Abstract
The regulation of body fat stores and blood glucose levels is critical for survival. This review highlights growing evidence that leptin action in the central nervous system (CNS) plays a key role in both processes. Investigation into underlying mechanisms has begun to clarify the physiological role of leptin in the control of glucose metabolism and raises interesting new possibilities for the treatment of diabetes and related disorders.
I. INTRODUCTION
Obesity, insulin resistance and type 2 diabetes are growing health concerns and the incidence and prevalence of these diseases is increasing world-wide (175). In the US alone, more than two-thirds of adults are classified as either overweight or obese while more than 23 million people have been diagnosed with type 2 diabetes (117). These numbers are expected to increase in the foreseeable future with costs for obesity in the US alone well in excess of $100 billion per year (156, 175). Obesity is associated with increased morbidity, because it is a major risk factor for diabetes, cardiovascular disease, and cancer. Diabetes, in turn greatly increases the risk of heart disease and both macro- and micro-vascular disease (175, 202). Given these statistics, the need for a better understanding of how body weight and glucose metabolism are regulated is compelling, as such information is essential if we are to develop new strategies for the safe and effective treatments for obesity and type 2 diabetes.
In studies dating back to the 19th century, the French physiologist Claude Bernard observed that puncturing the floor of the fourth ventricle (“piqure diabetique”) induced diabetes (18), suggesting that the brain plays an important role in the control of blood glucose. Following the discovery of insulin in the early twentieth century (8), however, research into the regulation of glucose metabolism focused primarily on insulin secretion and on its action in peripheral tissues such as liver, muscle and adipose tissue. One result of this research effort is clear evidence, accumulated over the last forty years, that obesity is associated with insulin resistance in these tissues and that this insulin resistance is a cardinal feature of type 2 diabetes (125). Consequently, pharmaceutical strategies for the treatment of type 2 diabetes have tended to focus on either increasing insulin secretion or improving insulin sensitivity. Yet while a large literature provides insight into the pathogenesis of obesity-associated insulin resistance, a parallel body of evidence suggests that the CNS plays an important role in the regulation of glucose metabolism, and that dysfunction of this control system may contribute to metabolic consequences of obesity (161).
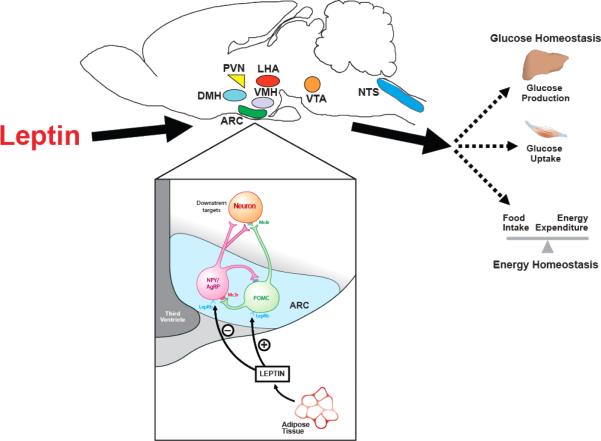
A. Model of CNS Control of Energy and Glucose Homeostasis
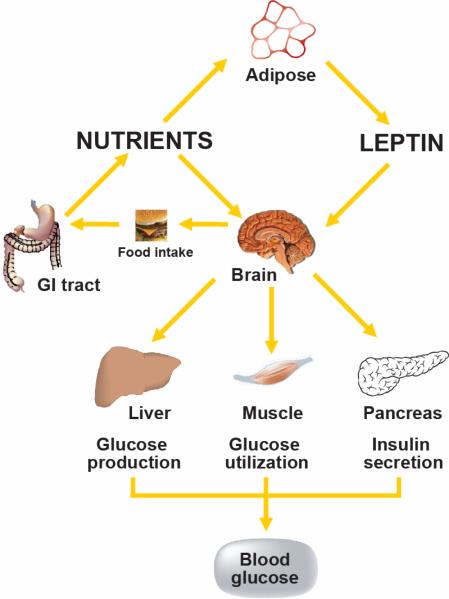
The brain continuously transduces input from neural, hormonal and nutrient-related signals into responses that maintain both energy- and glucose-homeostasis. Afferent signals such as the hormones insulin and leptin convey information to the brain regarding long-term energy stores, while information regarding short-term energy availability is conveyed by nutrient-related signals such as glucose and free-fatty acids (FFA). In response to this input, the brain makes adjustments to output systems that control food intake, energy expenditure, hepatic insulin sensitivity and glucose uptake (Figure 1). To restore homeostasis in the face of reduced energy stores or energy availability for example, the brain initiates responses that promote both positive energy balance (i.e. increased food intake and reduced energy expenditure) and increased nutrient availability (increased endogenous glucose production). Conversely, in times of nutrient abundance and excess energy storage, the brain initiates responses that both promote negative energy balance and limit endogenous glucose production. In view of anatomical overlap in brain areas involved in energy homeostasis and glucose metabolism, dysfunction along a single neuroregulatory pathway can potentially predispose to both obesity and type 2 diabetes; remedying such a defect could therefore be of benefit in the treatment of both disorders. The goal of this review is to highlight recent advances in our understanding of the CNS mechanisms governing glucose metabolism, with a special focus on the adipocyte hormone, leptin.
FIGURE 1. Model of CNS regulation of glucose homeostasis.
The CNS integrates signals from both long-term energy stores (e.g. leptin) and short-term availability (nutrients; e.g. free fatty acids) and orchestrates responses that coordinate hepatic glucose production, peripheral glucose uptake and insulin secretion to maintain glucose homeostasis.
A large and compelling body of evidence supports the hypothesis that hormonal signals generated in proportion to body fat stores act in the CNS via a negative feedback loop to promote energy homeostasis. Early support for this model was based on the adaptive response to conditions characterized by negative energy balance and weight loss. These include the increase of food intake and reduction of energy expenditure induced by energy deprivation or chronic energy restriction that enables the efficient recovery of lost weight, comprising an important protective mechanism for survival (164). In 1953, Kennedy first hypothesized that body adiposity was regulated by circulating factors released in proportion to adipose tissue mass that acted in the brain to maintain energy balance (92). While there was a concerted effort by researchers to identify circulating factors involved in energy homeostasis, it wasn't until 1994, when Zhang and colleagues successfully identified and cloned the ob gene and demonstrated that it is expressed selectively in adipose tissue and encodes a peptide hormone that was subsequently termed “leptin”, mutation of which caused obesity in the ob/ob mouse (212). Not long thereafter, the leptin receptor was cloned and its expression demonstrated in hypothalamus and other tissues (183), and the db mutation was localized to this gene locus (100). As expected, either intraperitoneal or intracerebroventricular (i.c.v.) administration of leptin, reduced food intake and body weight in ob/ob mice, whereas db/db mice failed to respond (30, 76, 141, 192). Taken together, these data provided compelling initial support for the hypothesis that an adipose-tissue derived circulating factor acts in the CNS to regulate energy balance, and that maintenance of normal body fat stores is dependent on the integrity of this endocrine system. The clinical relevance of this work was confirmed by evidence that severe obesity results in humans as well as in mouse models from mutation of genes encoding either leptin (118, 212) or its receptor (100, 110, 181).
B. Adiposity Signals: Insulin and Leptin
Several lines of evidence support the hypothesis that in addition to leptin, the pancreatic hormone insulin also acts in the brain as an “adiposity negative feedback signal”. Both hormones circulate at levels that vary in proportion to body fat stores (4, 39) and both enter the CNS in proportion to their plasma level (160), where they act on their respective receptors expressed in key brain areas that control energy balance, glucose metabolism and autonomic function (10, 11, 53). Administration of either peptide into the brain reduces food intake and body weight (30, 76, 200) while conversely, deficiency of either hormone or their respective receptors in the CNS results in hyperphagia, weight gain and insulin resistance (27, 174, 183, 212). In humans subjected to weight loss due to energy restriction, administration of leptin at doses that maintain pre-weight loss plasma levels blocks the reduction of sympathetic nervous system (SNS) activity and metabolic rate characteristic of the weight-reduced state (151). Taken together, these data support the hypothesis that energy homeostasis involves negative feedback signals that circulate in proportion to body fat and act centrally to inhibit food intake on the one hand, and to increase energy expenditure on the other. Although this review focuses primarily on leptin, its many areas of overlap with the actions of insulin in the brain warrant a focus on both hormones.
