Abstract
Background
The pathophysiology of psychogenic movement disorders, including psychogenic tremor (PT), is only emerging.
Case Report
This is a single case report of a patient who met diagnostic criteria for PT. He underwent positron emission tomography (PET) of brain with 18F-deoxyglucose at resting state. His PET study showed symmetrically increased 18F-deoxyglucose uptake in both posterior medial parietal lobes. There was no corresponding abnormality on structural imaging.
Discussion
Hypermetabolism of the medial aspects of posterior parietal lobes bilaterally may reflect abnormal activity of sensory integration that is important in the pathogenesis of PT. This further supports the idea that non-organic movement disorders may be associated with detectable functional brain abnormalities.
Keywords: Psychogenic tremor, positron emission tomography, parietal lobe, conversion reaction
Introduction
Psychogenic tremor (PT) is one of the most common manifestations of psychogenic movement disorders.1 Even though recent trends emphasize the need to establish laboratory criteria for PT, its diagnosis remains clinical and is principally based on exclusion of other possible tremor-causing conditions.2,3 Sudden onset, irregular and inconsistent oscillatory movements with distractibility and suggestibility have been suggested as the main diagnostic criteria for PT.4,5 Proposed diagnostic criteria also differentiate the degree of diagnostic certainty. Documented PT is characterized by symptom improvement after psychotherapy, suggestion or placebo, or the absence of symptoms when the patient is unaware of being observed. Clinically established PT is based on inconsistent tremor characteristics incongruent with organic disorders, and the presence of somatization signs or a documented psychiatric illness.1–3,5–7
Psychogenic or non-organic movement disorders are not under direct voluntary control unless they fall under the category of malingering or factitious disorders, where abnormal movements are intentionally feigned.3–5 Patients with factitious disorders simulate medical symptoms in order to assume the role of a patient, while malingering is associated with a clearly definable external goal, such as receiving compensation or avoidance of certain responsibilities or punishments.8 In contrast to voluntarily produced psychogenic disorders, subconsciously generated abnormal movements are most commonly classified in the Diagnostic and Statistical Manual of Mental Disorders, version IV (DSM-IV) conversion disorder or undifferentiated somatoform disorder.9 Historically, the cause of psychogenic movement disorders meeting diagnostic criteria for a conversion reaction or somatoform disorder has been attributed to various psychological stressors, converting anxiety into a physical symptom.1–3 However, their pathogenesis is only emerging, and recent functional imaging brain studies have suggested abnormal metabolic activity of several cortical areas, indicating the “organic” origin of these clinical abnormalities.6,10–12 Here we report the presence of abnormal focal cortical hypermetabolism detected by 18F-deoxyglucose positron emission tomography (PET) in a patient who met diagnostic criteria for documented PT.
Case report
A 38-year-old male was evaluated because of a 6-month history of shaking in both arms that started suddenly after he lost his job. Tremor was present all the time and he did not identify any aggravating or ameliorating factors. His examination demonstrated bilateral upper extremity tremor that varied in frequency, direction, and amplitude. The tremor was postural and present during various tasks, such as drinking and writing. He also had an intermittent rest tremor alternating between both sides. The tremor frequency was estimated between 10 Hz and approximately 12 Hz; moreover, the tremor frequency was influenced on both sides by directed motor activities in the contralateral arm, such as finger tapping. The remainder of his examination was entirely normal.
Even though he denied depression and did not endorse any vegetative symptoms, such as weight change or difficulties sleeping, he felt upset about his recent job loss. He denied any ongoing or pending litigation or any other actions related to his job termination. There was no family history of tremor or any other neurologic problems. Thyroid studies were within normal limits.
The diagnosis of psychogenic tremor as a form of conversion reaction was discussed with the patient and his wife, and he agreed to undergo psychological counseling. However, during the initial visit both the patient and his spouse were not very open to the possibility of a stress-induced neurologic problem.
