Abstract
Sleep disturbance is an early marker for bipolar disorder (BD) onset in youth. We characterized sleep quality in adolescents experiencing mania within the last 6–12 months. We examined the association between mood and sleep in 27 adolescents with BD and 24 matched healthy controls (HC). Subjects were assessed by parent and teen report of sleep, a semi-structured clinical interview, the Young Mania Rating Scale (YMRS), and the Childhood Depression Rating Scale (CDRS-R). Average BD youth YMRS (mean 20.3 ± 7.3) and CDRS-R (mean 42.4 ± 14.1) scores indicated they were still ill at time of assessment. Compared to HCs, adolescents with BD have distinct patterns of prolonged sleep onset latency, frequent nighttime awakenings, and increased total time awake. Mood symptoms, specifically excessive guilt, self-injurious behavior, and worsening evening mood, interfered with sleep. Further studies are needed to determine whether early regulation of sleep would improve long-term outcome in BD youth.
Keywords: Bipolar disorder, Adolescents, Sleep, Mood, Parent–child report
Introduction
Pediatric bipolar disorder (BD) is a chronic and debilitating illness associated with frequent mood episode relapse, psychosocial impairment, and substance use [1–7]. Children with bipolar spectrum disorders also function at a significantly poorer level and have twice the rate of attempted suicides than do individuals with unipolar depression [8, 9]. Thus, there is a critical need to better characterize markers of BD illness early in its course and to identify effective early interventions.
Sleep disturbance may be one such early marker for the onset and progression of BD in youth at familial risk for BD, with continued sleep disturbance contributing to relapse [10]. A systematic review of reports of prodromal symptoms by adults with BD indicated that more than 80% could identify symptoms of mania early with the most common symptom being some form of a sleep disturbance [11]. Sleep disturbance in children with BD is often characterized by increased sleep onset latency, multiple nighttime awakenings, variable total sleep time duration, and decreased need for sleep [12], the latter constituting the criteria for sleep disturbance in bipolar disorder as per DSM-IV-TR [13]. In fact, although decreased need for sleep is considered part of the bipolar syndrome, 40–95% of children with BD I, II, or cyclothymia [14] have been found to report a decreased need for sleep during mania or hypomania [14–21]. A meta-analysis concluded that 72% of children with bipolar spectrum disorders demonstrated decreased need for sleep during mania [22]. Sleep disturbances in BD children have also been associated with the depressive phase of the illness [23]. In one of the few studies conducted on pediatric BD depression, Parker, et al. found that depressed BD patients under 25 years old had a high prevalence of hypersomnia characterized by difficulty with morning awakening [24]. Thus, mood state may contribute to the development of sleep disturbance and how it is reported.
Sleep disturbances may also distinguish BD from other psychopathology. In a study by Gellar et al. [25] of 7–16-year-old children with DSM-IV criteria BD I or II disorder had a significantly higher rate of decreased need for sleep than did children with attention deficit hyperactivity disorder (ADHD) and healthy controls. Children with bipolar spectrum disorders have also been shown to exhibit increased sleep onset latency compared to healthy controls [21, 26–28]. Experimental evidence to support these distinct sleep patterns include actigraphic data that have demonstrated that BD youth exhibit decreased sleep efficiency, continuity of sleep, sleep duration, and increased nocturnal activity compared to children with ADHD [26]. In addition, polysomnography revealed more time in stage 3 sleep and shorter REM total durations in 6–7-year-old children with a BD profile by the child behavioral checklist (CBCL) than in a healthy comparison group [27]. Finally, an EEG study of adolescents with BD in the depressive phase of illness showed a significant increase in stage 1 sleep compared with unipolar depressed subjects [28]. In animal models, non-REM sleep has been shown to be necessary for regulating neurotransmitters and allowing their receptors to reset, maintaining their proper level of sensitivity. This process is thought to be necessary to regulate mood and increase learning ability [29]. An increase in stage 3 sleep leads to decreased REM sleep, and successive decreases in REM sleep may lead to a rebound of increased REM sleep, which is associated with depression in BD. In mania, adults with BD have sleep continuity disturbances and REM disinhibition, characterized by decreased latency to and increased phasic activity during REM sleep [30]. Therefore, disrupted sleep architecture is associated with mood symptoms in BD. Collectively, we would expect children with BD to have decreased sleep duration, more nighttime awakenings, and increased activity that may manifest as increased productivity or increased total time awake upon awakening.
One research limitation is the possibility of informant disagreement between parent and child. Informant agreement between parent and child is low in psychiatric disorders [31], and sleep disturbances often occur without parental awareness. Therefore, the study of informant agreement is particularly important in pediatric BD where sleep is a salient concern. Such informant disagreement may place adolescents with psychopathology at risk for an adverse outcome [32]. In children and adolescents with bipolar spectrum disorders, manic symptoms tend to be underreported [33], with low parent–child agreement for symptoms of mania and depression [23]. There is also a relative lack of the use of standardized psychiatric measurement to characterize sleep disorders [21]. Low correlations between parent and child reports of sleep behavior suggest that children report greater sleep-onset delay and more nighttime awakenings than do parents [34]. This high degree of reporting variability may be due to frequent and prolonged mood episodes [28]. Reporting variability may also be moderated by age [35]. Therefore, as mood states affect sleep disturbances, these sleep disturbances may not be accurately reported by either child or parent given previous studies suggesting low concordance, and both reports should be examined.
To minimize variability across age and clinical course, we sought to characterize the quality of sleep in postpubertal 13–18-year-old adolescents with bipolar I disorder who experienced a single initial episode of mania within 6–12 months of research participation and compare them to demographically similar healthy controls (HCs). We also sought to assess whether there was an association between current mood symptoms and sleep quality and whether there was parent-teen concordance for reported sleep. Based on prior studies, we hypothesized that BD and HC adolescents would differ in their patterns of sleep. Specifically, we hypothesized that teens with BD would have a greater number of nighttime awakenings, greater total time awake, and a longer sleep onset latency. We hypothesized further that HCs would have longer sleep time on the weekends versus during the week than would BD teens, given findings reported by Carskadon [36] of longer total sleep time during weekend nights in healthy teenagers. We also hypothesized these sleep variables would be associated with manic symptoms, such as decreased need for sleep, increased productivity, and increase in goal directed activity. Finally, we predicted that parent-teen concordance for reported sleep variables would be lower in the BD than in the HC group.