II. LEPTIN SIGNALING
A. Jak-STAT pathway
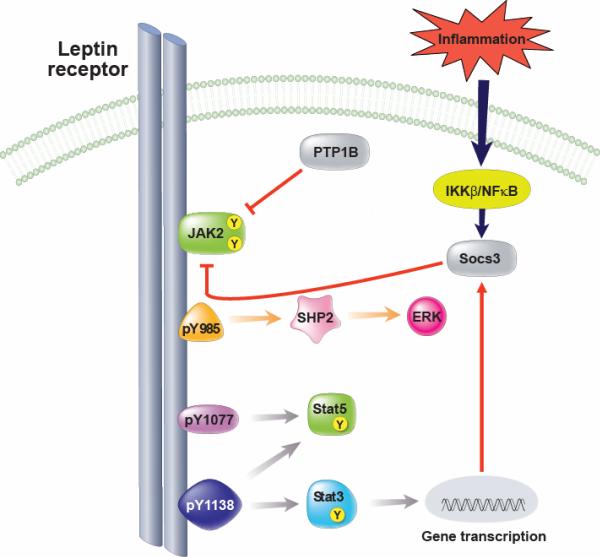
The leptin receptor contains an extracellular ligand-binding domain, a single transmembrane domain and a cytoplasmic signaling domain and is member of the interleukin (IL)-6 receptor family of class 1 cytokine receptors (182). Among the various isoforms of LepR, only the “long”, or “signaling” form, LepRb, appears to be competent for intracellular signal transduction and is critical for energy homeostasis. This assertion is based in part on evidence that mice that lack only LepRb (e.g. db/db mice) exhibit a phenotype similar to leptin-deficient ob/ob mice (100). Upon leptin binding to LepRb, the tyrosine kinase Janus kinase-2 (Jak2) is activated (69), resulting in the phosphorylation of three tyrosine residues on LepRb (Tyr985, Tyr1077, Tyr1138) that in turn recruit and activate a distinct set of downstream signaling proteins. Phosphorylation of Tyr985 recruits SH2-domain-containing phosphatase-2 (SHP-2) to LepRb, leading to the activation of the extracellular signal regulated (ERK) signaling pathway in cultured cells, as well as the recruitment of suppressor of cytokine signaling-3 (SOCS-3), a negative regulator of LepRb signaling (6, 21, 22). In contrast, phosphorylation of Tyr1138 recruits signal transduction and activator of transcription-3 (STAT3), which is then itself phosphorylated on tyrosine residues by Jak2 allowing it to dimerize and translocate to the nucleus where it functions as a transcription factor (6, 23, 186). Among the genes transcriptionally induced by STAT3 is SOCS-3, which causes negative feedback inhibition of leptin signaling by binding to Tyr985 of LepRb to block its signaling (Figure 2). Phosphorylation of Tyr1077 and Tyr1138 promote the recruitment, phosphorylation and transcriptional activation of STAT5 (71), and leptin may also activate signals generated by Jak2 phosphorylation that are independent of tyrosine phosphorylation sites on LepRb.
FIGURE 2. Leptin receptor signaling.
Leptin binding to LepRb mediates activation of the tyrosine kinase Janus kinase-2 (Jak2), resulting in phosphorylation of three tyrosine residues (Tyr985, Tyr1077, Tyr1138). Phosphorylation of Tyr985 recruits SHP-2, leading to the activation of the ERK pathway, and also binds to suppressor of cytokine signaling-3 (SOCS-3), a negative regulator of LepRb signaling. Phosphorylation of Tyr1077 mediates signal transduction and activator of transcription-5 (STAT5). Phosphorylation of Tyr1138 recruits and activates STAT3 and STAT5. Activation of STAT3 leads to increased SOCS-3 expression which acts as a negative feedback inhibitor of leptin signaling, in part, by binding to Tyr985 of LepRb. In addition, hypothalamic inflammation is also induced during high-fat feeding and increases SOCS-3 expression. Similar to SOCS-3, protein tyrosine phosphatase (PTP)1B may also inhibit LepRb signaling via dephosphorylation of Jak2.
To determine the functional significance of each of these LepRb tyrosine residues in vivo, an approach was taken to generate homologously targeted “knock-in” mice whereby sequences encoding substitution mutants of specific LepRb phosphorylation sites replace the endogenous Lepr allele. Mice bearing a mutant LepRb gene in which Tyr1138 is replaced by a serine residue (s/s mice) are unable to recruit STAT3 and are characterized by hyperphagia, obesity (14) and reduced energy expenditure (12), suggesting that the Tyr1138 →STAT3 pathway plays a crucial role in the regulation of regulation of energy homeostasis (12, 14). However, the phenotype of these s/s mice does not completely mimic that of either ob/ob or db/db mice with respect to glucose metabolism, immune function or reproduction (13, 14), implying that other LepRb signals must also contribute to leptin action.
To determine the contribution of LepRb Tyr985 to leptin action in vivo, mice were generated in which the endogenous LepRb allele was replaced with a mutant in which a serine residue is substituted for Tyr985. In contrast to the hyperphagic, obese phenotype of animals mutant for Tyr1138, mutation of Tyr985 in these (l/l) mice resulted in a lean phenotype characterized by reduced food intake and adiposity, and increased leptin sensitivity (24). These results support data from cultured cells (22) suggesting that Tyr985 attenuates leptin action in vivo, presumably by serving as the SOCS-3 binding site (24). Since SOCS-3 can also be induced via the IKKβ-NFκβ pathway during cellular inflammation, this mechanism can contribute to leptin resistance in this setting (211) and has been implicated in the pathogenesis of diet-induced obesity (DIO) (87, 119, 211). What remains uncertain based on the phenotype of both s/s and l/l mice is the extent to which Tyr1077 and/or Jak2-dependent signals independent of LepRb tyrosine phosphorylation mediate leptin action in vivo.
Recent evidence suggests that leptin is capable of activating STAT5 in the hypothalamic arcuate nucleus (ARC) in vivo (126), in addition to cultured cells (71), raising the possibility that LepRb→STAT5 signaling contributes to the physiological actions of leptin. This hypothesis is supported by evidence that neuronal deletion of STAT5 in mice (via RIP-cre or Nestin-cre) causes a mild-obesity phenotype (101). However, in addition to leptin, several other cytokines and growth factors activate STAT5 including granulocyte macrophage colony stimulating factor (GM-CSF), prolactin and growth hormone, amongst others (101). Second, the use of RIP-cre and Nestin-cre deletes some, but not all LepRb-expressing neurons as well as deleting many non-LepRb-expressing neurons. Generation of mice bearing LepRb Tyr1077 substitution will help to clarify the physiological role of leptin-mediated STAT5 signaling.
To investigate the potential role in leptin action for signals activated by Jak2 independent of LepRb phosphorylation, homologous recombination was used to replace Lepr with a truncated LepRb mutant (LepRbΔ65) (150). These Δ/Δ mice exhibit a phenotype that resembles db/db mice where energy homeostasis, neuroendocrine and immune function are concerned, but also are characterized by a delayed progression to diabetes, suggesting a modest improvement in glucose homeostasis (150). Taken together, these findings suggest that Jak2-autonomous LepRb signals are insufficient to mediate most of the physiological actions of leptin.
Studies from knock-in mice have therefore offered important insight into the physiological contribution of specific tyrosine residues on LepRb to leptin action in vivo. Although leptin clearly activates the Jak-STAT pathway in mediobasal hypothalamus (186) and although this pathway is clearly critical for leptin regulation of energy homeostasis (14), leptin also elicits neuronal responses in a time frame of seconds to minutes that cannot be explained by STAT3-mediated changes of gene transcription. For instance, in electrophysiological studies using freshly prepared hypothalamic slices, leptin rapidly changes the membrane potential and firing rate of neurons (42, 176), and leptin-induced inhibition of food intake is observed within an hour. These observations prompted a search for leptin-activated signal transduction mechanisms additional to the Jak-STAT pathway.
B. IRS-PI3K pathway
Similarities between insulin and leptin action in the CNS prompted investigation of the hypothesis that intracellular signaling by insulin and leptin converge in key neuronal subsets at the level of the insulin receptor substrate-phosphatidylinositol-3-OH kinase (IRS-PI3K) pathway (Figure 3). Initial support for this hypothesis was provided by electrophysiological studies in which leptin and insulin were shown to hyperpolarize a subset of “glucose-responsive” neurons in the ARC via activation of the ATP-sensitive potassium channel (KATP) (176, 177) via a mechanism that was blocked by a PI3K inhibitor (116, 177). Other early studies showed that like insulin, leptin activates the IRS-PI3K pathway in the hypothalamus, and pharmacological inhibition of PI3K activity in the brain blocks the ability of leptin to reduce food intake (129, 130). A potential mechanism linking the leptin receptor to IRS-PI3K signaling involves the protein SH2B1 (103), which appears to facilitate Jak2-mediated IRS phosphorylation in response to leptin receptor activation. Interesting, genome-wide scans have identified the SH2B1 gene locus as an obesity risk allele in human populations (195). Leptin has also been reported to activate nutrient-sensing enzymes including AMP-activated protein kinase (AMPK) (115) and mammalian target of rapamycin (mTOR) signaling pathways (41), although neither the neuronal subsets nor the molecular mechanism involved has been elucidated.
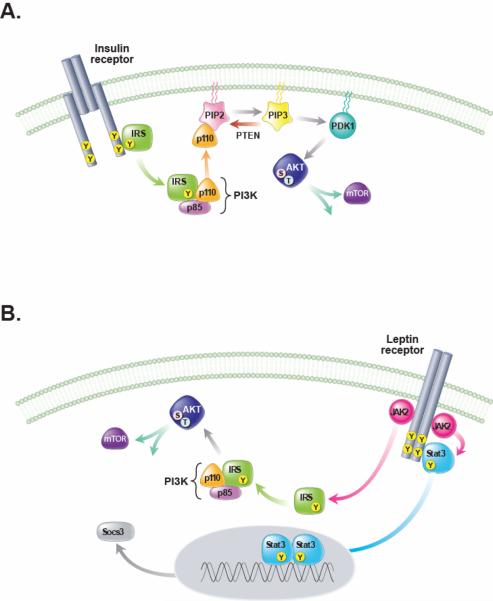
FIGURE 3. Leptin and insulin signal transduction pathways in the CNS.