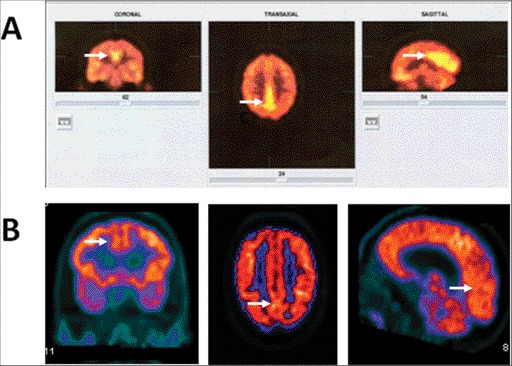
The patient was seen for a follow-up visit after 6 months. His tremor was unchanged and he reported more functional limitations in activities of daily living. He had not initiated psychological counseling as recommended. In the interim, the patient also sought an outside second opinion and had undergone additional diagnostic workup including multisequence (T1, T2, FLAIR (fluid-attenuated inversion-recovery), and diffusion), multiplanar magnetic resonance imaging (MRI) of the brain with and without contrast, genetic testing for Huntington disease, and PET with 18F-deoxyglucose brain scan. His brain PET study, performed in a tertiary medical center affiliated with a medical school, was obtained during a symptomatic period. The scan was done at a rest state following a standard protocol after IV application of 400 MBq of 18F-deoxyglucose. The PET study revealed abnormally increased uptake in the medial aspects of posterior parietal lobes bilaterally (Figure 1). The remainder of the cerebral cortex, including the motor strip, cerebellum, and basal ganglia showed a symmetrical uptake that was within normal limits. Correlation with MRI of the brain did not show any structural abnormalities in the cortical areas with abnormally increased 18F-deoxyglucose uptake. Testing for Huntington disease revealed that both alleles had a normal range of CAG repeats with heterozygosity at the HTT locus.
Figure 1. (A) An 18F-deoxyglucose positron emission tomography (PET) study with increased rest uptake in the medial posterior parietal lobe bilaterally seen in a patient with psychogenic tremor (arrows). (B) The corresponding areas of a 46-year-old patient with a history of essential tremor (ET) since the age of 10 years who developed Parkinson's disease (PD) 4 years ago. His clinical examination showed overlapping severe ET and tremor-dominant PD with rest tremor (right>left), and bilateral postural and kinetic tremor. We used the same PET protocol and he was in off state for his dopaminergic medications. Corresponding areas of the posterior parietal lobes showed no increased 18F-deoxyglucose uptake (arrows).
During this visit we reiterated the diagnosis of a psychogenic tremor and this time he was more open to accept this possibility. He was also started on citalopram 20 mg once a day and again, referred to a psychiatrist and counseling.
The patient was subsequently seen by a psychiatrist who continued the same dose of citalopram and confirmed the diagnosis of a conversion reaction. Neuropsychological testing included the Minnesota Multiphasic Personality Inventory (MMPI-2), which showed Hs subscore (hypochondriasis) 92, Hy (hysteria) 90, and D (depression) 65, consistent with a conversion reaction.13 A follow-up visit in our Movement Disorders Clinic after 4 months showed a significant improvement of his tremor, which was now only intermittent and induced by occasional anxiety. He also demonstrated better insights into his neurologic problem.
A follow-up visit after an additional 4-month period revealed a complete absence of tremor, which also coincided with him being rehired to the same job position. He also agreed to undergo a follow-up research 18F-deoxyglucose brain PET to compare his abnormal cortical activity after the disappearance of his psychogenic tremor. Approximately 2 weeks before the scheduled study he suffered severe polytrauma, which made the follow up PET study impossible.
Discussion
The presented patient met the diagnostic criteria proposed by Fahn and Williams14 for a documented PT based on its clinical characteristics and a complete resolution after the treatment of the active psychopathology. Serendipitously, he underwent an otherwise non-indicated brain 18F-deoxyglucose PET scan obtained during his symptomatic period, which demonstrated an abnormally increased uptake in both medial posterior parietal lobes. This did not correlate with any structural abnormalities on MRI brain scan and the only clinical abnormality was an active PT during the time of this PET study. He also did not take any psychoactive medication at the time of PET scan. The study was done at a rest mode without any motor activity, and, indeed, the areas of the primary motor cortex did not show any activation based on normal 18F-deoxyglucose uptake. Unfortunately, a follow-up study after the effective therapy of his tremor was not possible.