Methods
Participants
The university panel of medical research in human subjects approved this research protocol. After complete description of the study to the subjects and their parents, written informed consent was obtained from parents and youth 18 years of age, and written assent was obtained from youth under the age of 18. Twenty-seven post-pubertal participants ages 13–18 who had experienced a manic episode as defined by the Diagnostic and Statistical Manual, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association, DSM-IV Task Force, 2000) within the previous 12 months and fulfilling criteria for a diagnosis of BD I, were recruited from the Stanford University Pediatric Bipolar Disorders Program and surrounding community. Twenty-four healthy controls (HC) comparable in age, gender, and ethnicity, with no personal or family history of psychopathology, were also recruited from the 40 mile radius in the surrounding community via advertisement. Socioeconomic status (SES) was obtained using the Hollingshead scale [37]. For all subjects, exclusion criteria included those who had metal in their body or braces for a concurrent MRI study, head injury with loss of consciousness for over 5 min, IQ less than 80, seizures, or developmental disorder. Subjects were allowed to continue psychotropic medication. HC were excluded if they reported a history or current symptoms of BD, other Axis I psychiatric disorders, had a history of taking past or current psychotropic medications, or had a first-degree relative with any psychopathology.
Diagnostic and Clinical Assessments
All participants were evaluated for current and lifetime psychiatric disorders, using the Washington University in St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia (WASH-U KSADS) [38], administered separately to parents and children. The WASH-U KSADS has established symptom and diagnostic reliability (kappa > 0.9). A manic episode was defined by DSM-IV-TR (American Psychiatric Association, DSM-IV Task Force, 2000) criteria that lasted at least 1 week, and could not have been precipitated by exposure to recreational drugs, antidepressants, psychostimulants, or other medications or medical conditions. Diagnoses were determined by a consensus conference attended by board certified child and adolescent psychiatrists (KDC and MKS) and masters-level WASH-U KSADS interviewers after both parent and child interviews were completed. Symptom severity in BD subjects was assessed using the Young Mania Rating Scale (YMRS) [39], the Children’s Depression Rating Scale-Revised Version (CDRS-R) [40], and the Childhood Global Assessment Scale (CGAS) [41] by raters with established symptom reliabilities (ICC > 0.9).
Sleep Assessments
Sleep quality was quantified by both parent and teens using a sleep questionnaire based on the previously validated Pittsburgh Sleep Quality Index (PSQI) [42], which measures sleep variables during the past week, including total sleep time, number of nighttime awakenings, total time awake during such awakenings, and sleep onset latency. Variables were assessed separately for weeknight and weekend sleep patterns. Current WASH-U KSADS variables that affected sleep were chosen within each grouping of depressive and bipolar symptomatology. Table 1 lists variables selected from this semi-structured interview.
Table 1.
Selected WASH-U KSADS variables
| Depression | Bipolar |
|---|---|
| Depressed mood | Elation expansive mood |
| Crying | Decreased need for sleep |
| Irritability/anger | Unusually energetic |
| AM diurnal mood variation | Increase in goal directed activity |
| PM worse diurnal mood variation | Motor hyperactivity |
| Excessive inappropriate guilt | Increase in productivity |
| Feeling unloved/forlorn | |
| Hopelessness/helplessness | |
| Self pity | |
| Anhedonia | |
| Fatigue | |
| Psychomotor agitation | |
| Unable to sit still | |
| Pacing | |
| Psychomotor retardation | |
| Suicidal ideation | |
| Suicidal acts seriousness | |
| SIB seriousness nonSA | |
| SIB med lethality nonSA |
SIB Self-injurious behavior, SA Suicidal act
Analysis
Data were analyzed using SPSS version 18. Independent t-tests and Fisher exact tests were used where appropriate to analyze baseline differences between BD and HC groups. To assess sleep quality in BD versus HC groups, independent t-tests were conducted to examine group differences between adolescents with BD and HC or medicated versus non-medicated subgroups of the BD sample. Paired t-tests were conducted to compare parent and teen reports of weeknight versus weekend sleep patterns of BD and HC teens. WASH-U KSADS-derived symptom variables were examined separately from the bipolar and depression subsections. Each WASH-U KSADS-derived symptom variable was linearly regressed in a stepwise fashion against each sleep questionnaire variable. Covariates that were significant at P ≤ .05 were examined for correlations within their respective depression or bipolar groups. Correlations that were significant at P ≤ .05 were included in final multivariate regression models. One final model was run each for depression group variables and for bipolar group variables. To assess parent–child concordance in sleep ratings, paired t-tests were used to compare differences between parent and teen reports. To avoid risk of mood destabilization, BD subjects were allowed to continue to take psychotropic medications including mood stabilizers, atypical antipsychotics, or antidepressants. Medication history was recorded and used in exploratory analyses.
In the sleep questionnaire, participants were asked to report amount of nighttime sleep in hours and the time the subject went to bed, turned the lights out, and woke up. To account for possible discrepancies in reported sleep hours and the actual hour at which the subject went to sleep and woke up, a calculated sleep time variable was created using the following formula: calculated sleep was determined by subtracting the time the subject woke up from the greater of either time to bed or the time the lights went out. T-tests were then conducted on this calculated sleep measure to examine group differences.