A. Activation of the insulin receptor induces tyrosine phosphorylation of insulin receptor substrate (IRS) proteins which in turn activate phosphatidyl-inositol-3-kinase (PI3K), an enzyme that phosphorylates phosphatidyl-inositol-4,5-biphosphate (PIP2) to phosphatidyl-inositol-3,4,5-triiphosphate (PIP3) and activates several downstream molecules including 3-phosphoinositide-dependent protein kinase 1 (PDK1), protein kinase B (PKB, also referred to as Akt) and mammalian target of rapamycin (mTOR). B. Leptin binding to the extracellular domain of the LepRb activates Janus kinase-2 (JAK2), leading to binding and tyrosine phosphorylation of STAT3, which dimerizes, translocates to the nucleus and activates transcription of target genes SOCS-3, which can attenuate both insulin and leptin receptor signaling. Like insulin, leptin signaling can also activate PI3K, and downstream targets, presumably via tyrosine phosphorylation of IRS proteins by Jak2. Although leptin and insulin both have the potential to activate IRS-PI3K signaling in neurons and other cell types, important differences in the response to these two hormones exist. These likely arise from divergent roles for PI3K signaling within a given cell type due to differences in the intracellular microdomain in which PI3K signaling is activated, or from distinct cell subpopulations that respond to either leptin or insulin, but not both (e.g. POMC neurons). PTEN, phosphatase and tensin homologue.
We note here that although leptin and insulin both have the potential to activate IRSPI3K signaling in neurons and other cell types, both the subcellular localization and intracellular consequences of this activation can differ substantially depending on cell type and on whether activation is mediated by leptin vs. insulin. Stated differently, the assertion that similarities in the behavioral or metabolic actions of insulin and leptin in the brain reflect convergence of intracellular signaling via PI3K or other transduction mechanisms is an oversimplification, and additional studies are needed to better understand neuronal responses to these hormones and their consequences for energy homeostasis and glucose metabolism (157). Progress made using mouse genetics in this effort is discussed below under Leptin-Sensitive Neuronal Subsets.
III. CNS-MEDIATED EFFECTS OF LEPTIN ON GLUCOSE METABOLISM
A. Leptin and Glucose Metabolism
In addition to the well-established role played by leptin in the regulation of energy homeostasis (198), growing evidence implicates leptin in glucose homeostasis as well, particularly in the control of peripheral tissue insulin sensitivity. The earliest evidence for such a role comes from studies in mice with genetic leptin deficiency (ob/ob mouse) or leptin receptor deficiency (db/db mouse or fa/fa rat). Such animals exhibit not only hyperphagia and obesity, but insulin resistance and diabetes as well (37, 57, 181, 212). While increased food intake and body adiposity clearly contribute to impaired glucose metabolism in these rodent models, several observations suggest that leptin regulates glucose metabolism independently of its effects on energy balance. Not only is caloric restriction limited in its ability to improve insulin sensitivity and hyperglycemia in animals with genetic leptin or leptin-receptor deficiency (122, 203), leptin administration to leptin-deficient mice or humans ameliorates hyperglycemia and hyperinsulinemia (60, 141) even when differences of food intake are controlled by pair-feeding (80, 158).
Severe insulin resistance and diabetes are also observed in genetic disorders characterized by reduced leptin levels due to disorders of adipose tissue development. Such conditions, termed “lipodystrophy,” can result from any of several mutations that impair adipogenesis and thus severely limit the capacity to store triglyceride. In humans, the two most common genetic lipodystrophies are congenital generalized lipodystrophy (CGL), a condition in which body fat is absent from birth, and familial partial lipodystrophy (FPL), where the loss of body fat is progressive and variable, occurring during childhood and puberty (172). In addition to genetic lipodystrophy, acquired lipodystrophies also have been described (and are more common), including acquired partial lipodystrophy (APL), acquired generalized lipodystrophy (AGL), and that induced by highly active antiretroviral therapy (HAART) containing HIV-1 protease inhibitors in HIV-infected patients.
A pathophysiological sequence of events shared in common by these lipodystrophic disorders is that the loss of adipocytes and the inability to store fat leads to accumulation of excess nutrients (including triglyceride) in liver, muscle and other tissues, causing metabolic impairment. Several lines of evidence from both humans and rodent models suggest that leptin deficiency contributes to insulin resistance and diabetes in this setting (139, 168, 169). For one, transplantation of body fat from wild-type animals improves insulin sensitivity in a mouse model of lipodystrophy, whereas transplantation of fat from ob/ob mice has no effect (38, 63). As in leptin-deficient mice, the diabetes phenotype of these lipodystrophic mice is also ameliorated by leptin administration via a mechanism that is, at least in part, independent of its effect on food intake and body weight (64, 168). In humans, leptin therapy has been examined in several clinical trials in patients with lipodystrophy and these have demonstrated significant improvements in glycemia, dyslipidemia and hepatic steatosis (89, 90, 139, 142). At present, congenital lipodystrophy is the only clinical condition for which a compelling indication for leptin therapy has been established.
Another model of leptin deficiency is that which occurs in uncontrolled insulin-deficient diabetes (uDM), induced by the loss of insulin-secreting beta-cells. As the synthesis and storage of fat requires insulin, weight gain cannot occur in uDM, despite elevated food intake, known as “diabetic hyperphagia,” in this setting. Consequently, uDM is characterized by both hyperglycemia and hyperphagia (102), with progressive loss of body fat stores, and with a dramatic reduction of circulating levels of leptin as well as insulin (78). Since the excess calories consumed cannot be stored as fat, they contribute to hyperglycemia and are ultimately lost through the urine, and the ensuing diuresis causes dehydration leading to increased water intake (108). Although insulin deficiency is primarily responsible for hyperglycemia and weight loss in uDM, at least some of the beneficial effects of insulin treatment may in fact arise from an associated increase of leptin action since plasma leptin levels are normalized by insulin treatment in uDM rats (78). Similarly, reduction in the plasma level of both leptin and insulin is implicated in the pathogenesis of diabetic hyperphagia in rats treated with streptozotocin (STZ) (173), a chemical agent that selectively destroys pancreatic beta cells. A role for leptin deficiency in the hyperphagia characteristic of uDM was established by preventing the fall in plasma leptin levels by systemic administration of exogenous leptin at a dose that maintains normal physiological plasma leptin levels in rats with STZ-induced diabetes (173).
Similarly, leptin deficiency has recently been implicated in the progressive insulin resistance and associated neuroendocrine derangements seen in uDM. Systemic administration of exogenous leptin at a dose that maintains normal physiological plasma leptin levels prevented the development of severe, progressive insulin resistance in rats with uDM, an effect that could not be explained by leptin-induced changes of food intake or body weight (68). Moreover, the mechanism underlying this effect appeared to preferentially involve the liver, as physiological leptin replacement in uDM reduced hepatic triglyceride content and gluconeogenic gene expression, and also restored normal insulin signal transduction in liver, but not in skeletal muscle or WAT. This intervention also normalized circulating levels of glucagon and corticosterone, both of which are elevated in uDM. Thus, leptin deficiency plays a key role in neuroendocrine disturbances linked to hyperglycemia in uDM. Yet physiological leptin replacement only modestly reduced blood glucose levels in this setting (68), demonstrating that factors beyond leptin deficiency (and elevated levels of glucagon and corticosterone) are sufficient to explain hyperglycemia in this model. Thus, although prevention of leptin deficiency may ameliorate insulin resistance and related endocrine disorders and hence confer benefit to humans with diabetes, this intervention seems unlikely to substantially improve hyperglycemia in and of itself.
Interestingly, hyperleptinemia induced by either pharmacological leptin administration (34) or with adenoviral gene therapy (210) fully ameliorates hyperglycemia in STZ-induced diabetic rats, even in the face of extremely low plasma insulin levels. Although the mechanism underlying this effect requires additional study, it may involve a suppression of hepatic glucose production (HGP). In normal individuals, insulin-induced suppression of HGP is critical for the control of blood glucose levels. Consequently, in uDM, glucose production is unrestrained, and therefore contributes importantly to diabetic hyperglycemia. Several lines of evidence suggest that hyperglucagonemia contributes to the hyperglycemia in uDM, at least in part by driving the hepatic expression of the gluconeogenic genes, glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate kinase (Pepck) (47, 123).
This hypothesis was articulated by Yu, et al., who reported that the anti-diabetic effects of leptin in STZ-induced diabetes are accompanied by normalization of elevated glucagon levels (210). Moreover, glucagon receptor-null mice remain normoglycemic following treatment with alloxan to induce uDM, suggesting that intact glucagon signaling is required for diabetic hyperglycemia and hence that the leptin-mediated decrease of plasma glucagon levels may mediate its effects on blood glucose (191). Although this hypothesis merits further study, the observation that in rats with STZ-induced uDM, hyperglycemia is only slightly improved by physiological leptin replacement despite normalized plasma glucagon levels (68) suggests that the latter cannot fully explain the hypoglycemic effect of pharmacological leptin administration in this setting. Collectively, these data suggest that deficient endogenous leptin signaling underlies at least some manifestations of uDM, and raise the intriguing possibility that leptin therapy may one day constitute a useful adjunct to insulin in diabetes treatment.
