Abstract
Background
Cardiorespiratory fitness (CRF) in adults decreases with age and is influenced by lifestyle. Low CRF is associated with risk of diseases and the ability of older persons to function independently. We defined the longitudinal rate of CRF decline with aging and the association of aging and lifestyle with CRF.
Methods
We studied a cohort of 3429 women and 16 889 men, aged 20 to 96 years, from the Aerobics Center Longitudinal Study who completed 2 to 33 health examinations from 1974 to 2006. The lifestyle variables were body mass index, self-reported aerobic exercise, and smoking behavior. Cardiorespiratory fitness was measured by a maximal Balke treadmill exercise test.
Results
Linear mixed models regression analysis stratified by sex showed that the decline in CRF with age was not linear. After 45 years of age, CRF declined at an accelerated rate. For each unit of increase in body mass index, the CRF of women declined 0.20 metabolic equivalents (METs) (95% confidence interval, −0.21 to −0.19); that of men, 0.32 METs (−0.33 to −0.20). Current smokers of both sexes also had lower CRF (−0.29 METs [95% confidence interval, −0.40 to −0.19] for women and −0.41 METS [−0.44 to −0.38] for men). Cardiorespiratory fitness was positively associated with self-reported physical activity.
Conclusions
Cardiorespiratory fitness in men and women declines at a nonlinear rate that accelerates after 45 years of age. Maintaining a low BMI, being physically active, and not smoking are associated with higher CRF across the adult life span.
The U.S. population is aging1 and is becoming more obese2 and sedentary.3 It is well documented that the cardiorespiratory fitness (CRF) of men and women declines with age and that body composition and habitual physical activity are related to CRF. Using men and women from the Baltimore Longitudinal Study of Aging, Fleg et al4 reported that the age-related, longitudinal decline in CRF was not linear. They observed a decline in peak oxygen consumption per unit of time (V̇O2) of 3% to 6% per decade for the third and fourth decades of life, but after 70 years of age, the rate accelerated to more than 20% per decade. Furthermore, they reported that the accelerated rate of decline in CRF with aging was not affected by variation in habitual physical activity, but that at all ages the more active individuals were more fit.
An important public health research topic is the age-related rate of decline because CRF is associated with the risk of morbidity and mortality and with quality of life, preservation of function, and independence. Data from the Aerobics Center Longitudinal Study (ACLS) and other epidemiologic studies indicate that individuals with low CRF are much more likely to develop hypertension,5–7 diabetes,6,8–10 and metabolic syndrome6,11,12 and to have higher rates of death due to cardiovascular disease,13,14 cancer,15,16 and all causes13,14,16–18 during follow-up. A V̇O2max of no greater than 18 mL/kg per minute has been identified by the US Social Security Administration19 as indicating disability and is used as a threshold value of independent living.
The ACLS cohort includes large numbers of women and men with serial health examinations that provide lifestyle, clinical, and maximal treadmill exercise test data. The purposes of this study were (1) to define the role of age and lifestyle characteristics of body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), self-reported physical activity index (PAI), and smoking habit on the longitudinal change in CRF; and (2) to develop a model to define the combined role of aging and lifestyle on the longitudinal CRF change.
METHODS
STUDY POPULATION
The ACLS is an open cohort study. From 1974 to 2006, 3767 women and 18 083 men underwent at least 2 comprehensive medical examinations and maximal graded treadmill exercise tests at the Cooper Clinic in Dallas, Texas, and were enrolled in the ACLS. Participants came to the clinic for periodic preventive health examinations and for counseling regarding diet, exercise, and other lifestyle factors associated with increased risk of chronic disease. Participants were volunteers, not paid, and not recruited to the study as they would be for a clinical trial. Many were sent by their employers for the examination, some were referred by their physicians, and others were self-referred. Participants were excluded from the present study if they were younger than 20 years (n = 30); failed to achieve at least 85% of age-predicted maximal heart rate during the treadmill test (220 minus the age in years) (n = 113); had an abnormal finding on a resting or an exercise electrocardiogram (n = 388); reported a history of myocardial infarction (n = 57), stroke (n = 9), diabetes (n = 170), or hypertension (n = 737); or had missing information on any of the variables included in the analysis (n = 28). These criteria resulted in a study population of 3429 women and 16 889 men aged 20 to 96 years who were followed up from the date of the baseline examination until the date of the last examination. Because we excluded individuals with chronic disease, those who failed to achieve at least 85% of their estimated maximal heart rate on the treadmill test, and those with abnormal exercise test results, the study population was healthier than the overall cohort.