Results
Descriptive Analyses
Table 2 presents group demographic information. 70.3% of BD subjects were medicated at the time of assessment, with 51.9% of BD youth taking an atypical antipsychotic for an average of 4.1 weeks, 48.1% were taking a mood stabilizer for an average of 4.0 weeks, and 14.8% were taking an antidepressant for an average of 15.2 weeks. Two children were also taking benzodiazepines. Historically, 33.3% had taken a selective serotonin reuptake inhibitor (SSRI) for an average of 3.8 weeks, 22.2% had taken an antidepressant other than an SSRI for an average of 1.9 weeks, and 7.4% had taken a conventional antipsychotic for an average of less than 1 week. Of the BD group, 25.9% had a concomitant ADHD diagnosis, and 25.9% were taking a stimulant for an average of 5.1 weeks at time of assessment. One person was currently taking (24 weeks) and another had a trial (12 weeks) of atomoxetine.
Table 2.
Demographic data
| Bipolar (BD) (n = 27) | Healthy Controls (HC) (n = 24) | P | |
|---|---|---|---|
| Age | 15.6 ± 1.5 | 14.9 ± 1.4 | 0.18 |
| Gender (females) | 15 | 14 | 1.00 |
| Race | 67% Caucasian | 58% Caucasian | 0.17 |
| 22% Mixed race | 13% Mixed race | ||
| 4% Hispanic | 8% Hispanic | ||
| 4% Pacific Islander | 17% Asian | ||
| 4% Unknown | 4% Black | ||
| Hollingshead SES | 4.7 ± 0.5 | 4.6 ± 0.7 | 0.43 |
Analyses of Sleep Variables
HC subjects had significantly higher mean CGAS scores than did BD subjects (93.9 versus 54.7, P < .001). In contrast, BD subjects had significantly higher YMRS and CDRS scores than did HC subjects (YMRS: 20.3 for BD versus .18 for HC, P < .001; CDRS: 42.4 for BD versus 17.8 for HC, P < .001). Within the BD group, 24% were in a current manic state at the time of assessment, 28% were in a depressive state, and 28% were experiencing mixed symptomatology.
Parent Reporting of Sleep Variables
There were significant differences between parent reports for the BD and HC groups in weeknight sleep onset latency (t(25) = −2.9, P = .01) and weeknight number of times awake (t(24.6) = −2.4, P = .02), with the BD group having more difficulty falling asleep and waking up more times during the night. There was also a significant difference in weekend night sleep onset latency, with the BD group having more trouble falling asleep than the HC group (t(22) = −2.6, P = .02). The BD group also spent more time awake during weekend nighttime awakenings than did the HC group (t(13) = −2.2, P = .04). Table 3 presents the results for differences in parent report between HC and BD groups.
Table 3.
Parent reporting
| Bipolar disorder (BD) versus healthy controls (HC)—weeknights
|
BD versus HC—weekend nights
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean
|
t-test | df | P value | Mean
|
t-test | df | P value | ||||
| BD | HC | BD | HC | ||||||||
| Latency to sleepa (BD = 21, HC = 18) | 41.5 ± 35.4 | 18.1 ± 11.8 | −2.9 | 25.0 | 0.008 | Latency to sleepa (BD = 21, HC = 17) | 44.8 ± 51.6 | 15.0 ± 10.5 | −2.6 | 22.0 | 0.017 |
| No of times awake (BD = 16, HC = 14) | 1.1 ± 1.1 | 0.4 ± 0.6 | −2.4 | 24.6 | 0.024 | No of times awake (BD = 18, HC = 20) | 3.0 ± 3.9 | 3.0 ± 4.7 | 0.0 | 35.8 | 1.000 |
| Total time awakea (BD = 16, HC = 14) | 52.3 ± 109.7 | 11.4 ± 17.8 | −1.5 | 15.9 | 0.162 | Total time awakea (BD = 14, HC = 13) | 25.0 ± 42.0 | 0 | −2.2 | 13.0 | 0.044 |
| Reported sleepb (BD = 20, HC = 20) | 8.4 ± 2.0 | 8.1 ± 0.9 | −0.6 | 26.4 | 0.551 | Reported sleepb (BD = 20, HC = 19) | 9.4 ± 1.8 | 9.8 ± 1.3 | 0.6 | 34.8 | 0.548 |
| Calculated sleepb (BD = 21, HC = 20) | 8.6 ± 1.8 | 8.4 ± 0.9 | −0.5 | 30.0 | 0.597 | Calculated sleepb (BD = 21, HC = 19) | 9.9 ± 1.5 | 9.7 ± 1.5 | −0.5 | 37.3 | 0.602 |
In minutes
In hours
Comparison of Parent Reporting of Weeknight Versus Weekend Night Sleep Variables
Parents of HC and BD subjects reported significantly greater sleep in HC and BD groups on the weekends than on the weeknights for both calculated sleep (HC: t(18) = −3.5, P = .002, BD: t(18) = −3.1, P = .006) and reported sleep (HC: t(18) = −5.6, P < .01, BD: t(17) = −2.3, P = .037). These differences occurred even though 80% of BD subjects were still experiencing residual mood symptoms that may affect sleep quality.
Teen Reporting of Sleep Variables
BD and HC teens differed in weekend night total time awake (BD: t(24.9) = −2.0, P = .05). No other significant differences were found (see Table 4). Figure 1 shows the average hours of sleep by age of BD youth compared to HC.
Table 4.
Teen reporting
| Bipolar disorder (BD) versus healthy controls (HC)—weeknights
|
BD versus HC—weekend nights
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean
|
t-test | df | P value | Mean
|
t-test | df | P value | ||||
| BD | HC | BD | HC | ||||||||
| Latency to sleepa (BD = 24, HC = 21) | 35.6 ± 40.1 | 20.7 ± 17.7 | −1.6 | 32.5 | 0.110 | Latency to sleepa (BD = 24, HC = 21) | 39.1 ± 62.5 | 14.3 ± 16.8 | −1.9 | 26.8 | 0.073 |
| No of times awake (BD = 24, HC = 21) | .75 ± 1.1 | .52 ± .75 | −0.8 | 41.1 | 0.413 | No of times awake (BD = 24, HC = 21) | 0.8 ± 1.1 | 0.4 ± 0.8 | −1.1 | 37.6 | 0.298 |
| Total time awakea (BD = 24, HC = 20) | 26.0 ± 63.5 | 1.9 ± 3.2 | −1.9 | 23.1 | 0.075 | Total time awakea (BD = 24, HC = 20) | 17.6 ± 35.0 | 2.9 ± 6.8 | −2.0 | 24.9 | 0.054 |
| Reported sleepb (BD = 23, HC = 20) | 8.9 ± 1.9 | 8.8 ± 1.3 | −0.2 | 38.6 | 0.812 | Reported sleepb (BD = 23, HC = 20) | 9.9 ± 1.4 | 9.4 ± 2.6 | −0.8 | 29.6 | 0.403 |
| Calculated sleepb (BD = 24, HC = 21) | 9.2 ± 2.1 | 8.4 ± .9 | −1.6 | 33.0 | 0.126 | Calculated sleepb (BD = 24, HC = 21) | 10.0 ± 1.5 | 9.6 ± 1.8 | −0 .7 | 39.2 | 0.514 |
In minutes
In hours
Fig. 1.