B. Sites and CNS Mechanisms Underlying Leptin Action on Glucose Metabolism
To determine the mechanism by which leptin influences glucose metabolism, the acute effects of a pharmacological dose of leptin on peripheral and hepatic insulin action were studied using the euglycemic-hyperinsulinemic clamp technique. In non-diabetic rats, an acute intravenous (i.v.) leptin infusion enhanced insulin's ability to suppress glucose production without affecting glucose uptake. This enhanced insulin-mediated suppression of glucose production was due to a marked suppression of hepatic glycogenolysis, whereas gluconeogenesis was paradoxically increased (152). In wild-type mice, i.v. leptin increased glucose turnover and glucose uptake but decreased hepatic glycogen content (91). In ob/ob mice, i.v. leptin also increased glucose turnover and stimulated glucose uptake in brown adipose tissue (BAT), brain and heart, but not in skeletal muscle or white adipose tissue (WAT) (29). Among several lines of evidence implicating the brain in the actions of leptin on glucose metabolism is the observation that the effect is similar whether leptin is administered i.v. or via i.c.v. infusion (at much lower doses) in both rats (106) and mice (91). Despite clear evidence that leptin has direct actions on pancreatic β-cells, skeletal muscle, fat, and other tissues, low-dose i.c.v. administration of leptin corrects the insulin resistance and diabetes phenotype of both leptin deficient ob/ob and lipodystrophic mice (2). Similarly, i.c.v. administration of leptin normalizes blood glucose levels in STZ-diabetic rats at doses that are ineffective when administered peripherally (43, 82, 104, 190). Moreover, we recently found that continuous infusion of leptin directly into the brain normalizes blood glucose levels in STZ-induced diabetic rats via a mechanism that cannot be explained by reduced food intake, increased urinary glucose losses or a recovery of pancreatic β-cells. Instead, leptin action in the brain potently suppressed hepatic glucose production while increasing tissue glucose uptake despite persistent, severe insulin deficiency (67). This leptin action is distinct from its previously reported effect to increase insulin sensitivity in the liver (66, 146), and offers compelling evidence of a novel mechanism through which the brain normalizes diabetic hyperglycemia without the need for insulin (67). Taken together, these data provide compelling evidence that the CNS mediates key effects of leptin on glucose metabolism.
Using in situ hybridization and immunohistochemical techniques, leprb mRNA and protein have both been detected in several hypothalamic areas involved in the control of energy balance and autonomic function, including the ARC, ventromedial nucleus (VMH) and dorsomedial nucleus (DMN), with lesser amounts in the paraventricular nucleus (PVN) and the lateral hypothalamic area (LHA) (10, 53). Beyond the hypothalamus, functional leptin receptors are expressed in the hippocampus, hindbrain (including the nucleus of the solitary tract (NTS)), pyriform cortex and other olfactory processing areas, and in mesolimbic areas involved in reward including the ventral tegmental area (VTA) and substantia nigra (SN) (61, 72).
To investigate the role of the ARC in the action of leptin on peripheral glucose metabolism, gene targeting and gene therapy techniques have been used to selectively restore leptin signaling to this brain area in leptin receptor-deficient animals. Coppari and colleagues studied mice in which the endogenous Lepr allele was replaced by a mutant construct through which gene expression is blocked by insertion of a “floxed” stop cassette (Leprneo/neo mice). Microinjection of a virus expressing cre recombinase was then used in these mice to restore Lepr expression in a brain region-specific manner. With this approach, unilateral, selective restoration of leptin signaling to the ARC was found to have only modest effects on food intake and body weight, while it markedly improved hyperinsulinemia and normalized blood glucose levels (40). We developed a complementary approach using the leptin receptor-deficient Koletsky (fak/fak) rat, in which genetic obesity arises from a point mutation that results in the absence of all forms of the leptin receptor (181). These animals are characterized by obesity, hyperphagia, insulin resistance and glucose intolerance but are not hyperglycemic (57). Using adenoviral gene therapy, we found that selective restoration of leptin receptor signaling to the ARC significantly improved peripheral insulin sensitivity via a mechanism that was not dependent on reduced food intake or body weight (122). Furthermore, the effect of leptin signaling in the ARC to improve insulin sensitivity was attenuated by i.c.v. administration of the PI3K inhibitor, LY294002, while it was mimicked by ARC-directed expression of a constitutively active mutant of Akt/protein kinase B (PKB), a key downstream mediator of PI3K (122). These findings suggest that leptin signaling limited to the ARC is sufficient to promote normal insulin sensitivity, and that the neuronal signaling mechanism underlying this leptin effect involves PI3K.
C. Role of Hypothalamic PI3K – Insight from CNS Insulin Action
The hypothesis that PI3K signaling contributes to the effects of hypothalamic leptin action on glucose metabolism is further supported by studies in which the endogenous leptin receptor was replaced with a mutant allele (leprS1138) that prevents LepRb-STAT3 signaling without affecting other transduction pathways. As expected, these mice exhibit hyperphagia and obesity, yet their impairment of glucose metabolism is mild compared to either ob/ob or db/db mice (13). Furthermore, since defective glucose metabolism in these mice is prevented by chronic caloric restriction (13), whereas this does not occur in calorically-restricted db/db mice (203), leptin receptor-mediated STAT3 signaling appears to be critical for energy homeostasis, but not for glucose homeostasis. Leptin-stimulated PI3K signaling likely contributes to this mechanism, based on findings noted above (122), and is consistent with recent evidence that Jak2-mediated signals that are independent of LepRb tyrosine phosphorylation contribute to the modulation of glucose homeostasis by leptin (150).
Like leptin, insulin also activates the IRS-PI3K pathway in hypothalamic neurons, and PI3K is also required for the ability of insulin to inhibit food intake (129). While insulin is well known for its actions in peripheral tissues such as liver, muscle and fat in the control glucose metabolism, several studies identify a role for insulin action in the brain as well. For example, mice with neuron-specific deletion of either the insulin receptor (IR) or insulin receptor-substrate-2 (IRS-2) are characterized by mild obesity and insulin resistance (27, 97, 105). Moreover, while selectively restoring insulin receptors to the liver and pancreas of insulin receptor-deficient mice rescues them from perinatal lethality, restoration of insulin signaling to the brain as well prevents the development of diabetes (136).
Using a complementary approach, Koch and colleagues acutely inactivated insulin receptor signaling either in all tissues including brain (IRΔwb) or in a manner that was restricted to peripheral tissues (IRΔper). The IRΔper mouse model used cre-recombinase fused to the estrogen receptor to acutely induce insulin receptor-deficiency in peripheral tissues, through peripheral administration of the synthetic estrogen receptor antagonist, tamoxifin. To acutely induce insulin receptor-deficiency in both peripheral tissues and brain, a second mouse model was created using conditional RNAi-mediated gene knockdown through the expression of a ubiquitous IR-specific shRNA under the transcriptional control of the human H1/U6 promoter that contains a tetracycline-operated sequence (tetO). Upon doxycycline administration, the tetracycline repressor (tetR) which silences the shRNA is sequestered from the promoter, allowing ubiquitous transcription of the IR shRNA. Using each of these elegant approaches, inducible inaction of the IR in both peripheral tissues and brain consistently causes a more pronounced diabetes phenotype than when receptor deletion occurs in peripheral tissues alone, thus implicating neuronal insulin action in the control of glucose homeostasis (95).
Consistent with a role for CNS insulin action in the control of glucose metabolism, either intrahypothalamic administration of antisense oligonucleotides to reduce insulin receptor signaling or local infusion of the PI3K inhibitor, LY294002, causes hepatic insulin resistance in rats (132). Conversely, administration of insulin into either the third ventricle or the mediobasal hypothalamus improves insulin sensitivity via enhanced suppression of hepatic glucose production, with no effect on glucose uptake (135). Moreover, these effects of insulin are blocked by infusion of the PI3K inhibitor and also appear to require activation of KATP channels (135, 145). Similarly, increasing hypothalamic PI3K signaling, a major intracellular mediator of insulin action, by local overexpression of IRS-2 increases peripheral insulin sensitivity in rats with uDM induced by STZ (65). Collectively, these studies suggest that neuronal PI3K signaling is an important determinant of glucose metabolism, and that the CNS action of insulin is mediated via this mechanism.
D. CNS Nutrient-Sensing
In addition to insulin and leptin, the CNS is capable of sensing and responding to input from nutrient-related signals. Several lines of evidence suggest that one hypothalamic signal of nutrient availability is the intracellular accumulation of long-chain fatty acyl-CoA (LCFA) molecules. Consistent with this hypothesis, pharmacological interventions designed to block LCFA oxidation by increasing malonyl CoA levels (e.g. oleic acid, fatty acid synthase (FAS) inhibitor) in the CNS reduce food intake and HGP (99, 107, 133, 147), while conversely, pharmacological interventions that reduce hypothalamic malonyl CoA levels cause obesity and insulin resistance (79). Nutrient-sensing involving in the CNS play an important role in the regulation of energy balance and glucose metabolism and are described in greater detail elsewhere (31, 98).
The enzyme AMP-activated protein kinase (AMPK) is a ubiquitous fuel sensor that is activated when cellular fuel availability is low. AMPK phosphorylates and inhibits acetyl Co-A carboxylase (ACC), preventing the conversion of acetyl CoA to malonyl CoA, leading to a fall in malonyl CoA levels (179). This, in turn, increases energy utilization (e.g. fat oxidation) by disinhibiting the enzyme carnitine palmitoyltransferase-1 (CPT-1) (154). In the hypothalamus, AMPK activity is sensitive not only to changes of nutrient (e.g., glucose) availability, but is inhibited by central administration of either insulin or leptin (115, 208). Further, inhibition of hypothalamic AMPK (using an adenovirus expressing the dominant negative form of AMPK) lowers food intake and HGP, while increasing hypothalamic AMPK has the opposite effect (113, 209), mimicking some hypothalamic actions of leptin and insulin. These findings collectively implicate cellular nutrient-sensing mechanisms as downstream mediators of at least some leptin and insulin effects.