The total number of tests completed was 11 749 for women and 70 978 for men. The number of tests completed by each participant ranged from 2 to 23 (mean, 3.4; median, 2.0) for women and 2 to 33 (mean, 4.2; median, 3.0) for men. The mean (median) number of tests per year of follow-up time was 1.8 (1.3) for women and 1.6 (1.2) for men. The mean (median) length of follow-up was 6.1 (4.0) years for women and 6.6 (4.0) years for men. Participants were predominantly white, well educated, and belonged to the middle and upper socioeconomic strata. Participants signed an informed consent for the clinical examinations and follow-up, and the study was reviewed and approved annually by the institutional review board of the Cooper Institute.
CLINICAL EXAMINATION
A Balke maximal exercise test on a treadmill was used to measure CRF.17,20 Patients were encouraged to give a maximal effort, and the test end point was volitional exhaustion or termination by the physician for medical reasons. The speed and elevation of the final minute of the treadmill test was used to convert treadmill performance to metabolic equivalents (METs).21 One MET is an oxygen uptake of 3.5 mL/kg per minute, the energy expenditure at a resting state. Exercise treadmill duration on this protocol is highly correlated (r ≥ 0.92) with measured peak oxygen uptake in women22 and men.23 Treadmill time expressed in METs is analogous to maximal aerobic power (peak V̇O2) and is used herein as an objective laboratory measure of CRF. The examination included the measurement of resting and exercise electrocardiograms and blood pressure. Trained laboratory technicians, with physician supervision, administered the exercise tests and other procedures according to a standardized manual of operations. Resting heart rate was measured with the participants recumbent after a 5-minute rest and was obtained from the electrocardiogram.24,25 Maximal heart rate was the highest value during exercise recorded from the electrocardiogram during the exercise test. The mean (SD) percentage of age-predicted maximal heart rate achieved during exercise was 102.3 (6.6) beats/min in women and 103.0 (6.8) beats/min in men.
The lifestyle-related variables included in the present analyses were body composition, PAI, and smoking behavior. Height and weight were measured on a physician’s scale and stadiometer at the time of each treadmill test. The BMI was used as a proxy for body composition. Smoking habits were obtained from a standardized questionnaire. The patient was classified as a nonsmoker or current smoker at the time of each examination. The self-reported PAI consisted of 5 categories based on the patients’ responses to questions in the medical history questionnaire about their regular physical activity habits during the past 3 months. If they reported participation in an activity, the patient was asked to provide additional information about the type of activity, number of times per week, duration of each exercise session, and distance or time spent in the activity. No regular activity was considered sedentary (PAI=0). Among those who reported any regular exercise, the following 4 levels of activity were defined: high, those who walked or jogged more than 20 miles per week (PAI = 4); moderate, those who walked or jogged between 10 and 20 miles per week (PAI = 3); low, those who walked or jogged up to 10 miles per week (PAI = 2); and other, those who participated in some other regular physical activity such as bicycling, swimming, racquet sports, and other strenuous sports, but not walking or jogging (PAI = 1). Walking and jogging were chosen as the basis for the PAI used herein because it was the most common activity for this population. This method of PAI classification has been discussed in previous reports from our group.16,25 We used a PAI of moderate level in all models to illustrate the effect of physical activity. Assuming individuals walked at a rate of 3.3 mph, a PAI of 3 represents a level of aerobic exercise of 200 minutes or more per week. This level of exercise is slightly higher than the recent consensus recommendation for physical activity in adults by the Department of Health and Human Services.26
STATISTICAL ANALYSES
Linear mixed models (LMM) regression was used to analyze the longitudinal data. A random intercept growth model27,28 was used to define the longitudinal age-associated change in CRF. The patient’s age was the measure of time for the growth model. The major advantage of LMM is that it accommodates unbalanced, unequally spaced observations over time, making it an ideal method to model longitudinal data. Stata software, version 10 xtmixed program,29 was used for all LMM analyses. The dependent variable for each LMM analysis was maximal treadmill time expressed in METs. The first model examined the nonlinear longitudinal change in CRF due to aging. The second model added the lifestyle variables to the age model. A log-likelihood ratio test was used to determine whether the model improved the fit.27–29 The variables of the LMM fixed-effects portion of the model were age, age2, BMI, PAI, and smoking behavior. Age and BMI were continuous variables, and smoking behavior and PAI were categorical. The smoking referent was current nonsmoking, and the physical activity referent was a PAI of 0. Each fixed-effect regression coefficient was tested with a z statistic to determine whether it was significantly different from 0.27,29 The random part of the LMM consisted of the serial treadmill tests. Published ACLS results have shown that low and moderate CRF increase the risk of adverse health outcomes in women and men 60 years or older. The cut points for low and moderate fitness are 6 and 7 METs in women and 7 and 8 METs in men.30,31 The LMM modeled data were used to examine the influence of lifestyle and aging on reaching these CRF cut points. The analyses were stratified by sex. Statistical tests were 2 sided, and P < .05 was accepted to indicate statistical significance.