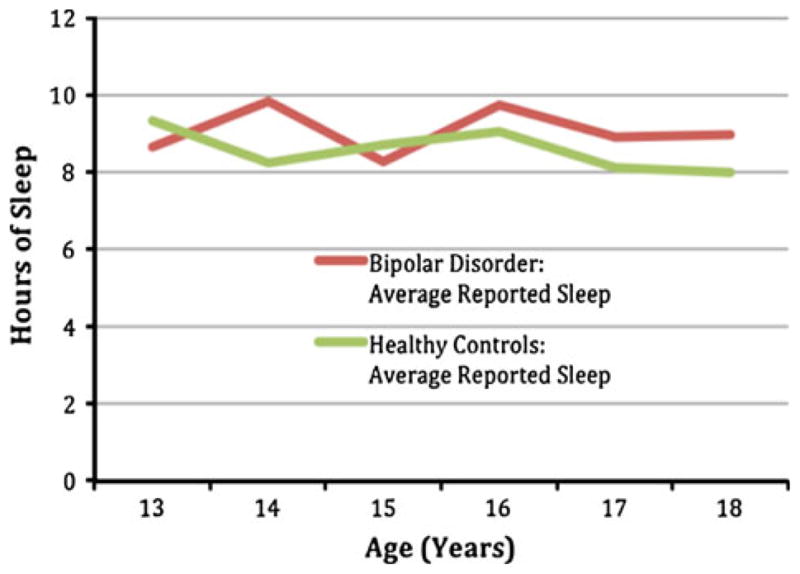
Average teen reported sleep in hours by age
Comparison of Teen Reporting of Weeknight Versus Weekend Night Sleep Variables
HC teens reported significantly greater sleep during weekends than during weeknights (t(20) = −3.2, P = .004). HC teens also had significantly longer sleep onset latency during weeknights than during weekend nights (t(20) = 3.1, P = .006). BD teens also reported longer sleep on weekends than on weeknights (t(20) = −2.3, P = .03); No significant difference was found in the BD group for sleep onset latency (t(21) = −.24, P = .81) or number of times awake (t(22) = 0, P = 1.0) during weeknights versus weekend nights.
Association of WASH-U-KSADS Variables with Sleep Quality
Tables 5 and 6 present associations of WASH-U KSADS variables with sleep variables as reported by parents and teens. Greater sleep onset latency was associated with parent and teen report of a longer period of worsening evening mood (parent: P = .002 for week-nights, P = 0.009 for weekend nights, teen: P = .025 for weekend nights). Increasing excessive/inappropriate guilt (P = .001 weeknights and P = 0.0033 weekend nights) and greater seriousness of non-suicidal self-injurious behavior (P = .002 weekend nights) were also associated with greater sleep onset latency per parent report. The greater the degree of medical lethality of non-suicidal self-injurious behavior (P = .026 weekend nights) and a greater feeling of being unloved (P = .025 weekend nights) were both associated with longer sleep onset latency per teen report.
Table 5.
Association of WASH-U KSADS to sleep variables parent reporting
| Estimated effect
|
Standard error
|
t-Statistic
|
P value
|
|||||
|---|---|---|---|---|---|---|---|---|
| Weeknight | Weekend night | Weeknight | Weekend night | Weeknight | Weekend night | Weeknight | Weekend night | |
| Latency to sleep | ||||||||
| PM worse diur mood vara | 13.9 | 17.9 | 4.0 | 6.4 | 3.4 | 2.8 | 0.002 | 0.009 |
| Excessive/inappropriate guilta | 18.9 | 17.4 | 5.2 | 7.8 | 3.6 | 2.2 | 0.001 | 0.033 |
| SIB seriousness—non SAa | NA | 31.9 | NA | 9.5 | NA | 3.4 | NA | 0.002 |
| No. of nighttime awakenings | ||||||||
| Elation/expansive mood | 0.4 | NA | 0.2 | NA | 2.7 | NA | < 0.001 | NA |
| Pacing | 0.5 | NA | 0.5 | NA | 2.7 | NA | < 0.001 | NA |
| Total time awake | ||||||||
| Inc in goal directed activity | 41.7 | NA | 10.6 | NA | 3.9 | NA | 0.001 | NA |
| Inc in productivity | NA | 16.1 | NA | 5.3 | NA | 3.0 | NA | 0.006 |
| Pacing | 43.0 | NA | 15.1 | NA | 2.9 | NA | 0.009 | NA |
| Psychomotor retardation | NA | 11.8 | NA | 4.8 | NA | 2.4 | NA | 0.024 |
| Reported sleep | ||||||||
| Depressed mood | 0.4 | NA | 0.2 | NA | 2.9 | NA | 0.007 | NA |
| Calculated sleep | ||||||||
| Decreased need for sleep | −0.3 | NA | 0.1 | NA | −2.2 | NA | 0.036 | NA |
| Depressed mood | 0.4 | NA | 0.2 | NA | 2.6 | NA | 0.010 | NA |
| Suicidal ideation | NA | 0.7 | NA | 0.2 | NA | 2.9 | NA | 0.006 |
PM worse diur mood var PM worse diurnal mood variation, SIB self-injurious behavior, SA suicidal act, NA not associated
Individual depression variables were not all correlated to each other. Results reflect the following groupings based on significant correlations: (a) PM worse diurnal mood variation and irritability/anger (P <.001), (b) irritability/anger and excessive/inappropriate guilt (P = .001), (c) excessive/inappropriate guilt, irritability/anger, and self-injurious behaivor-seriousness-nonSA (P ≤ .001)
Table 6.
