 (3.4) | 13.1 (14.2) | |
| Median | 6 | 5 | 8 | 0.01 |
| Hospitalization costs, $ | ||||
| Mean (SD) | 23,308 (32,738) | 13,704 (10,816) | 40,822 (49,238) | |
| Median | 15,796 | 9938 | 28,864 | 0.002 |
Abbreviations: CD, Crohn’s disease; MPR, medication possession ratio; SD, standard deviation.
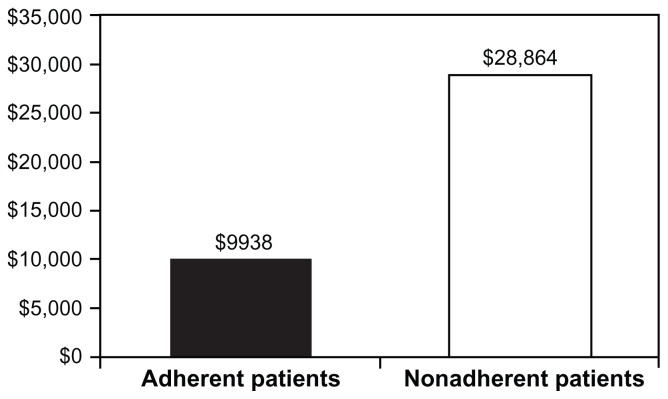
Among the subset of patients with at least one hospitalization, patients demonstrating adherence to infliximab had significantly shorter median length of hospital stay compared with those without adherence (5 versus 8 days; P = 0.01). Patients with adherence also trended toward fewer mean ± SD hospitalizations than patients without adherence, although the difference was not statistically significant (1.2 ± 0.5 versus 1.3 ± 0.5). Mean ± SD inpatient costs, however, were signif icantly lower for the adherent cohort than for the nonadherent cohort ($13,704 ± 10,816 versus $40,822 ± 49,238; P = 0.002). Median costs are presented in Figure 1.
Figure 1.
Median Crohn’s disease-related costs of hospitalization among patients with Crohn’s disease and with at least one hospitalization.
Note: P = 0.002, adherent versus nonadherent patients.
Discussion
Nonadherence with treatment regimens in chronic diseases is a ubiquitous problem. Nonadherence with oral therapies in gastrointestinal disease and its subsequent impact on medical costs is well documented.23–35 Reported nonadherence rates for oral medications in inflammatory bowel disease range between 7% and 72%, with most studies reporting nonadherence rates between 30% and 45%.28 Few studies, however, have evaluated the impact of adherence on biologic therapies in inflammatory bowel disease, and even fewer studies have focused on the impact of adherence with maintenance therapy specifically in patients with CD. To our knowledge, as of the date of this publication, this is the first study to evaluate the impact of a continuous measure of adherence with infliximab maintenance therapy, as measured by MPRs over 12 months, in CD treatment.
In the current study, 23% of patients with CD met the criteria for nonadherence with infliximab maintenance therapy at 12 months. Kane et al29 reported a higher nonadherence rate of 34% with maintenance infliximab treatment over 12 months in a retrospective study of patients with CD from the Integrated Health Care Information Service claims database. That study, however, did not evaluate adherence using MPR; adherence was measured by the number of infusions administered over one year. The adherence rate of 77% in the current study is more consistent with the adherence rate of 70%–80% with TNF-α inhibitors in patients with rheumatoid arthritis over 12 months, as reported by Tang et al.30
Medication adherence, as defined by the International Society for Pharmacoeconomics and Outcomes Research, is the “extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen.”36 As per the methodological standards of the International Society for Pharmacoeconomics and Outcomes Research, medication adherence may be estimated by an MPR calculation; however, unlike oral or patient self-administered medications, intravenous medications administered by health care professionals (versus patients) may have a greater degree of physician input and control on the prescribed interval and dose. This poses a challenge to patient adherence research for a treatment like infliximab yet offers a view on overall adherence compared with prescribing recommendations; this adherence estimate is the result of treatment-related shared decision-making between the patient and health care professional.