RESULTS
Table 1 gives the descriptive statistics of all observations of women and men, contrasted by age group. Ages ranged from 20 to 83 years for the women and 20 to 96 years for the men. The men were taller, heavier, and had higher levels of CRF. The BMI of the men was higher than that for the women across all of the age groups. Just 20.5% of female observations exceeded a BMI of 25 or greater compared with 56.1% of men. For both men and women, the treadmill time was shorter and the prevalence of smoking was lower in the older groups. Approximately 21% of women and men reported that they did not engage in any regular physical activity (sedentary), whereas 22.9% of women and 26.6% of men reported that they were moderately active (PAI of 3 and 4).
Table 1.
Descriptive Statistics for All Observations of Women and Men According to Age Group: Aerobics Center Longitudinal Study, 1974–2006a
| Variable | All Observations | Observations by Age Group, y
|
||||
|---|---|---|---|---|---|---|
| <40 | 40–49 | 50–59 | 60–69 | ≥70 | ||
| Women | ||||||
| No. of observations | 11 749 | 2214 | 4056 | 3633 | 1582 | 264 |
| Age, y | 48.3 (10.3) | 34.3 (4.4) | 44.6 (2.9) | 54.1 (2.8) | 63.3 (2.7) | 72.7 (2.7) |
| Height, cm | 164.3 (5.9) | 165.1 (6.1) | 164.6 (5.9) | 164.1 (5.8) | 163.3 (5.7) | 160.8 (5.7) |
| Weight, kg | 61.9 (10.0) | 60.3 (10.2) | 62.1 (10.4) | 62.6 (9.8) | 62.4 (9.0) | 60.3 (8.9) |
| BMI | 22.9 (3.4) | 22.1 (3.4) | 22.9 (3.5) | 23.2 (3.4) | 23.4 (3.3) | 23.3 (3.2) |
| CRF, METs | 10.0 (2.0) | 11.1 (2.1) | 10.4 (1.9) | 9.6 (1.8) | 8.8 (1.6) | 8.1 (1.5) |
| Maximal HR, %b | 102.3 (6.6) | 99.9 (5.3) | 101.9 (6.1) | 103.1 (6.7) | 104.9 (7.6) | 104.0 (8.7) |
| PAI, % of participantsc | ||||||
| 0 | 21.3 | 20.4 | 20.5 | 21.6 | 22.6 | 30.7 |
| 1 | 25.4 | 24.9 | 24.8 | 26.0 | 25.9 | 28.4 |
| 2 | 30.4 | 34.3 | 30.9 | 29.2 | 26.9 | 25.4 |
| 3 | 16.7 | 14.1 | 17.0 | 17.3 | 19.2 | 10.6 |
| 4 | 6.2 | 6.3 | 6.7 | 5.9 | 5.5 | 4.9 |
| Current smoker, % of participants | 5.5 | 9.4 | 6.1 | 4.1 | 2.8 | 1.1 |
|
| ||||||
| Men | ||||||
| No. of observations | 70 978 | 12 595 | 25 101 | 22 412 | 9041 | 1829 |
| Age, y | 49.0 (10.1) | 34.8 (3.8) | 44.7 (2.8) | 54.1 (2.8) | 63.4 (2.7) | 73.4 (3.5) |
| Height, cm | 179.0 (6.4) | 179.9 (6.6) | 179.5 (6.4) | 178.6 (6.2) | 177.5 (6.2) | 175.4 (6.3) |
| Weight, kg | 83.0 (12.0) | 82.9 (12.6) | 83.9 (12.1) | 83.5 (11.8) | 80.8 (10.9) | 75.8 (10.0) |
| BMI | 25.9 (3.3) | 25.6 (3.4) | 26.0 (3.3) | 26.1 (3.2) | 25.6 (3.0) | 24.6 (2.8) |
| CRF, METs | 12.3 (2.3) | 13.2 (2.4) | 12.7 (2.2) | 12.0 (2.1) | 11.2 (2.0) | 10.0 (1.9) |