Association of WASH-U KSADS to sleep variables teen reporting
| Estimated effect
|
Standard error
|
t-Statistic
|
P value
|
|||||
|---|---|---|---|---|---|---|---|---|
| Weeknight | Weekend Night | Weeknight | Weekend Night | Weeknight | Weekend Night | Weeknight | Weekend Night | |
| Latency to sleep | ||||||||
| SIB med lethality-nonSA | 26.2 | NA | 8.3 | NA | 3.2 | NA | 0.003 | NA |
| PM worse diurnal mood var | NA | 16.2 | NA | 7.0 | NA | 2.3 | NA | 0.026 |
| Feeling unloved | NA | 14.7 | NA | 6.3 | NA | 2.3 | NA | 0.025 |
| No. of nighttime awakenings | ||||||||
| Increased productivity | 0.3 | NA | 0.1 | NA | 2.3 | NA | 0.024 | NA |
| PM worse diurnal mood var | 0.4 | NA | 0.1 | NA | 2.6 | NA | 0.015 | NA |
| Psychomotor retardation | NA | 0.3 | NA | 0.1 | NA | 2.4 | NA | 0.025 |
| Pacing | NA | 0.4 | NA | 0.2 | NA | 2.2 | NA | 0.033 |
| Total time awake | ||||||||
| Decreased need for sleep | 14.3 | 6.9 | 4.6 | 2.6 | 3.1 | 2.6 | 0.004 | 0.014 |
| SIB med lethality-nonSA | 50.0 | 25.3 | 13.5 | 6.7 | 3.7 | 3.8 | 0.001 | 0.001 |
| Psychomotor retardation | NA | 11.5 | NA | 3.5 | NA | 3.3 | NA | 0.002 |
| Calculated sleep | ||||||||
| Terminal insomnia | 1.2 | NA | 0.4 | NA | 2.9 | 0.006 | NA | |
| Suicidal ideation | NA | 0.5 | NA | 0.2 | NA | 2.1 | NA | 0.045 |
SIB med lethality-nonSA Self-injurious behavior, medical lethality, non suicidal act, PM worse diur mood var PM worse diurnal mood variation, NA Not associated
Per teen report, increased pacing (P = .033 weekend nights) and more psychomotor retardation (P = 0.025 weekend nights) were simultaneously associated with greater total number of nighttime awakenings, as was more increased productivity (P = .024 week-nights), and a longer period of worsening evening mood (P = .015 weeknights). A higher number of nighttime awakenings (P < .001 weeknights) was associated with both increased pacing (P < .001 weeknights) and greater elation/expansive mood (P < .001 weeknights) per parent report.
Greater total time awake during nighttime awakenings was associated with more decreased need for sleep (weeknights: P = .004 and weekend nights: P = .014) and a higher degree of medical lethality of non-suicidal self-injurious behavior (weeknights and weekend nights: P = .001) per teen report. Greater total time awake on weekend nights (teens: P = .002, weekend nights, parents: P = .024) was associated with increased psychomotor retardation per both teen and parent report. Increased total time awake was also associated with more pacing (P = .009 weeknights), a greater increase in goal directed activity (P = .001 weeknights), and a higher increase in productivity (P = .006 weekend nights) per parent report.
Greater calculated sleep time was associated with more suicidal ideation (teens: P = .05, parents: P = .006) as reported by both parent and teens for weekend nights. Greater weeknight calculated sleep was also associated with more depressed mood (P = .01) per parent report.
Similarities and Differences in Parent-Teen Reporting
Parents and teens differed significantly in their reports of the number of times awake during weekend nights in both the BD (mean .76 ± 1.2 in teens, 3.12 ± 4.01 in parents, P = .034,) and HC (mean .45 ± .76 in teens, 3.0 ± 4.7 in parents, P = .03) groups.
Discussion
The present study was designed to characterize sleep quality and to examine the association between mood and sleep in BD I youth with recent manic episodes. In the acute manic phase, children with bipolar I disorder experience a decreased need for sleep, clinically seen with difficulty falling asleep and frequent nighttime awakenings. The goal of treatment is often to make these children sleep and often an increase in sleep time is seen due to medications and/or recovery from manic episode. Sleep may therefore be a response marker and a therapeutic target in mania. The results of this study indicate that, compared to demographically similar healthy controls, adolescents with bipolar I disorder are indeed characterized by distinct patterns of sleep disruption. Consistent with our hypothesis, BD teens had longer sleep onset latency, a greater number of nighttime awakenings, and greater total time awake during these awakenings than did typically developing HC teens. BD and HC participants slept more during weekend nights than during weeknights, but mood symptoms contributed to sleep disturbances within the BD group. For example, total time awake was associated with decreased need for sleep, increase in goal directed activity, and an increase in productivity in the BD group. The total number of nighttime awakenings was associated with elevated/expansive mood, an increase in productivity, and worsening evening mood, which was also associated with longer sleep onset latency. Our hypothesized discrepancy between parent and teen report of sleep quality was found with the number of nighttime awakenings reported.
Our findings are consistent with those of previous studies indicating a decreased need for sleep as a common presenting symptom for pediatric BD [14–21]. Consistent with previous studies of bipolar spectrum children [21, 26–28], we found a significant difference between BD and control groups for increased sleep onset latency. We also found, however, decreased need for sleep and longer sleep onset latency to be associated with quantified sleep variables. Our finding of significantly greater number of times awake for the BD group compared to controls is consistent with actigraphic data of decreased sleep continuity and efficiency [26]. Similar to [24], we found that depressed mood was positively associated with increased sleep length time. Mood symptoms associated with PSQI based sleep variables included worsening evening mood, excessive/inappropriate guilt, feeling unloved, and self-injurious behavior. While it is logical that a worse mood in the evening would lead to difficulties sleeping, these relations should be explored further, as they may place a child at increased risk for progression of both sleep dysfunction and mood symptomatology.