Functional abnormalities of various brain regions have been previously reported in patients with PT consistent with conversion reactions using either functional MRI or single photon emission tomography (SPECT) assessing regional cerebral blood flow (rCBF).6,10–12 These studies, however, were done comparing resting and activation states. SPECT studies using 99mTc-ethyl ysteinate ligand showed increased rCBF in the left inferior frontal gyrus and left insula in PT patients at rest when compared to patients with essential tremor (ET). ET patients had increased resting rCBF in the cerebellum and left inferior frontal gyrus, and a motor task of imitation of drinking with a cup resulted in activation of the supplementary motor cortex and the contralateral primary motor cortex.12 Patients with PT demonstrated a regional hypoactivity on fMRI in the right temporoparietal junction compared to normal individuals.6,10,11
We hypothesize that a hypermetabolic state of medial aspects of posterior parietal lobes, detected in our patient in a rest state, is related to PT and caused by abnormal activation of this cerebral region in PT. This corresponds primarily to Brodmann areas 23 and 31, and also encompasses the posterior part of the cingulate gyrus. The posterior parietal cortex is implicated in planning of movements by integrating input from sensory and visual systems.15,16 Sensory prediction of the motor outcome is associated with normal, self-generated movements, and the comparisons of predicted and actual movements based on the propricoceptive feedback are performed mostly in the posterior parietal lobe.17 An excessive cortical arousal, as reflected by cortical hypermetabolism with increased glucose uptake, may reflect an abnormal modulation of the sensory integrative functions of the posterior parietal lobe and result in abnormal interactions of internal sensory predictions with the actual sensory state.18 Thus, these abnormal modulatory interactions between limbic and sensorimotor networks may be a crucial component of the pathophysiology of non-organic movement disorders.19,20
Our data may further expand known brain areas implicated in the pathophysiology of PT. This report, in spite of its limitations as a single case report and the use of resting PET scan only, suggests that hyperactivity of posterior medial parietal lobe and posterior cingulate gyrus may be important for the development of psychogenic tremor.
Footnotes
Funding: PH is supported by K02NS057666 (NIH/NINDS).
Competing Interests: The author reports no conflict of interest.
References
- 1.Bhatia KP, Schneider SA. Psychogenic tremor and related disorders. J Neurol. 2007;254:569–574. doi: 10.1007/s00415-006-0348-z. [DOI] [PubMed] [Google Scholar]
- 2.Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5:695–700. doi: 10.1016/S1474-4422(06)70523-3. [DOI] [PubMed] [Google Scholar]
- 3.Lang AE, Voon V. Psychogenic movement disorders: Past developments, current status, and future directions. Mov Disord. 2011;26:1175–1182. doi: 10.1002/mds.23571. [DOI] [PubMed] [Google Scholar]
- 4.Schwingenschuh P, Katschnig P, Seiler S, et al. Moving toward “laboratory supported” criteria for psychogenic tremor. Mov Disord. 2011;26:2509–2515. doi: 10.1002/mds.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol. 2009;22:430–436. doi: 10.1097/WCO.0b013e32832dc169. [DOI] [PubMed] [Google Scholar]
- 6.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams PJ, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol. 1995;65:233–257. [PubMed] [Google Scholar]
- 8.McCullumsmith CB, Ford CV. Simulated illness: the factitious disorders and malingering. Psychiatr Clin North Am. 2011;34:621–641. doi: 10.1016/j.psc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.) Washington, DC: 2002. [Google Scholar]
- 10.Stone J, Zeman A, Simonotto E, et al. fMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med. 2007;69:961–969. doi: 10.1097/PSY.0b013e31815b6c14. [DOI] [PubMed] [Google Scholar]
- 11.Ghaffar O, Staines R, Feinstein A. Unexplained neurologic symptoms: An fMRI study of sensory conversion disorder. Neurology. 2006;67:2036–2038. doi: 10.1212/01.wnl.0000247275.68402.fc. [DOI] [PubMed] [Google Scholar]
- 12.Czanecki K, Jones DT, Burnett MS, Mullan B, Matsumoto JY. SPECT perfusion patterns distinguish psychogenic from essential tremor. Park Relat Disord. 2011;17:328–332. doi: 10.1016/j.parkreldis.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Tellegen A, Ben-Porath YS, McNulty JL, Arbisi PA, Graham JR, Kaemmer B. The MMPI-2 Restructured Clinical Scales: Development, validation, and interpretation. Minneapolis, MN: University of Minnesota Press; 2003. [Google Scholar]
- 14.Fahn S, Williams PJ. Psychogenic dystonia. Adv Neurol. 1988;50:431–455. [PubMed] [Google Scholar]
- 15.Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res. 2003;153:239–245. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- 16.Desmurger M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- 17.Desmurger M, Sirugu A. A parietal premotor network for movement intention and motor awareness. Trends Cogn Sci. 2009;13:411–419. doi: 10.1016/j.tics.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Vuilleumier P. Hysterical conversion and brain function. Prog Brain Res. 2005;150:309–329. doi: 10.1016/S0079-6123(05)50023-2. [DOI] [PubMed] [Google Scholar]
- 19.De lange FP, Roelofs K, Toni I. Motor imagery: a window into the mechanism and alterations of the motor system. Cortex. 2008;44:494–506. doi: 10.1016/j.cortex.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Cojan Y, Waber L, Carruzzo A, Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage. 2009;47:1026–1037. doi: 10.1016/j.neuroimage.2009.05.023. [DOI] [PubMed] [Google Scholar]