| Maximal HR, %b | 103.0 (6.8) | 100.6 (5.4) | 102.4 (6.0) | 103.8 (6.9) | 105.3 (7.9) | 106.7 (9.3) |
| PAI, % of participantsc | ||||||
| 0 | 21.1 | 23.8 | 21.1 | 20.0 | 19.3 | 23.8 |
| 1 | 21.8 | 20.9 | 21.4 | 21.9 | 23.7 | 21.3 |
| 2 | 30.5 | 31.3 | 30.8 | 31.1 | 28.7 | 24.0 |
| 3 | 18.2 | 15.6 | 17.9 | 19.0 | 20.4 | 21.9 |
| 4 | 8.4 | 8.5 | 8.8 | 8.1 | 7.9 | 9.0 |
| Current smoker, % of participants | 11.7 | 16.3 | 13.3 | 10.6 | 5.1 | 3.4 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CRF, cardiorespiratory fitness; HR, heart rate; METs, metabolic equivalents; PAI, physical activity index.
Unless otherwise indicated, data are expressed as mean (SD).
Indicates percentage of age-adjusted maximal HR attained on the treadmill test (maximal HR on the treadmill test/age-predicted maximal HR [220 minus the age in years]).
A rating of 0 indicates no reported activities; 1, Participated in some other activity such as bicycling, swimming, racquet sports, and other strenuous sports but not walking or jogging; 2, walked or jogged up to 10 miles/wk; 3, walked or jogged between 10 and 20 miles/wk; 4, walked or jogged more than 20 miles/wk. Because of rounding, percentages may not total 100.
Table 2 presents the LMM analyses for women and men. The nonlinear model for aging alone is given in LMM I. The lifestyle variables, which provided a more accurate fit than LMM I (P < .001), are provided in LMM II. All regression coefficients for the men’s and women’s models were statistically significant, showing that the lifestyle variables were related to CRF independent of aging (ie, after accounting for age). The LMM regression coefficients for each PAI category became progressively larger. The women’s PAI regression weights were 0.27 METs (95% confidence interval [CI], 0.22–0.33) for a PAI of 1, 0.36 METs (0.30–0.41) for a PAI of 2, 0.77 METs (0.70–0.83) for a PAI of 3, and 1.22 METs (1.13–1.32) for a PAI of 4. The PAI regression weights for men were slightly higher than those for the women. The PAI regression weights for men were 0.37 METs (95% CI, 0.35–0.40) for a PAI of 1, 0.51 METs (0.48–0.53) for a PAI of 2, 1.03 METs (1.00–1.06) for a PAI of 3, and 1.48 METs (1.44–1.52) for a PAI of 4. Each unit of increase in BMI was associated with a decrease in treadmill time of 0.20 METs for women (95% CI, −0.21 to −0.19) and 0.32 METs (−0.33 to −0.20) for men. Smoking behavior was related to CRF independent of age, PAI, and BMI. Being a current smoker was associated with lower CRF, at −0.29 METs (95% CI, −0.40 to −0.19) for women and −0.41 METs (−0.44 to −0.38) for men.
Table 2.