The most notable finding in this study is the economic impact of adherence with maintenance infliximab on hospitalizations and inpatient costs. A significantly lower proportion of adherent patients were hospitalized compared with nonadherent patients. In addition, adherent patients who were hospitalized had a significantly shorter length of hospital stay and significantly lower hospital costs. Another retrospective study used a medical claims database to evaluate the impact of adherence with oral aminosalicylate medications in non-specified gastrointestinal disease. Shaya et al25 demonstrated that nonadherent patients incurred higher costs for hospital admission, outpatient visits, and office visits. Nonadherent patients incurred an additional annual cost of $1875 in total health care expenditures; however, medication adherence was not measured in that study using the MPR method as used in the current study, and the database used in that study was limited to enrollees of a private insurance plan in Maryland.
In the Kane et al29 study that evaluated the impact of adherence on health care utilization and costs (as measured by the number of infusions over one year), nonadherence was also associated with significantly greater CD-related medical costs (94%) and hospitalization costs (250%) as well as outpatient costs (68%) when compared with adherent patients. Although the Integrated Health Care Information Service claims database used in the Kane study is a large database covering 25 million managed care lives, it is a smaller and less diverse database than the IMS LifeLink Database used in the current study. The IMS LifeLink Database includes over 90 health plans from across the US and contributes data from 60 million covered lives. It is considered a more diverse database in terms of health plans and may therefore be more nationally representative of this patient population.
The maintenance of remission and prevention of exacerbations that lead to costly hospitalizations and surgeries are the main goals in CD treatment. Hospitalizations for CD account for approximately half of all direct medical costs (53%–66%), and approximately half of all hospitalized patients will undergo a surgical procedure.7,31–33 The estimated length of hospital stay in the US is 8.0 days for nonsurgical treatment and 9.6 days for surgical treatment.33 The average cost per hospitalization was $37,459 in the US (2006 adjusted dollars) and is rising.7 The cost of hospitalizing patients with CD in the US increased from $762 million to $1330 million dollars (inflation-adjusted) between 1998 and 2004.34 Most importantly, the cost of illness in patients with inflammatory bowel disease who require hospitalization can be 20 times higher than that of ambulatory patients who remain in remission.35 Therefore, the economic burden that results from not maintaining remission in patients with CD is substantial. Therapy that can limit the need for hospitalization and/or surgery may reduce the overall costs of care and improve health-related quality of life in patients with CD.
Several clinical trials and retrospective studies have shown that scheduled maintenance treatment with infliximab resulted in a significant reduction in the number and duration of CD-related hospitalizations, fewer surgeries and procedures, fewer developed antibodies, and higher rates of mucosal healing compared with episodic treatment using infliximab.14–19 A recent retrospective 3-year study of 104 patients with CD demonstrated that patients who continued an uninterrupted maintenance dosing regimen of infliximab had fewer incidences of hospitalization, surgery, and disability than patients who had an irregular or interrupted regimen of infliximab prior to initiating a scheduled maintenance regimen.37 The data from this study expand the findings from previous infliximab studies by demonstrating the importance of maintaining adherence with infliximab in reducing the costs associated with CD-related hospitalizations and inpatient costs in a larger sample.
Only a few CD-related health care services in this study were impacted over 12 months by nonadherence with infliximab. Significant differences in radiology and ancillary outpatient services were noted between adherent and nonadherent patients. Given that ancillary services included billing for intravenous administration of infliximab, it is not surprising that the adherent group with the greater number of infusions would also require more ancillary services. These data also suggest that nonadherence to infliximab impacts utilization of outpatient services to a lesser extent than inpatient costs. Although the administrative and pharmacy costs of any biologic agent may be higher than oral medication costs, these data suggest that adherence to maintenance infliximab is a value-added treatment for CD. The economic benefits of adherence to infliximab may be realized in terms of reduced hospitalizations, length of hospital stay, and inpatient costs. The cost of infliximab administered in the inpatient setting was included in the overall inpatient cost results. The cost of infliximab administered in the outpatient setting was not included. Future analyses may include an assessment of the impact of infliximab on total CD-related health care resource utilization and costs and not limited only to the inpatient setting.
One of the advantages of using an administrative claims database to report pharmacoeconomic outcomes is the ability to examine primary health care data in a real-life setting rather than in a highly controlled clinical trial setting. Another advantage of the current study design over previous adherence studies is that medians (versus means) are reported. The presence of outliers is an inherent flaw in pharmacoeconomic cost analyses. Reporting medians in this type of study reduces the influence of outliers on the dataset.