E. Leptin-Sensitive Neuronal Subsets
Since its discovery, the neuronal mechanism(s) by which leptin acts in the brain has been an area of intense focus. Peripheral administration of leptin activates neuronal populations throughout the brain, as measured either by pSTAT3 activation (a marker of LepRb activation) or induction of c-fos expression (which can occur both in “first order” neurons activated directly by leptin and in “second order” neurons in a leptin-activated circuit), including regions throughout the hypothalamus such as the ARC, VMH, PVN, LHA and dorsomedial hypothalamus (DMH), and extrahypothalamic areas including the VTA, NTS and thalamus, amongst others (50, 52, 186). Ongoing studies have employed mouse genetics and other strategies to determine the functional role of each of these leptin-sensitive neuronal subsets in the actions of leptin on glucose metabolism, energy balance, immune function and reproduction (128).
Two of the most well characterized leptin-sensitive neuronal populations implicated in the regulation of food intake, energy expenditure and glucose metabolism are contained within the hypothalamic ARC. One of these subsets expresses neuropeptide Y (NPY) and agouti related peptide (AgRP), peptides that stimulate food intake and inhibit energy expenditure, thereby promoting positive energy balance (75, 153, 178). As these NPY/AgRP neurons are inhibited by both leptin and insulin, reduced neuronal input from these hormones (as occurs, for example, during fasting and uDM) increases hypothalamic expression of these peptides (158, 163).
Adjacent to these cells is a neuronal subset that expresses pro-opiomelanocortin (POMC). Unlike NPY/AgRP neurons, POMC neurons are stimulated by leptin (33, 162) whereas the effects of insulin are more complex and context-dependent (35, 204). The POMC cleavage product, alpha-melanocyte stimulating hormone (α-MSH) is released by these neurons and acts on melanocortin receptors (Mc3r/Mc4r) to inhibit food intake, increase energy expenditure and promote weight loss (59). Therefore, in conditions of reduced leptin signaling (e.g. fasting or diet-induced weight loss), POMC neurons are inhibited while NPY/AgRP neurons are activated, a combination of responses that promote positive energy balance and the recovery of lost weight. Hence, NPY/AgRP neurons stimulate feeding not only by releasing NPY, and thereby activating Y1 and Y5 receptors, but by reducing melanocortin signaling via release of AgRP, an endogenous melanocortin-3/4 receptor antagonist (137, 171). Moreover, NPY/AgRP neurons inhibit POMC neurons through release of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) (42) and recent work suggests that loss of GABAergic signaling by AgRP neurons projecting to the parabrachial nucleus causes profound anorexia (201).
While each of these two neuronal subpopulations is critical for normal energy homeostasis, both also appear to be important in the regulation of glucose homeostasis. Central administration of both NPY and the melanocortin-3/4 receptor antagonist, SHU9119, cause insulin resistance and glucose intolerance via mechanisms independent of their effects on food intake (1, 109, 188). Conversely, chronic central administration of the melanocortin agonist, melanotan II (MTII) has the opposite effect (134).
Several additional studies have sought to clarify the role of leptin signaling via the Jak-STAT and the IRS-PI3K pathway in ARC neurons. Electrophysiological studies first demonstrated that both insulin and leptin activate ATP-sensitive potassium channels and thereby hyperpolarize a subset of “glucose-responsive” neurons via a mechanism that requires PI3K (176, 177). Consistent with this, leptin inhibits the firing rate of NPY/AgRP neurons (189), while simultaneously depolarizing POMC neurons (42), and also activates PI3K signaling in the latter cells (204). In contrast to leptin, however, insulin hyperpolarizes and silences both AgRP and POMC neurons (96) and activates PI3K signaling in both these neuronal populations (204). In POMC neurons, therefore, both insulin and leptin activate PI3K signaling, but leptin leads to membrane depolarization and an increase in firing rate, whereas insulin evidently has the opposite effects. Complicating matters further, leptin reduces PI3K signaling in AgRP cells, whereas insulin increases it (204), and yet both hormones inhibit AgRP neuronal firing. Consequently, it has been difficult to reconcile how two different hormones share the same signaling cascade, yet have opposite effects on neuronal firing. To further clarify the role of various intracellular signaling transduction mechanisms in leptin action in vivo, Cre-LoxP technology has been utilized to delete leptin receptors, STAT3 or components of the IRS-PI3K pathway in each of these neuronal populations.
Mice with selective deletion or inactivation of either the insulin receptor or IRS-2 in POMC cells (35, 96) exhibit no obesity phenotype, unlike what is observed with pan-neuronal deletion of these proteins. When both subunits of PI3K (p85α, p110α) were deleted from POMC neurons in mice, leptin failed to depolarize and increase the firing rate of these neurons, and insulin's ability to hyperpolarize POMC neurons was similarly abolished. However, while the anorexic effects of leptin were blunted in these mice, body weight was not altered (84). To further examine the role of the IRS-PI3K pathway in POMC neurons on energy and glucose homeostasis, studies were conducted in which signaling molecules downstream of PI3K were deleted selectively in these cells.
Following its activation, PI3K phosphorylates phosphatidyl-inositol-4,5-biphosphate (PIP2) to phosphatidyl-inositol-3,4,5-triiphosphate (PIP3) and activates several downstream molecules including 3-phosphoinositide-dependent protein kinase 1 (PDK1) and protein kinase B (PKB, also referred to as Akt) (Figure 3A). While selective inactivation of PDK1 in POMC neurons caused a mild-obesity phenotype (15), selective inactivation of the PIP3 phosphatase, PTEN (phosphatase and tensin homologue) in these neurons unexpectedly caused a mild-obesity phenotype, despite increased PIP3 signaling (143). Yet deletion of PTEN in all leptin receptor-expressing cells (e.g., POMC cells along with many other cell types) protects against DIO and improves insulin resistance during high-fat (HF) feeding (144). These seemingly contradictory findings remain incompletely explained, but may reflect multiple, divergent roles for PI3K signaling within a given cell subpopulation, and divergent effects of leptin vs. insulin arising due to differences in the intracellular microdomain in which PI3K signaling is activated. Thus, while it has been established that anorexia induced by icv leptin can be blocked by inhibiting PI3K signaling using a pharmacological approach (130), the contribution of the PI3K signaling pathway in specific hypothalamic neurons to leptin regulation of energy balance and glucose metabolism has been less clear.
To address this conundrum, Elmquist and colleagues recently provided both histological and electrophysiological evidence that leptin and insulin act in distinct subpopulations of POMC cells, rather than acting on the same subset of POMC cells (197). This conclusion is based on evidence that the subset of POMC cells in which leptin-induced c-fos activity is detected is separate from those POMC neurons that express the insulin receptor, and they confirmed that acute effects of insulin in POMC cells differs from that of leptin in ways that suggest an anatomical separation of insulin- and leptin-responsive POMC cells (197). These data support a model in which “crosstalk” between insulin and leptin involves distinct subpopulation of POMC cells, rather convergent signal transduction in the same neurons (Figure 3A,B).
Taken together, these findings offer a plausible explanation for how both insulin and leptin activate the same intracellular signaling enzyme -- PI3K -- and yet have divergent effects on POMC neuronal activity. To further clarify the physiological roles of insulin and leptin signaling in POMC neurons, mice lacking both leptin and insulin receptors in POMC neurons (Pomc-Cre, Leprflox/flox IRflox/flox mice) were compared to mice lacking either insulin receptors alone or leptin receptors alone in these cells (83). Consistent with previous reports, body weight was not affected by deletion of insulin receptors alone in POMC neurons (96), whereas mice lacking leptin receptors from POMC neurons exhibit a mild-obesity phenotype (5). However, the combined deletion of insulin receptors and leptin receptors in POMC neurons ameliorated the obesity phenotype of mice lacking leptin receptors from POMC neurons (83). While previous studies had reported either no effect (5) or only a mild effect on glucose homeostasis in male mice lacking leptin receptors in POMC neurons (165), female mice lacking both insulin- and leptin-receptors in POMC neurons exhibited marked hepatic insulin resistance, as measured using the euglycemic-hyperinsulinemic clamp technique (83), suggesting that actions of both insulin and leptin on discrete subpopulations of POMC neurons act synergistically to suppress HGP.
While leptin-induced activation of STAT3 is critical for normal energy homeostasis (12, 14), selective deletion of STAT3 in either POMC (205) or NPY/AgRP (70) neurons results in a mild-obesity phenotype, as occurs when leptin receptors are selectively deleted from these cells (5, 46, 187). Conversely, overexpression of STAT3 in AgRP neurons results in a lean phenotype that is resistant to DIO due to an increase in locomotor activity and energy expenditure (112). Deletion of leptin receptors from both NPY/AgRP and POMC ARC neurons results in a phenotype that is nearly additive with respect to the increase of body weight and adiposity observed following deletion of leptin receptors from individual neuronal subsets, but the overall effect is still modest compared to obesity caused by leptin deficiency (187). This outcome is not surprising, given that leptin receptors are expressed in many other hypothalamic areas, as well as in extrahypothalamic sites that likely contribute to leptin's anorectic effects. Consequently, leptin receptors must be restored widely throughout the CNS using several neuron-specific promoters to normalize obesity, fertility and glucose metabolism in db/db mice (44).