Maximum Likelihood Estimates for Models Including the Random Intercept of the Women and Men: Aerobics Center Longitudinal Study, 1974–2006
| Variable | Women
|
Men
|
||||||
|---|---|---|---|---|---|---|---|---|
| LMM I
|
LMM II
|
LMM I
|
LMM II
|
|||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed part | ||||||||
| Constant | 9.05a | 0.26 | 12.77a | 0.25 | 10.46a | 0.12 | 17.26a | 0.12 |
| Age | 0.10a | 0.01 | 0.10a | 0.01 | 0.14a | 0.005 | 0.16a | 0.004 |
| Age2 | −0.002 | 0.0001 | −0.002a | 0.0001 | −0.002a | 0.0001 | −0.002a | 0.0001 |
| BMI | −0.20a | 0.01 | −0.32a | 0.003 | ||||
| PAIb | ||||||||
| 1 | 0.27a | 0.03 | 0.37a | 0.01 | ||||
| 2 | 0.36a | 0.03 | 0.51a | 0.01 | ||||
| 3 | 0.77a | 0.03 | 1.03a | 0.01 | ||||
| 4 | 1.22a | 0.05 | 1.48a | 0.02 | ||||
| Current smoker | −0.29a | 0.05 | −0.41a | 0.02 | ||||
| Random part | ||||||||
| SD intercept | 1.66 | 0.02 | 1.42 | 0.02 | 1.97 | 0.01 | 1.57 | 0.01 |
| SD error | 0.88 | 0.01 | 0.82 | 0.01 | 1.05 | <0.01 | 0.92 | <0.01 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LMM I, linear mixed models due to aging; LMM II, LMM due to aging controlled for lifestyle; PAI, physical activity index; SD error, standard error of the LMM model; SD intercept, standard deviation of the individual variance of the random part of the model.
P < .001.
See the footnote in Table 1 for an explanation of the PAI ratings.
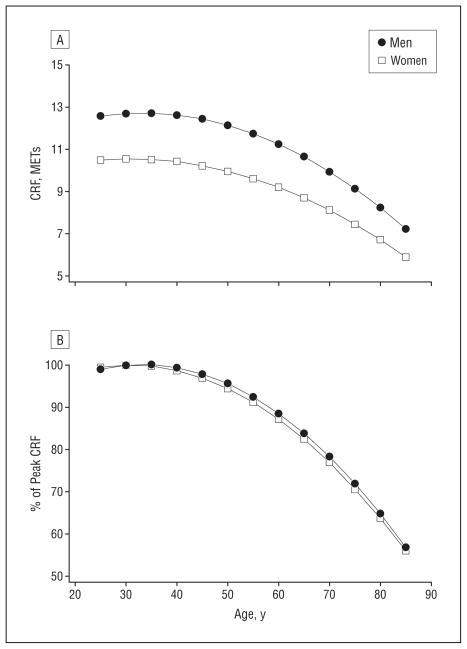
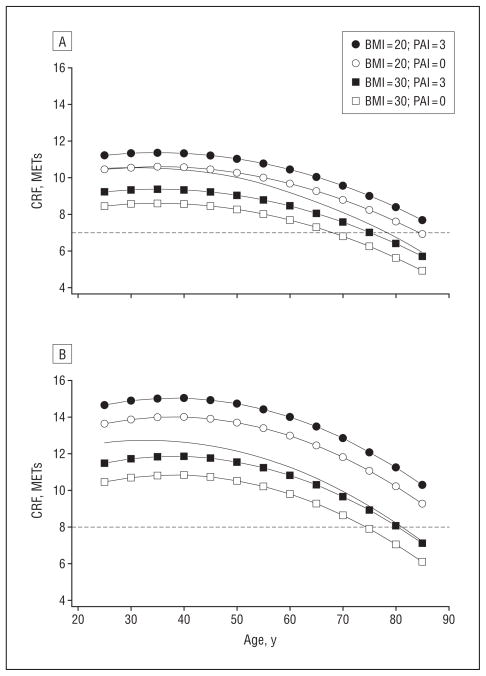
Figure 1 gives the nonlinear longitudinal decline in CRF associated with aging for men and women. Figure 1A shows the decline in METs and demonstrates the well-documented sex difference in CRF. The MET data shows that the CRF of men and women declined at an accelerated rate after 45 years of age and the decline for men was greater than that for women. Figure 1B shows the age decline in CRF represented as a percentage of peak CRF. The women and men’s changes in CRF expressed as a percentage of peak CRF were nearly identical. Figure 2 illustrates the role of lifestyle on the age-related change in CRF. Provided in the figure is the longitudinal change in CRF from 25 to 85 years of age for BMI values of 20 and 30 associated with PAI values of 0 (inactive) and 3 (moderate). Provided for reference as a dashed line is the cut point for moderate CRF, which is 7 METs for women and 8 METs for men. The trends in Figure 2 were developed with LMM II, and it was assumed that the patients did not smoke. The BMI values were arbitrarily selected to illustrate the combined associations of BMI and level of physical activity on CRF.