The limitations of a claims database are well known and include the same biases that occur in any retrospective observational study, including inability to determine reasons for lack of adherence or discontinuation of drug therapy, lack of socioeconomic information, lack of clinical data from medical charts that might be associated with health care costs, and lack of detailed information on disease activity and duration. No statistical adjustments were performed when comparing health care utilization and costs between the adherent and nonadherent groups because baseline characteristics were mostly similar between cohorts; however, similarities in observable characteristics at baseline may not always exist in every adherent and nonadherent CD population. It is recommended that statistical adjustments be made in any CD population where baseline characteristics differ. Unfortunately, no method or mechanism was used in this administrative claims database analysis to adjust for unobservable characteristics (eg, travel distance to site for infliximab infusion or infliximab supply availability to health care professionals) that may have had a role in determining the level of observed adherence. Information regarding the dose of infliximab given at each infusion was not available, so while the infusion patterns were consistent with prescribing recommendations, the actual dose was unknown. Finally, this database contained information largely from commercial payers in the US; therefore, the results may not be generalizable to patients covered by Medicare or Medicaid or to patients outside the US.
Conclusion
In this study, adherence with maintenance infliximab over 12 months was associated with a lower rate of CD-related hospitalizations. Furthermore, among those who were hospitalized, adherence had an observed beneficial economic impact, as evidenced by lower CD-related inpatient costs and a shorter length of hospital stay. Additional comparative studies are needed to compare adherence with more frequently versus less frequently administered therapies for the treatment of CD and their resulting effects on clinical and health economic outcomes.
Footnotes
Disclosure
Financial support for this work was provided by Janssen Scientific Affairs, LLC. The authors acknowledge Kim Poinsett-Holmes at Poinsett Publications, Inc., for assistance with the preparation and editing of this manuscript, as well as Gianna Paone at Janssen Services, LLC, for editorial and submission support. Kim Poinsett-Holmes was reimbursed by Janssen Scientific Affairs, LLC, for her editorial assistance in this work. CTC is an employee of Janssen Scientific Affairs, LLC, as was HCW at the time of the analysis and manuscript preparation (currently employed at S2 Statistical Solutions, Inc., Cincinnati, OH, USA). These data have been previously presented, in part, at the Academy of Managed Care Pharmacy Meeting, October 13–15, 2010, St Louis, MO, and at the 2010 Advances in Inflammatory Bowel Diseases, Crohn’s & Colitis Foundation’s Clinical & Research Conference, December 9–12, 2010, Hollywood, FL.
References
- 1.Loftus EV, Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16(1):51–60. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Von Wietersheim J, Kessler H. Psychotherapy with chronic inflammatory bowel disease patients: a review. Inflamm Bowel Dis. 2006;12(12):1175–1184. doi: 10.1097/01.mib.0000236925.87502.e0. [DOI] [PubMed] [Google Scholar]
- 3.Akobeng AK. Crohn’s disease: current treatment options. Arch Dis Child. 2008;93(9):787–792. doi: 10.1136/adc.2007.128751. [DOI] [PubMed] [Google Scholar]
- 4.Karwowski CA, Keljo D, Szigethy E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(11):1755–1764. doi: 10.1002/ibd.20919. [DOI] [PubMed] [Google Scholar]
- 5.Katz JA. Management of inflammatory bowel disease in adults. J Dig Dis. 2007;8(2):65–71. doi: 10.1111/j.1443-9573.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 6.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135(6):1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu AP, Cabanilla LA, Wu EQ, Mulani PM, Chao J. The costs of Crohn’s disease in the United States and other Western countries: a systematic review. Curr Med Res Opin. 2008;24(2):319–328. doi: 10.1185/030079908x260790. [DOI] [PubMed] [Google Scholar]