The impact of leptin signaling in POMC neurons on energy homeostasis and glucose metabolism was also recently investigated by Bjorbaek and colleagues by reactivating leptin signaling selectively in POMC neurons of mice that otherwise lack leptin receptors. Expression of leptin receptors selectively in POMC neurons of Leprdb/db mice (HA-LRb/Pomc-Cre/Leprdb/db) reduced food intake and body weight, while normalizing blood glucose levels independently of changes in energy balance (88). These data provide further evidence that leptin signaling in POMC neurons plays an important in leptin's ability to regulate glucose metabolism. Similarly, while mice with selective inactivation of the insulin receptor in AgRP neurons maintain normal body weight, they exhibit hepatic insulin resistance, implying a role for insulin action in AgRP expressing cells in the maintenance of normal liver insulin sensitivity (96).
In addition to leptin action in ARC neurons, several lines of evidence suggest that the VMH also plays an important role in leptin's effects on glucose metabolism. Neurons in the VMH express the leptin receptor (53), are activated by leptin (as measured by induction of either pSTAT3 or cFos) (124), and leptin rapidly depolarizes and increases the firing rate of steroidogenic factor-1 (SF1)-containing neurons in this brain area (180). In addition, microinjection of leptin directly into the VMH promotes glucose uptake in peripheral tissues via a mechanism involving the SNS (77, 114), whereas selective deletion of leptin receptors from SF-1 positive neurons results in an obese, insulin-resistant phenotype, an effect that is exaggerated when leptin receptor deficiency is present in both POMC and VMH neurons (20, 46). Neurons in the VMH are implicated in the regulation of melanocortin signaling (180), whereas melanocortins also regulate the function of VMH neurons. For example, BDNF-containing neurons in the VMH are regulated by both nutritional state and by Mc4r signaling (206). Furthermore, mice with reduced expression of the BDNF receptor, TrkB, exhibit a phenotype characterized by obesity, hyperphagia and increased body length, similar to that of Mc4r deficient mice, and leptin administration induces Bdnf expression in the VMH (206). Given that a subset of VMH neurons send strong excitatory inputs to POMC neurons in the ARC (180), these data implicate a subset of VMH neurons as components of the melanocortin pathway and suggest that leptin signaling in this brain area participates in the control of glucose metabolism.
The VMH is also implicated in the control of glucagon secretion. Studies dating back to the 1960's and 1970's demonstrated that electric stimulation of the VMH increases glucagon secretion, promotes liver glycogen breakdown and raises blood glucose levels via the ANS (166, 167). Moreover, VMH neurons are critical for sensing and responding to hypoglycemia (111). Thus, leptin signaling in the VMH offers a plausible mechanism to explain leptin-mediated normalization of hyperglycemia and hyperglucagonemia in STZ-induced diabetes (210).
F. Effect of CNS Leptin to Regulate Glucose Metabolism
Investigation into how leptin signaling in the brain improves peripheral insulin sensitivity has been hampered to some extent by divergent outcomes from studies that employed a pharmacological approach in normal animals versus those using a gene therapy approach in animals that lack leptin receptors. In normal rats, i.c.v. leptin administration stimulates hepatic gluconeogenesis, but fails to affect overall hepatic glucose production owing to a simultaneous decrease of glycogenolysis (106). Subsequent studies demonstrated that the effect of i.c.v. leptin on gluconeogenesis and expression of the gluconeogenic enzyme, Pepck were blocked when activation of neuronal melanocortin signaling was inhibited using the melanocortin-3/4-receptor antagonist, SHU9119, while leptin suppression of glycogenolysis remained intact, resulting in a net decrease of glucose production (73). Thus, pharmacological leptin stimulation of gluconeogenesis appears to involve a melanocortin-dependent pathway while leptin inhibition of glycogenolysis is proposed to occur via a melanocortin-independent pathway. In addition, the acute effects of hypothalamic leptin signaling on liver glucose flux require leptin receptor signaling through STAT3 (28). Since i.c.v. leptin infusion reverses insulin resistance induced in rats by short-term exposure to a HF diet by reducing both glycogenolysis and gluconeogenesis (146), these data suggest that that metabolic response of normal rats to i.c.v. leptin is context-dependent, and further, that CNS responses may contribute to the pathogenesis of peripheral insulin resistance during HF feeding.
Recent work has begun to clarify how hypothalamic leptin action affects peripheral glucose metabolism. Using the euglycemic-hyperinsulinemic clamp technique to measure changes of glucose metabolism induced by restoring leptin receptor signaling to the ARC of leptin receptor-deficient Koletsky rats, improved insulin sensitivity was shown to involve enhanced insulin-induced suppression of glucose production, rather than changes of glucose uptake (66). These effects of restored ARC leptin signaling were associated with enhanced insulin signal transduction via PI3K in liver, but not skeletal muscle and were also associated with reduced hepatic expression of key gluconeogenic genes, G6Pase and Pepck (66). Thus, leptin action in the ARC increases peripheral insulin sensitivity primarily via effects on the liver, and like insulin, this effect is PI3K-dependent and involves inhibition of hepatic gluconeogenic gene expression.
One potential mechanism whereby leptin could alter hepatic insulin sensitivity is via inhibition of the hepatic enzyme, stearoyl-coenzyme A desaturase 1 (SCD-1), which catalyzes the synthesis of monounsaturated fatty acids (MUFA, such as oleic acid) from saturated FFA (e.g. palmitate, stearate) and is essential for de novo lipogenesis. Hepatic Scd-1 mRNA levels are markedly increased in ob/ob mice, an effect implicated in the dramatic increase of hepatic triglyceride synthesis and steatosis characteristic of these animals (36). Since i.c.v. (as well as systemic) leptin treatment inhibits hepatic Scd-1 gene expression via a mechanism that cannot be attributed to reduced food intake, leptin regulation of hepatic Scd-1 gene expression may involve a central site of action (36). Moreover, hepatic insulin resistance induced by HF feeding is blocked by reducing hepatic SCD-1 expression using antisense oligodeoxynucleotides (ASO) (74), and whole-body Scd-1 deficient mice are protected against DIO and its metabolic sequelae. In addition, introduction of SCD-1 deficiency in lipodystrophic aP2-nSREBP-1c mice corrects hepatic steatosis, although it does not ameliorate diabetes (2), and loss of SCD-1 in ob/ob mice reduces body weight and hepatic steatosis but worsens diabetes (62). Thus, although SCD1 regulation likely contributes to effects of leptin on hepatic TG and lipoprotein synthesis, its role in leptin regulation of hepatic glucose metabolism is uncertain.
Another potential mediator of leptin control of glucose metabolism is IGF-binding protein-2 (IGFBP2). Using microarrays, hepatic expression of Igfbp2 was shown to be up-regulated by leptin, and IGFBP2 plasma levels were also increased following leptin treatment in both ob/ob and wild-type mice (80). Moreover, adenoviral-induced overexpression of IGFBP2 normalized blood glucose levels in ob/ob mice via improved hepatic insulin sensitivity, accompanied by reduced hepatic expression of the gluconeogenic genes, G6Pase and Pepck, along with a significant reduction of hepatic steatosis (80). Over-expression of IGFBP2 also reduced blood glucose levels in models of leptin and insulin resistance/deficiency including agouti, DIO and STZ-induced diabetic mice. However, the question of whether IGFBP2 is required to mediate effects of leptin on glucose metabolism remains unanswered, and the mechanism whereby leptin induces Igfbp2 is also unknown. Additional uncertainty stems from early observations that insulin is a major inhibitory regulator of hepatic Igfbp2, such that expression of the latter is markedly increased in animals with insulin-deficient diabetes (67, 68, 194). Since insulin treatment of diabetic rodents lowers both HGP and Igfbp2 gene expression (26, 138, 194), the notion that IGFBP2 plays a major role in leptin-mediated inhibition of HGP seems counterintuitive. Additional studies are warranted address these questions.
In addition to effects on HGP, at pharmacological doses, central administration of leptin in normal animals increases whole body glucose turnover and glucose uptake in peripheral tissues including skeletal muscle, heart and brown adipose tissue (BAT), but not white adipose tissue (WAT), without changes in plasma insulin or glucose levels (91). The hypothalamus is implicated in these effects since microinjection of leptin directly into the VMH increases insulin-independent glucose uptake in skeletal muscle, heart and BAT (114) via a mechanism involving the sympathetic nervous system (SNS) (77). The observation that these effects of leptin were blocked by the Mc3/4r antagonist, SHU9119 suggests that this effect of leptin is dependent on activation of melanocortin receptors (185). By comparison, leptin injection into the ARC increases glucose uptake only in BAT, while injection into either the LHA (114), DMH or PVN has no effect (185). These findings support a model in which autonomic responses triggered by leptin action in the brain play a key role in changes of peripheral glucose metabolism.
One attractive candidate for mediating the effect of central leptin action on systemic glucose clearance is via autonomic activation of AMPK in peripheral tissues. AMPK is a master sensor of cellular energy expressed in key metabolic tissues including liver, skeletal muscle, adipose tissue and hypothalamus (179), and is activated when fuel availability is reduced, as during exercise, which in turn stimulates fat oxidation while increasing glucose uptake in muscle via an insulin-independent mechanism (154). Moreover, recent evidence suggests leptin action in the hypothalamus activates AMPK in skeletal muscle via a mechanism involving the sympathetic branch of the autonomic nervous system (115).