Figure 1.
Age-related cardiorespiratory fitness (CRF) changes in women and men. Changes are expressed in metabolic equivalents (METs) (A) and as a percentage of peak CRF (B).
Figure 2.
Quadratic trends in cardiorespiratory fitness (CRF) for selected body mass index values (BMI; calculated as weight in kilograms divided by height in meters squared) and self-reported physical activity index (PAI). The conditions are defined with linear mixed models (LMM) II, controlling for lifestyle, for women (A) and men (B). An explanation of PAI is given in the “Clinical Examination” subsection of the “Methods” section. Provided for reference are the nonlinear regression line at which lifestyle is not statistically controlled (solid line) and the Aerobics Center Longitudinal Study moderate CRF levels (for women, 7 metabolic equivalents [METs] and for men, 8 METs) associated with higher risk of adverse health outcomes (dashed line).
Table 3 gives the decline in CRF for men and women per decade of age (≥40 years) for LMM I and lifestyle-controlled (LMM II) models. These data show the accelerated rate of decline in CRF with aging. The graphs provided in Figure 2 for women (A) and men (B) were similar and documented 3 important trends. First, with lifestyle factors statistically controlled, CRF declined at a nonlinear rate. Second, assuming that BMI and activity level did not change, the CRF advantage of being lean and active was constant during the age range studied. Third, inactive patients with a high BMI can be expected to reach the health-related ACLS level of moderate CRF at a younger age than inactive patients with a lower BMI.
Table 3.
Change in CRF per LMM I and LMM II: Aerobics Center Longitudinal Study, 1974–2006a
| Age Group, y | Women
|
Men
|
||
|---|---|---|---|---|
| LMM I | LMM II | LMM I | LMM II | |
| 40–49 | −0.45 | −0.29 | −0.48 | −0.30 |
| 50–59 | −0.77 | −0.59 | −0.89 | −0.73 |
| 60–69 | −1.08 | − −0.89 | −1.31 | −1.16 |
| 70–79 | −1.40 | −1.18 | −1.71 | −1.59 |
Abbreviations: CRF, cardiorespiratory fitness; LMM I, linear mixed models due to aging; LMM II, LMM due to aging controlled for lifestyle.
Data are expressed as metabolic equivalents.
COMMENT
These results showed that the longitudinal decline in CRF of women and men in the ACLS was not linear. These ACLS results confirmed that lifestyle was related to CRF independent of aging. With lifestyle statistically controlled, the nonlinear decline in CRF with aging remained. The LMM II results showed that being active, keeping a normal BMI, and not smoking were associated with substantially higher levels of CRF during the adult life span studied. Being inactive and having a high BMI were associated with a lower age at which an individual could be expected to reach threshold CRF levels associated with substantially higher health risks. The large number of men and women and the age variability of the ACLS cohort support the stability and fidelity of these findings. Although the ages of about 66% of all of the men and women in the ACLS ranged from 40 to 60 years, 1846 women and nearly 11 000 men were 60 years or older at the time of testing.
The major finding of these ACLS data was consistent with the results reported by Fleg and associates,4 who studied 435 men and 375 women in the Baltimore Longitudinal Study of Aging. Their patients ranged in age from 21 to 87 years, and each underwent testing 2 or more times, providing more than 2000 observations. Fleg et al4 measured oxygen uptake using the same Balke treadmill test protocol as in the ACLS. Both studies defined a maximal test result as one for which the patient’s measured exercise heart rate was 85% or more of the age-predicted maximal heart rate. Although measured peak V̇O2 is the preferred CRF measure, maximal treadmill performance is highly correlated (r ≥ 0.92) with peak V̇O222,23,32 Even with the different CRF measures, both studies produced similar results: after 45 years of age, CRF declined at an accelerated rate. Fleg and associates4 combined the data of men and women into 1 model and reported that the rate of decline for men at each decade was larger than that for women. We did not test for a sex difference, but Figures 1 and 2 suggest that, after approximately 45 years of age, the rate of decline in CRF expressed in METs was steeper for men than for women. These results are consistent with the 10-year longitudinal data reported by Stathokostas et al,33 who reported a mean longitudinal yearly change in V̇O2max of −0.19 mL/kg per minute per year for 28 women and −0.43 mL/kg per minute per year for 34 men. Figure 1B documents that the rates of decline (expressed as a percentage of peak CRF) for men and women were almost identical. At 80 years of age, the CRF of women and men was 64% and 65% of peak aerobic capacity, respectively. A major difference between our results and the results of Fleg et al4 is that the ACLS model defines the influence of common lifestyle variables on the longitudinal decline in CRF associated with age. The model provides a means to assess the relative CRF from readily obtainable, inexpensive clinical data.