Many of the BD subjects also were experiencing manic, mixed, or depressive symptoms at the time of assessment, all of which may be associated with sleep disturbances. The finding of greater sleep time on the weekends is consistent with Carskadon’s findings that teens sleep more on the weekends than on weeknights [36]. We found this to be true even for BD teens, the majority of whom were still in a mood episode. However, it is difficult to conclude whether the increase in weekend sleep time the BD group reported was sufficient enough recovery sleep, as the amount of reported and calculated weekend sleep was comparable to the HC group. Further studies using outcomes specifically measuring sleep recovery with and without mood symptoms are warranted.
Over two-thirds of the BD sample was medicated, which can also affect sleep as treating sleep disturbance in BD is a common clinical practice, and many of the medications used to treat BD cause somnolence. In addition, previous studies suggest that treating sleep disturbances in mania leads to more positive outcomes, including shorter inpatient stays [43–45]. Surprisingly, the medicated subsample (N = 21) of the BD group did not sleep significantly more hours than the unmedicated (N = 6) subjects, nor were any significant group differences found in analyzing the sleep variables of the medicated subsample. In addition, only three of our patients were euthymic at time of assessment. Larger sample sizes would be needed to better interpret subgroup analyses of medication, sleep, and mood between medicated and non-medicated BD. Whether the teens required better control of current mood symptomatology to improve sleep or whether they needed more sleep to improve mood requires further investigation.
With BD children at higher risk for suicidality and impulsivity, does impulsivity and negative mood states lead to sleep disturbances? Conversely, does lack of sleep or sleep disturbances increase impulsivity and negatively affect mood? While it is clear that self-injurious behavior is often impulsive [46], our finding that self-injurious behavior was associated with increased sleep onset latency indicates that this sort of impulsive behavior combined with mixed mood states may lead to sleep disturbances. Previous literature supports the idea that adults in mixed states have increased impulsivity, substance use, and suicide attempts [47]. In a study of BD youth 7–17 years old, non-suicide attempt SIB was associated with greater lifetime number of mixed episodes [48]. However, neither of these studies directly relates impulsivity or mood states with specific sleep patterns. Among the few studies examining the relations among impulsivity, mood, and sleep, Schmidt et al. [49, 50] showed that insomnia (initial, middle, and/or terminal) in undergraduate students was associated with impulsivity characterized by actions completed without thinking when upset. This impulsivity led to greater negative bedtime thoughts and emotions that were then associated with more severe insomnia. Therefore, based on these latter studies and our finding, it may be useful to address negative bedtime thoughts and emotions with behavioral therapies to decrease impulsivity and improve sleep [49, 50].
HC parents may have showed more reporting discrepancy than BD parents because the former do not observe their teen’s sleep patterns as vigilantly as do their counterparts, particularly in this group of newly diagnosed BD youth with perhaps higher parental concern if the teens are reporting sleep disturbance. With all subjects, more discrepancies were found between teen and parent reporting for weekends rather than weeknights, suggesting that parents may be more aware of sleep patterns in their teens when their children have to wake up in time to attend school. Significant correlations between reported and calculated sleep for parent-teen BD reporting suggest that bedtimes and awakening times are accurately noted.
Our findings echo the conclusion of Lofthouse et al., who found that parent–child concordance is low when reporting on sleep symptoms in youth with bipolar spectrum disorders [21]. Tillman et al. also showed low parent–child concordance in a population of bipolar spectrum disordered children ages 7–14 for reports of symptoms of mania, including the symptom of decreased need for sleep (kappa .07) [23]. Owens et al. found that healthy elementary school aged children reported greater nighttime awakenings than did parents, consistent with our findings for all the subjects in our study [34]. No studies to our knowledge have examined discrepancies between parent and teen reporting using quantified sleep variables, or examined the associations between mood and these sleep variables. Our study is also the first to focus on adolescents with a recent onset of fully syndromal mania, rather than the spectrum of bipolar disorders with variable onset and course described in previous bipolar sleep studies in youth.
Limitations to this study include the fact that data are based on self-report. However, the WASH-U KSADS semi-structured clinical interview provided another source of supporting evidence of our results. We based our sleep analysis on a previously validated sleep scale (PSQI [42]) but did not use actigraphic data to provide more objective measures of sleep-related activity. It is important to note, however, that Goldberg et al. demonstrated that self-reported measures of sleep in BD adults have been shown to have internal consistency, construct validity, discriminant validity, and test–retest reliability [51]. Subgroup comparisons were limited by sample size, such that the effect size of analyses of mood state, sleep, and medications used in medicated versus unmedicated patients, of which there were few, was too small to provide a clinically significant interpretation. However, the sample of BD subjects ascertained for this study uniformly had BD I with few co-occurring psychiatric illnesses or other sources of heterogeneity. Other areas of sleep dysfunction that may potentiate mood symptoms and vice versa were not explored. For example, parasomnias such as confusional arousals are associated with a 13 times higher occurrence in adults with BD in a general population sample [52]. It would be important to include a study of other characteristics of sleep dysfunction as this may also affect nighttime awakenings and total time awake. Finally, this study represents sleep symptoms exhibited only at around the time of assessment and based on retrospective report of past mood symptoms. Future studies should examine such symptoms longitudinally to determine their course and influence on outcome.