However, the mechanism whereby leptin signaling in the CNS communicates to peripheral tissues to stimulate glucose uptake is not well understood. To identify the neural circuits whereby leptin promotes glucose uptake in skeletal muscle, a recent study used LepRb-GFP reporter mice in combination with muscle-specific injection of an mRFP-expressing pseudorabies virus (PRV) (3). They found that while injection of PRV in muscle led to PRV labeling within multiple areas of the hypothalamus and hindbrain, the only area of co-localization between leptin receptor expressing cells and muscle-derived PRV was within the NTS and the retrochiasmatic area (RCh) (3), an area that projects to leptin-sensitive neurons in the intermediolateral (IML) nucleus of the spinal cord (51). While populations may yet exist in other brain areas, these data implicate that the NTS and RCh are key sites whereby leptin action in the brain activates autonomic relays that affect skeletal muscle.
G. Extrahypothalamic Neurocircuits Involved in Leptin Regulation of Glucose Metabolism
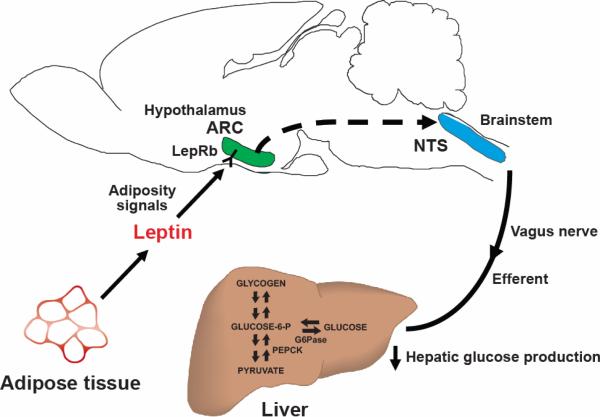
One mechanism proposed to explain how leptin reduces food intake is by enhancing the CNS response to gut-derived satiety peptides, such as cholecystokinin (CCK) and glucagon-like-peptide-1 (GLP-1) (196) that are released upon food ingestion and activate vagal afferent fibers that terminate in the NTS (9, 25, 55, 121). Available evidence suggests that leptin signaling in forebrain areas such as the ARC activates a descending projection to the NTS, which in turn, enhances the hindbrain response to input from CCK (122). Interestingly, a parallel neurocircuit is implicated in the mechanism that links hypothalamic leptin action to the control of hepatic insulin sensitivity, in which leptin-sensitive forebrain neurons regulate the activity of neurons in the NTS and adjacent dorsal motor nucleus of the vagus (DMX). Contained within the DMX are vagal motor neurons that supply the liver and other tissues, and their activity is tonically regulated by input from the NTS. The NTS, in turn, receives input from both afferent vagal fibers and from descending projections from the hypothalamus and other brain areas (Figure 5).
FIGURE 5. Model of the hypothalamic regulation of hepatic glucose production.
Leptin-sensing neurons in the hypothalamic ARC receive input regarding energy stores and in response to this input, pathways that increase hepatic vagal tone to the liver are activated, increasing hepatic insulin sensitivity. ARC, arcuate nucleus; LepRb, leptin receptor; NTS, nucleus of the solitary tract; G6Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate kinase.
Consistent with this hypothesis, both local administration of insulin and inhibition of fat oxidation in the ARC increase hepatic insulin sensitivity via a mechanism that requires intact hepatic vagal innervation, and also activates hindbrain neurons within both the NTS and DMX (147). Moreover, the beneficial effects of restoring hypothalamic leptin signaling to Koletsky rats on hepatic insulin sensitivity were blocked by selective hepatic vagotomy (66). However, selective hepatic vagotomy failed to affect hepatic glucose metabolism in leptin receptor-deficient Koletsky rats that did not receive leptin gene therapy, and previous studies have demonstrated that selective removal of vagal tone to the liver by itself in genetically normal rats has no effect on insulin-induced suppression of HGP. Similarly, while abundant evidence suggests that vagal afferents play a critical role in the ability of gut-derived satiety signals, such as CCK, to promote the termination of a meal, selective vagotomy by itself fails to affect energy homeostasis (19). Thus, additional studies are required to determine the physiological relevance of this circuit in the control of glucose metabolism.
H. Indirect Effects of CNS Leptin on Glucose Metabolism
While several lines of evidence suggest that leptin action in the brain improves glucose metabolism independent of its effects on energy homeostasis, the effect of leptin to reduce food intake and body weight (and body fat mass) also likely contributes to the overall effects of leptin to improve glucose metabolism, since weight loss as small as 5–10% can substantially improve insulin sensitivity in obese patients (16).
Yet another potential mechanism whereby leptin signaling in the brain can influence insulin action is via effects on lipid metabolism. Infusion of leptin directly into the mediobasal hypothalamus during an euglycemic-hyperinsulinemic clamp suppressed the expression of key de novo lipogenic enzymes in WAT and also activated lipolysis and suppressed FFA uptake into this tissue (28). Surprisingly, while the ability of hypothalamic leptin to regulate food intake, glucose metabolism and reproductive function requires intact STAT3 signaling, these effects of leptin on adipose tissue metabolism are STAT3-independent, appearing instead to involve hypothalamic PI3K and SNS outflow (28).
Pertinent to this discussion is the phenotype of mice in which PI3K signaling in leptin-receptor expressing neurons in the CNS is increased by deleting PTEN using cre-loxP technology, alluded to earlier. Selective PTEN ablation in leptin-expressing neurons results in a phenotype characterized by reduced adiposity and increased energy expenditure due, at least in part, to increased fatty acid oxidation in WAT (144), associated with increased sympathetic nerve activity to WAT and leading to a remodeling of WAT to take on characteristics of BAT, including increased mitochondrial content and UCP1 expression (144).
Leptin-mediated activation of the melanocortin pathway may contribute to these potent effects on adipose tissue metabolism. Pharmacological inhibition of melanocortin receptors in the CNS increases WAT lipid uptake, triglyceride synthesis and fat accumulation, whereas activation of neuronal melanocortin receptors stimulates lipid mobilization independent of changes in food intake. Moreover, while reduced melanocortin signaling promotes insulin sensitivity and glucose uptake in WAT, it reduces glucose utilization in BAT and muscle (131), favoring increased TG synthesis and storage in the body.
V. PERSPECTIVES
A. Leptin Resistance and Obesity Pathogenesis
While absolute leptin deficiency causes obesity (118, 212), most obese humans and animal models exhibit elevated circulating plasma leptin levels (39). This is not surprising, given that leptin is synthesized and secreted by adipocytes in direct proportion to total body fat mass (39). Yet an abundant literature supports the hypothesis that body fat is regulated by a negative feedback loop described earlier that adjusts food intake and energy expenditure as needed to promote the stability of body fuel stored as fat. These observations raise a fundamental paradox: if body fat is homeostatically regulated, increases of body fat should activate adaptive responses involving increased leptin secretion that protect against weight gain. How, then, do obese individual's maintain elevated weight in the face of elevated leptin levels?
One potential explanation is that under normal conditions, this homeostatic system defends against both weight loss and weight gain, but that common forms of obesity develop due to genetic or acquired defects that impair the response to elevated leptin levels (159). Obesity in both humans and in rodents placed on a HF diet are characterized by hyperleptinemia, and the ability of leptin to suppress food intake and reduce body weight is attenuated (49, 81). Consistent with this observation, the ability of leptin to activate pSTAT3, a marker of leptin signaling (49), is impaired in rodent models of DIO, specifically in the hypothalamic ARC (56, 124). This concept is commonly referred to as “leptin resistance”, and is somewhat analogous to “insulin resistance” whereby elevated insulin levels are required to maintain blood glucose levels in the normal range. Indeed, the same cellular mechanisms that impair insulin signaling in tissues such as liver and muscle of obese individuals may also contribute to the pathogenesis of leptin resistance.
The identification of cellular mechanisms that can explain impaired leptin signaling in DIO is an area of intense current research. One model proposes that during positive energy balance and excess body fat gain, leptin resistance is caused by elevated plasma leptin levels, resulting in chronic overstimulation of the leptin receptor and associated SOCS-3 induction (22, 54, 155). Consistent with this hypothesis, heterozygous SOCS-3 deficient mice display increased leptin sensitivity and are protected against the development of obesity on a HF diet (87). This effect appears to be neurally mediated, as neuron-specific SOCS-3 conditional knockout mice exhibit a similar phenotype (119), suggesting that in neurons, SOCS-3 plays a physiological role to favor positive energy balance and weight gain. Moreover, mutation of LRb Tyr985 prevents SOCS-3 action on the leptin receptor and thereby results in mice which display increased leptin sensitivity, are lean and are resistant to DIO (24). An important consideration in the interpretation of this work, however, is that pro-inflammatory signaling induced in the hypothalamus during HF feeding (see below) also increases SOCS-3 expression. So while a compelling argument exists in support of a causal role of hypothalamic SOCS-3 in the pathogenesis of common forms of leptin resistance, whether SOCS-3 induction is due to hyperleptinemia, pro-inflammatory cellular responses or some combination of these awaits clarification.
Interestingly, mice expressing a constitutively-active version of STAT3 selectively in POMC neurons causes a hyperphagic, obese phenotype accompanied by central insulin and leptin resistance (58), offering further (albeit indirect) evidence that induction of SOCS-3 in these cells impairs energy homeostasis. Moreover, while up-regulation of SOCS-3 selectively in POMC neurons causes leptin resistance and obesity, somewhat surprisingly, over-expression of SOCS-3 in leptin receptor-positive neurons did not (149). Thus, SOCS-3 may play a cell-type specific role in the regulation of energy balance. Again, it is important to recognize that SOCS-3 is induced not only by pSTAT3, but also by inflammatory signaling through the IKKβ-NFκB pathway, when interpreting these outcomes.