Low CRF is associated not only with higher morbidity and mortality but also with loss of independence. The CRF value identified by the US Social Security Administration19 as the threshold for the CRF level of not being able to live independently is 18 mL/kg per minute or less (≤5.1 METs). The finding that BMI and a habitual level of physical activity are important determinants of CRF raises a lifestyle concern for the older adult population. A high percentage of US adults do not engage in regular physical activity,3 and data from the 2003–2004 National Health and Nutrition Examination Survey2 show that obesity (BMI≥30) prevalence in US women and men are 33.2% and 31.1%, respectively. Nearly 7% of women and 3% of men are extremely obese (BMI ≥40). These results suggest that, with the increased prevalence of obesity, more inactive men and women will reach the Social Security Administration threshold of disability at a younger age. These findings demonstrate the role lifestyle has on quality of life and function. These data indicate the need for physicians to recommend to their patients the necessity to maintain their weight, engage in regular aerobic exercise, and abstain from smoking. Assuming one walks at a comfortable speed of approximately 3 mph, a 30-minute walk each day of the week would amount to approximately 10 miles per week. Data in Table 1 document that about 22.9% of women and 26.6% of men reported that they exercised this much or more.
The major strengths of this study are the use of very large samples of women and men who varied in age across the adult life span and an objective measure of CRF. A major limitation is that the ACLS cohort consists of mostly white patients who are well educated, are members of the middle and upper socioeconomic strata, and had access to health care. Another limitation is that, although BMI and PAI are commonly used in public health research, they are not precise measures of body composition and habitual exercise behavior. While BMI is an objective measure, it does not distinguish between fat and fat-free mass. The PAI is a self-report measure and is subject to misclassification. In addition, the PAI is a single measure representing exercise behavior for a 3-month period before the examinations. Even with this limitation, the PAI regression coefficients weights were all statistically significant and the weights became progressively larger with each increase in activity category. Cross-sectional research34,35 documents that self-report measures of physical activity are correlated with measured peak V̇O2. We do not have information on the occurrence of independent living to compare with the MET cut point. Future longitudinal studies need to investigate this issue.
Generalization of the findings of this retrospective study may apply only to well-educated, white, middle–to upper–socioeconomic status individuals who have access to health care. However, the ACLS population is not substantially different from other large and well-characterized cohorts of free-living women and men in regard to self-reported exercise habits, fitness, chronic disease, and several important clinical variables.36 An advantage of the homogeneity of our sample is that it strengthens the internal validity. Finally, the PAI may result in some misclassification, as is the case with all self-reports of lifestyle habits.
In summary, CRF declines at a nonlinear rate that accelerates after 45 years of age in both men and women. Maintaining a normal weight, being physically active, and not smoking are associated with higher CRF across the adult life span.
Acknowledgments
Funding/Support: The ACLS was supported by grants AG06945 and HL62508 from the National Institutes of Health.
Role of the Sponsor: The funding organization played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of data; or the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Drs Blair and Sui had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Jackson, Hébert, Church, and Blair. Acquisition of data: Blair. Analysis and interpretation of data: Jackson, Sui, Hébert, and Church. Drafting of the manuscript: Jackson and Church. Critical revision of the manuscript for important intellectual content: Sui, Hébert, Church, and Blair. Statistical analysis: Jackson, Sui, Hébert, and Church. Obtained funding: Blair. Administrative, technical, and material support: Hébert.
Additional Contributions: The Cooper Clinic physicians and technicians collected the baseline data, and the staff at the Cooper Institute provided data entry and data management. Raymond Fowler, PhD, provided constructive comments on an early draft of the manuscript, and no compensation has been provided.