Despite these limitations, this study characterized distinct sleep disturbances in adolescents with bipolar I disorder including longer sleep onset latency, greater number of nighttime awakenings, and greater total time awake during these awakenings compared to typically developing youth. Prospective assessments of sleep in teens with recent manic episodes will aid in clarifying how sleep quality changes with different mood states and whether sleep quality in euthymic BD subjects is more similar to that in HC participants. Creating a separate validated consensus rating of parent and child report as is done in other assessments such as the WASH-U KSADS, YMRS, and CDRS-R may also provide an accurate measure of sleep in this population. Future studies including objective actigraphic and polysomnographic data would be useful to associate with the subjective findings of sleep disturbance and mood found in this study. These objective studies would also clarify the effect of medications on sleep and mood. While historically difficult to study manic patients in monitored sleep studies, it would be useful to conduct such studies while the child was experiencing a depressed or mixed state, given that many in our BD group were in such states. Studies of both macroarchitecture to describe sleep onset latency, REM, and specific sleep stages, and microarchitecture using quantitative EEG [53] would aid in further clarifying and understanding the biological mechanisms [54] by which bipolar mood states affect sleep, and therefore direct clinical interventions. Beyond advising families of youth with BD to track sleep patterns daily, use goal setting to regulate the sleep-wake cycle, and provide psychoeducation regarding good sleep hygiene, few studies have examined the application of current sleep therapies to youth with BD. Interpersonal and social rhythm therapy (IPSRT) focuses on addressing circadian rhythm disruptions via sleep and social routines. In a recent study of IPSRT in adolescents with BD, Hlastala et al. found significant decreases in manic, depressive, and general psychiatric symptoms and overall increase in global functioning over 20 weeks [55]. Additionally, cognitive behavioral therapy (CBT) for insomnia, family-focused intervention, CBT for BD, and light therapy all address elements of sleep dysfunction and may be appropriate for regulation of the sleep –wake cycle in BD [53]. Medications to help motivate and awaken depressed BD patients and to aid with insomnia can also help regulate this cycle [56]. By discovering when and how sleep architecture is disturbed and how it is associated with mood, the optimum time for intervention with these possible therapies can be determined.
Summary
In summary, this study sought to characterize sleep quality in adolescents with recent manic episodes and to examine the association between mood and sleep in 27 post-pubertal adolescents with BD and 24 matched HC. Adolescents with BD exhibited longer sleep onset latency, increased number of nighttime awakenings, and greater total time awake during these nighttime awakenings. Sleep onset latency was associated with worsening evening mood, excessive guilt, and SIB. Number of nighttime awakenings was also associated with poor mood in the evenings as well as with elevated/expansive mood, and with increase in productivity. Total time awake was associated with decreased need for sleep and SIB. It will be important to determine whether interventions targeted toward specific domains of sleep dysfunction improve overall long-term outcome and prevent progression of BD into adulthood.
Acknowledgments
The authors would like to thank Melissa Pease, Erica Weitz, Elizabeth Adams, and Tenah Acquaye for their assistance with recruiting and running participants, and Allison Libby for help with data entry.
Contributor Information
Donna J. Roybal, Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, Stanford, CA, USA
Kiki D. Chang, Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, Stanford, CA, USA
Michael C. Chen, Department of Psychology, Stanford University, Stanford, CA, USA
Meghan E. Howe, Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, Stanford, CA, USA
Ian H. Gotlib, Department of Psychology, Stanford University, Stanford, CA, USA
Manpreet K. Singh, Email: mksingh@stanford.edu, Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, Stanford, CA, USA. Division of Child and Adolescent Psychiatry, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305-5719, USA
References
- 1.Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter TD, Mundo E, Parikh SV, Kennedy JL. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatry Res. 2003;37:297–303. doi: 10.1016/s0022-3956(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 3.Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B. Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2002;159:927–933. doi: 10.1176/appi.ajp.159.6.927. [DOI] [PubMed] [Google Scholar]
- 4.Geller B, DelBello MP. Bipolar disorder in childhood and early adolescence. Guilford Press; New York: 2003. [Google Scholar]
- 5.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Togen M, Angst J. Textbook in psychiatric epidemiology. Wiley-Liss; New York: 2002. [Google Scholar]
- 7.Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the course and outcome of bipolar youth (COBY) study. Am J Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein TR, Birmaher B, Axelson D, Goldstein BI, Gill MK, Esposito-Smythers C, et al. Psychosocial functioning among bipolar youth. J Affect Disord. 2009;114:174–183. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 10.Harvey AG. The adverse consequences of sleep disturbance in pediatric bipolar disorder: implications for intervention. Child Adolesc Psychiatry Clin N Am. 2009;18:321–338. viii. doi: 10.1016/j.chc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- 12.Staton D. The impairment of pediatric bipolar sleep: hypotheses regarding a core defect and phenotype-specific sleep disturbances. J Affect Disord. 2008;108:199–206. doi: 10.1016/j.jad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 14.Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 15.Ballenger JC, Reus VI, Post RM. The “atypical” clinical picture of adolescent mania. Am J Psychiatry. 1982;139:602–606. doi: 10.1176/ajp.139.5.602. [DOI] [PubMed] [Google Scholar]
- 16.Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- 17.Findling RL, Gracious BL, McNamara NK, Youngstrom EA, Demeter CA, Branicky LA, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3:202–210. [PubMed] [Google Scholar]
- 18.Bhangoo RK, Dell ML, Towbin K, Myers FS, Lowe CH, Pine DS, et al. Clinical correlates of episodicity in juvenile mania. J Child Adolesc Psychopharmacol. 2003;13:507–514. doi: 10.1089/104454603322724896. [DOI] [PubMed] [Google Scholar]