- 8.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targan SR, Hanauer SB, van Deventer SJH, et al. the Crohn’s Disease cA2 Study Group. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. N Engl J Med. 1997;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 10.Hanauer SB, Feagan BG, Lichtenstein GR, et al. the ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 11.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 12.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340(18):1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein GR, Hanauer SB, Sandborn WJ the Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104(2):465–483. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128(4):862–869. doi: 10.1053/j.gastro.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Colombel JF, Rutgeerts P, Yan S, et al. Infliximab (Remicade) maintenance treatment results in lower hospitalization rate in Crohn’s disease patients. Poster presented at Digestive Disease Week; May 19–22, 2002; San Francisco, CA. [Google Scholar]
- 16.Williams JB, Cross RK, Thameen D, et al. Long-term infliximab maintenance infusion regimens and rates of hospitalization, surgery and disability in Crohn’s disease patients [Abstract] Gastroenterology. 2005;128(4 Suppl 2):A-589. [Google Scholar]
- 17.Williams JB, Weber LR, Beaulieu DB, et al. Long-term infliximab maintenance infusion regimens and rates of hospitalization, surgery, and disability in Crohn’s disease [Abstract] Gastroenterology. 2006;130(4 Suppl 2):A-143. [Google Scholar]
- 18.Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58(4):492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 19.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126(2):402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 20.PharMetrics Inc. LifeLink Health Plan Claims Database. Watertown, MA: IMS Health; 2010. [Google Scholar]
- 21.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 22.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 23.Bokemeyer B, Teml A, Roggel C, et al. Adherence to thiopurine treatment in out-patients with Crohn’s disease. Aliment Pharmacol Ther. 2007;26(2):217–225. doi: 10.1111/j.1365-2036.2007.03365.x. [DOI] [PubMed] [Google Scholar]
- 24.Mantzaris GJ, Roussos A, Kalantzis C, Koilakou S, Raptis N, Kalantzis N. How adherent to treatment with azathioprine are patients with Crohn’s disease in long-term remission? Inflamm Bowel Dis. 2007;13(4):446–450. doi: 10.1002/ibd.20041. [DOI] [PubMed] [Google Scholar]
- 25.Shaya FT, El Khoury AC, Wong W, et al. Persistence with pharmacotherapy for gastrointestinal disease: associated costs of health care. PT. 2006;31(11):657–665. [Google Scholar]
- 26.Higgins PD, Rubin DT, Kaulback K, Schoenfield PS, Kane SV. Systematic review: impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther. 2009;29(3):247–257. doi: 10.1111/j.1365-2036.2008.03865.x. [DOI] [PubMed] [Google Scholar]
- 27.Kane S, Shaya F. Medication non-adherence is associated with increased medical health care costs. Dig Dis Sci. 2008;53(4):1020–1024. doi: 10.1007/s10620-007-9968-0. [DOI] [PubMed] [Google Scholar]
- 28.Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105(3):525–539. doi: 10.1038/ajg.2009.685. [DOI] [PubMed] [Google Scholar]
- 29.Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn’s disease patients. Adv Ther. 2009;26(10):936–946. doi: 10.1007/s12325-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 30.Tang B, Rahman M, Waters HC, Callegari P. Treatment persistence with adalimumab, etanercept, or infliximab in combination with methotrexate and the effects on health care costs in patients with rheumatoid arthritis. Clin Ther. 2008;30(7):1375–1384. doi: 10.1016/s0149-2918(08)80063-x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen RD, Larson LR, Roth JM, Becker RV, Mummert LL. The cost of hospitalization in Crohn’s disease. Am J Gastroenterol. 2000;95(2):524–530. doi: 10.1111/j.1572-0241.2000.01779.x. [DOI] [PubMed] [Google Scholar]
- 32.Blomqvist P, Ekbom A. Inflammatory bowel disease: health care and costs in Sweden in 1994. Scand J Gastroenterol. 1997;32(11):1134–1139. doi: 10.3109/00365529709002993. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein CN, Papineau N, Zajaczkowski J, Rawsthorne P, Okrusko G, Blanchard JF. Direct hospital costs for patients with inflammatory bowel disease in a Canadian tertiary care university hospital. Am J Gastreoenterol. 2000;95(3):677–683. doi: 10.1111/j.1572-0241.2000.01845.x. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen GC, Tuskey A, Dassopoulos T, Harris ML, Brant SR. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13(12):1529–1535. doi: 10.1002/ibd.20250. [DOI] [PubMed] [Google Scholar]
- 35.Bassi A, Dodd S, Williamson P, Bodger K. Cost-of-illness of inflammatory bowel disease in the United Kingdom: a single-centre retrospective study. Gut. 2004;53(10):1471–1478. doi: 10.1136/gut.2004.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 37.Stein DJ, Ananthakrishnan AN, Issa M, et al. Impact of prior irregular infliximab dosing on performance of long-term infliximab maintenance therapy in Crohn’s disease. Inflamm Bowel Dis. 2010;16(7):1173–1179. doi: 10.1002/ibd.21164. [DOI] [PubMed] [Google Scholar]