References
- 1.Lurie N. Healthy People 2010: setting the nation’s public health agenda. Acad Med. 2000;75(1):12–13. doi: 10.1097/00001888-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee [published correction appears in Circulation. 2007;115(5):e172] Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 5.Barlow CE, LaMonte MJ, FitzGerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163(2):142–150. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 6.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290(23):3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 7.Sawada S, Tanaka H, Funakoshi M, Shindo M, Kono S, Ishiko T. Five year prospective study on blood pressure and maximal oxygen uptake. Clin Exp Pharmacol Physiol. 1993;20(7–8):483–487. doi: 10.1111/j.1440-1681.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT, Craig CL, Gauvin L. Adiposity, physical fitness and incident diabetes: the Physical Activity Longitudinal Study. Diabetologia. 2007;50(3):538–544. doi: 10.1007/s00125-006-0554-3. [DOI] [PubMed] [Google Scholar]
- 9.Lynch J, Helmrich SP, Lakka TA, et al. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non–insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med. 1996;156 (12):1307–1314. [PubMed] [Google Scholar]
- 10.Sui X, Hooker SP, Lee IM, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31(3):550–555. doi: 10.2337/dc07-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25(9):1612–1618. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- 12.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112(4):505–512. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 13.Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 14.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 15.Evenson KR, Stevens J, Cai J, Thomas R, Thomas O. The effect of cardiorespiratory fitness and obesity on cancer mortality in women and men. Med Sci Sports Exerc. 2003;35(2):270–277. doi: 10.1249/01.MSS.0000053511.02356.72. [DOI] [PubMed] [Google Scholar]
- 16.Kampert JB, Blair SN, Barlow CE, Kohl HW., III Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6(5):452–457. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 17.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 18.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353(5):468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 19.Social Security Administration. Disability Evaluation Under Social Security. Baltimore, MD: Social Security Administration Office of Disability Programs; 2005. SSA publication 64–039. [Google Scholar]
- 20.Balke B. A simple field test for assessment of physical fitness: rep 63-6. Rep Civ Aeromed Res Inst US. 1963 Apr;:1–8. [PubMed] [Google Scholar]
- 21.ACSM. Guidelines For Exercise Testing and Prescription. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 22.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103(3):363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 23.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92(1):39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 24.Cheng YJ, Lauer MS, Earnest CP, et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003;26(7):2052–2057. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y, Macera CA, Davis DR, Ainsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: is physical activity a risk factor? J Clin Epidemiol. 2000;53(3):315–322. doi: 10.1016/s0895-4356(99)00168-7. [DOI] [PubMed] [Google Scholar]
- 26.2008 Physical activity guidelines for Americans. US Department of Health & Human Services; 2008. [Accessed October 7, 2008]. Web site. http://www.health.gov/paguidelines/ [Google Scholar]
- 27.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 2. College Station, TX: Stata Press; 2008. [Google Scholar]
- 28.Twisk J. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. New York, NY: Cambridge University Press; 2003. [Google Scholar]
- 29.STATA User’s Guide: Release 10. College Station, TX: StataCorp; 2007. [Google Scholar]
- 30.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165(12):1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298(21):2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster C, Jackson AS, Pollock ML, et al. Generalized equations for predicting functional capacity from treadmill performance. Am Heart J. 1984;107(6):1229–1234. doi: 10.1016/0002-8703(84)90282-5. [DOI] [PubMed] [Google Scholar]
- 33.Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH. Longitudinal changes in aerobic power in older men and women. J Appl Physiol. 2004;97(2):781–789. doi: 10.1152/japplphysiol.00447.2003. [DOI] [PubMed] [Google Scholar]
- 34.Jackson AS, Beard EF, Wier LT, Ross RM, Stuteville JE, Blair SN. Changes in aerobic power of men, ages 25–70 yr. Med Sci Sports Exerc. 1995;27(1):113–120. [PubMed] [Google Scholar]
- 35.Jackson AS, Wier LT, Ayers GW, Beard EF, Stuteville JE, Blair SN. Changes in aerobic power of women, ages 20–64 yr. Med Sci Sports Exerc. 1996;28(7):884–891. doi: 10.1097/00005768-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness: evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129(6):1145–1156. doi: 10.1093/oxfordjournals.aje.a115236. [DOI] [PubMed] [Google Scholar]