- 19.Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61:459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- 20.Faedda GL, Baldessarini RJ, Glovinsky IP, Austin NB. Pediatric bipolar disorder: phenomenology and course of illness. Bipolar Disord. 2004;6:305–313. doi: 10.1111/j.1399-5618.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 21.Lofthouse N, Fristad M, Splaingard M, Kelleher K. Parent and child reports of sleep problems associated with early-onset bipolar spectrum disorders. J Fam Psychol. 2007;21:114–123. doi: 10.1037/0893-3200.21.1.114. [DOI] [PubMed] [Google Scholar]
- 22.Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 2005;7:483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 23.Tillman R, Geller B, Craney JL, Bolhofner K, Williams M, Zimerman B. Relationship of parent and child informants to prevalence of mania symptoms in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161:1278–1284. doi: 10.1176/appi.ajp.161.7.1278. [DOI] [PubMed] [Google Scholar]
- 24.Parker G, Malhi G, Hadzi-Pavlovic D, Parker K. Sleeping in? The impact of age and depressive sub-type on hypersomnia. J Affect Disord. 2006;90:73–76. doi: 10.1016/j.jad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Geller B, Zimerman B, Williams M, Delbello MP, Bolhofner K, Craney JL, et al. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 26.Faedda GL, Teicher MH. Objective measures of activity and attention in the differential diagnosis of psychiatric disorders of childhood. Essent Psychopharmacol. 2005;6:239–249. [PubMed] [Google Scholar]
- 27.Mehl RC, O’Brien LM, Jones JH, Dreisbach JK, Mervis CB, Gozal D. Correlates of sleep and pediatric bipolar disorder. Sleep. 2006;29:193–197. doi: 10.1093/sleep/29.2.193. [DOI] [PubMed] [Google Scholar]
- 28.Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Rao R, et al. Heterogeneity in EEG sleep findings in adolescent depression: unipolar versus bipolar clinical course. J Affect Disord. 2002;70:273–280. doi: 10.1016/s0165-0327(01)00396-2. [DOI] [PubMed] [Google Scholar]
- 29.Siegel JM. Why we sleep. Sci Am. 2003;289:92–97. doi: 10.1038/scientificamerican1103-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riemann D, Voderholzer U, Berger M. Sleep and sleep-wake manipulations in bipolar depression. Neuropsychobiology. 2002;45(Suppl 1):7–12. doi: 10.1159/000049255. [DOI] [PubMed] [Google Scholar]
- 31.Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull. 1987;101:213–232. [PubMed] [Google Scholar]
- 32.Ferdinand RF, van der Ende J, Verhulst FC. Parent-adolescent disagreement regarding psychopathology in adolescents from the general population as a risk factor for adverse outcome. J Abnorm Psychol. 2004;113:198–206. doi: 10.1037/0021-843X.113.2.198. [DOI] [PubMed] [Google Scholar]
- 33.Youngstrom EA, Findling RL, Calabrese JR. Effects of adolescent manic symptoms on agreement between youth, parent, and teacher ratings of behavior problems. J Affect Disord. 2004;82(Suppl 1):S5–S16. doi: 10.1016/j.jad.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. J Dev Behav Pediatr. 2000;21:27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Grills AE, Ollendick TH. Multiple informant agreement and the anxiety disorders interview schedule for parents and children. J Am Acad Child Adolesc Psychiatry. 2003;42:30–40. doi: 10.1097/00004583-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- 37.Hollinghead A. Four-factor index of social status. Yale University; New Haven: 1975. [Google Scholar]
- 38.Geller B, Williams M, Zimerman B, Frazier J. Washington University in St Louis Kiddie Schedule for affective disorders and schizophrenia (WASH-U-KSADS) Washington University; St. Louis: 1996. [DOI] [PubMed] [Google Scholar]
- 39.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 40.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 42.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Nowlin-Finch NL, Altshuler LL, Szuba MP, Mintz J. Rapid resolution of first episodes of mania: sleep related? J Clin Psychiatry. 1994;55:26–29. [PubMed] [Google Scholar]
- 44.Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Res. 1996;65:121–125. doi: 10.1016/s0165-1781(96)02909-5. [DOI] [PubMed] [Google Scholar]
- 45.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- 46.Cloutier P, Martin J, Kennedy A, Nixon MK, Muehlenkamp JJ. Characteristics and co-occurrence of adolescent non-suicidal self-injury and suicidal behaviours in pediatric emergency crisis services. J Youth Adolesc. 2010;39:259–269. doi: 10.1007/s10964-009-9465-1. [DOI] [PubMed] [Google Scholar]
- 47.Swann AC, Moeller FG, Steinberg JL, Schneider L, Barratt ES, Dougherty DM. Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disord. 2007;9:206–212. doi: 10.1111/j.1399-5618.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein TR, Birmaher B, Axelson D, Ryan ND, Strober MA, Gill MK, et al. History of suicide attempts in pediatric bipolar disorder: factors associated with increased risk. Bipolar Disord. 2005;7:525–535. doi: 10.1111/j.1399-5618.2005.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt RE, Van der Linden M. The aftermath of rash action: sleep-interfering counterfactual thoughts and emotions. Emotion. 2009;9:549–553. doi: 10.1037/a0015856. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt RE, Gay P, Ghisletta P, VDLM Linking impulsivity to dysfunctional thought control and insomnia: a structural equation model. J Sleep Res. 2010;19:3–11. doi: 10.1111/j.1365-2869.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- 51.Goldberg JF, McLeod LD, Fehnel SE, Williams VS, Hamm LR, Gilchrist K. Development and psychometric evaluation of the bipolar functional status questionnaire (BFSQ) Bipolar Disord. 2010;12:32–44. doi: 10.1111/j.1399-5618.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 52.Ohayon MM, Priest RG, Zulley J, Smirne S. The place of confusional arousals in sleep and mental disorders: findings in a general population sample of 13, 057 subjects. J Nerv Ment Dis. 2000;188:340–348. doi: 10.1097/00005053-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- 54.McClung CA. Clock genes and bipolar disorder: implications for therapy. Pharmacogenomics. 2007;8:1097–1100. doi: 10.2217/14622416.8.9.1097. [DOI] [PubMed] [Google Scholar]
- 55.Hlastala SA, Kotler JS, McClellan JM, McCauley EA. Interpersonal and social rhythm therapy for adolescents with bipolar disorder: treatment development and results from an open trial. Depress Anxiety. 2010;27:457–464. doi: 10.1002/da.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frye MA, Grunze H, Suppes T, McElroy SL, Keck PE, Jr, Walden J, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry. 2007;164:1242–1249. doi: 10.1176/appi.ajp.2007.06060981. [DOI] [PubMed] [Google Scholar]