Similar to SOCS-3, the protein tyrosine phosphatase (PTP)1B inhibits leptin and insulin signaling in cultured cells and in vivo via dephosphorylation of Jak2 (48, 93). Consistent with this, HF feeding increases PTP1B expression in the hypothalamus and pharmacological inhibition of PTP1B in the brain improves leptin sensitivity in aged-leptin resistant rats (120). Moreover, neuronal (17) or POMC-specific (7) PTP1B knockout mice are protected from DIO and exhibit increased leptin sensitivity. These data suggest that both SOCS-3 and PTP1B are possible mediators that contribute to reduced LepRb signaling in obesity.
A growing literature implicates cellular inflammation in the pathogenesis of obesity-associated metabolic impairment in peripheral tissues (85, 86, 170). Excess body adiposity has been associated with activation of macrophages and associated inflammation in a variety of peripheral tissues including WAT, liver, skeletal muscle and the vasculature and eventually is accompanied by increased circulating plasma levels of pro-inflammatory cytokines (193, 207). Recent evidence suggests that consumption of a HFD also induces inflammation in the hypothalamus, an effect that itself could favor weight gain by impairing the neuronal response to input from leptin and insulin (184). Consistent with this notion, rats fed a HFD for 20-weeks exhibit both leptin resistance and hypothalamic inflammation, as measured by elevated mRNA and protein levels of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) resulting from activation of IKKβ and JNK-1 (45, 148, 211). Beyond these correlative observations, recent work has begun to assess whether hypothalamic inflammation is a cause of DIO, or is simply a consequence.
Consistent with hypothalamic inflammation driving HF-induced obesity, activation of hypothalamic IKKβ/NF-κB signaling using a gene therapy approach promotes hyperphagia and weight gain and reduces leptin and insulin signaling in mice (211). Conversely, interventions that reduce hypothalamic inflammation, either by reducing neuronal IKKβ signaling using a gene therapy approach (211) or by pharmacological administration of a IKKβ inhibitor, reduce food intake and body weight in DIO animals (148) and maintain central leptin and insulin sensitivity. Thus, hypothalamic inflammation appears to be both necessary and sufficient for HF-induced obesity. This notion does not exclude a pathogenic role for hyperinsulinemia, since both inflammatory signaling and leptin share in common the ability to induce SOCS-3.
Further evidence of a role for SOCS-3 in the pathogenesis of DIO stems from mice with cell-type specific deletion of IKKβ in AgRP neurons, which are protected against DIO and exhibit reduced expression of SOCS-3 in the mediobasal hypothalamus (211). Moreover, increasing SOCS-3 expression in the mediobasal hypothalamus using a lentiviral approach blocked the protection against DIO conferred by AgRP-neuron-specific IKKβ knockout (211). Although the extent to which leptin resistance induced by hypothalamic inflammation depends upon SOCS-3 remains to be established, these data suggest that both hyperleptinemia and inflammation may contribute to central leptin resistance via an overlapping mechanism involving SOCS-3.
Yet another mechanism that may contribute to hypothalamic inflammation and central leptin and insulin resistance is endoplasmic reticulum (ER) stress. Recent studies demonstrate that ER stress and the unfolded protein response (UPR) are activated in the hypothalamus of obese mice and that this effect is sufficient to inhibit leptin signaling (140). In support of the hypothesis that ER stress is involved in pathways that mediate nutritional activation of hypothalamic IKKβ/NF-κB, central administration of an ER stress inhibitor suppressed hypothalamic NF-κB levels in HF-fed rats and lowered both food intake and body weight in DIO animals (211). Conversely, induction of ER stress in the brain of mice and rats increases food intake and body weight and blocks the anorexic effects of insulin and leptin (140, 199). In addition, neuronal deletion of X-box binding protein-1 (XBP-1) in mice increased food intake and body adiposity, and is accompanied by hypothalamic leptin resistance (140).
To compare the contribution of hyperleptinemia versus a HFD itself to the development of leptin resistance a recent study treated ob/ob mice with systemic leptin continuously at a dose designed to “clamp” plasma levels to that of wild-type controls fed standard chow (94). Following the switch to a HFD, wild-type and leptin-infused ob/ob mice displayed similar weight gain despite markedly higher plasma leptin levels in the former group (94). Moreover, wild-type mice exposed to a HFD were resistant to the ability of leptin to reduce food intake and body weight and to induce hypothalamic pSTAT3 relative to leptin-infused ob/ob mice fed a HFD. The authors' concluded from these studies that hyperleptinemia is required for the development of leptin resistance (94). However, the important question of whether hyperleptinemia is sufficient to induce leptin resistance was not examined in this study (since ob/ob mice did not receive leptin at doses that induce hyperleptinemia) and perhaps more importantly, the study design presumes that wild-type and leptin-infused ob/ob mice are fundamentally indistinguishable where leptin sensitivity and energy homeostasis are concerned, despite clear evidence that ob/ob mice are more sensitive to leptin than normal mice (30, 76). Additional studies are therefore needed to determine the extent to which elevated plasma leptin levels contribute to leptin resistance and associated weight gain during HF feeding.
One conclusion stemming from this area of science is that viable mechanisms have finally emerged with the potential to link altered hypothalamic function to sustained weight gain in models of DIO (127). A second inference is that no single mechanism is likely predominate in the pathogenesis of obesity-associated leptin resistance and, like insulin resistance in peripheral tissues, it seems probable that numerous mechanisms contribute in ways that are challenging to disarticulate.
B. Physiological Relevance
The regulation of blood glucose levels is a complex and highly integrated process involving the brain and peripheral tissues. In this review, we have highlighted evidence that the adiposity signal leptin plays an important role in the control not only of energy homeostasis, but of glucose homeostasis as well, and that the CNS is implicated in both leptin effects. Yet most approaches used to investigate the role of leptin in glucose homeostasis employ either genetic models (e.g. ob/ob mice, db/db mice or fak/fak rats) or pharmacological doses of leptin in normal animals. While this research has generated a wealth of information and has advanced our understanding of how leptin acts in the CNS and how CNS leptin action is communicated to the periphery, whether these leptin effects play a physiological role in glucose homeostasis on a minute-to-minute, hour-to-hour and day-to-day basis in genetically normal humans and animal models, and whether such effects are neurally-mediated, are key questions that remain incompletely answered.
Other technological considerations warrant comment. To examine the physiological mechanisms whereby insulin inhibits HGP in vivo, many experiments employed the pancreatic clamp, a technique that employs the infusion of somatostatin to block endogenous insulin and glucagon secretion, along with i.v. infusion of insulin and glucagon to maintain basal levels. One limitation of this approach relates to the fact that endogenous insulin is secreted into the hepatic portal vein, such that the liver is exposed to insulin levels higher than in the systemic circulation. Since most studies do not account for this difference (with the consequence that systemic and hepatic portal vein levels of insulin are matched), the question of whether neural sensing of insulin (or leptin) participate in the control of HGP has been challenged based on evidence that under true physiological conditions, insulin action on the liver is mediated primarily via a direct effect (32). While most investigators can agree that the brain senses changes of circulating insulin levels, whether insulin action in the brain plays a physiological role in the day-to-day regulation of glucose production by the liver or other tissues remains to be firmly established, and the same may be said about the role of leptin in glucose metabolism.
VI. CONCLUDING REMARKS
In conclusion, a large and growing literature supports the hypothesis that input to the CNS from afferent signals such as leptin is integrated with that from a variety of other hormonal and nutrient-related signals to co-ordinate efferent responses that control food intake, energy expenditure, hepatic insulin sensitivity and glucose uptake in the service of energy and glucose homeostasis. Evidence of substantial overlap in the CNS circuits that regulate energy balance and those that regulate glucose metabolism raises the possibility that reduced secretion, sensing, or responsiveness to these afferent signals promotes both weight gain and insulin resistance. Therapies that target the CNS and restore sensitivity of neuronal circuits to key afferent signals have the potential to improve currently available treatments for obesity and diabetes.
FIGURE 4. CNS neurocircuits regulating energy and glucose homeostasis.
A number of hypothalamic and extrahypothalamic sites have been implicated in the action of leptin to regulate energy and glucose homeostasis. The hypothalamic ARC contains neuropeptide Y and agouti-related peptide (NPY/AgRP) neurons that stimulate food intake and are inhibited by leptin, and pro-opiomelanocortin (POMC) neurons that reduce food intake and are stimulated by leptin. NPY/AgRP neurons also inhibit POMC neurons via synaptic release of the neurotransmitter γ-aminobutyric acid (GABA). ARC, arcuate nucleus; LepRb, leptin receptor; Mc3r/Mc4r, melanocortin-3/4 receptor; VMH, ventromedial hypothalamus; LHA, lateral hypothalamic area; PVN, paraventricular nucleus; DMH, dorsomedial hypothalamus; VTA, ventral tegmental area; NTS, nucleus of the solitary tract.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants (MWS) DK068384, DK083042 and DK052989, and by the Nutrition Obesity Research Center (N.O.R.C., DK035816), the Diabetes Endocrine Research Center (D.E.R.C., P30 DK17047) and the Mouse Metabolic Phenotyping Center (M.M.P.C; U24 DK076126) at the University of Washington. G.J. Morton is supported by an American Heart Association Scientist Development Grant.
REFERENCES
- 1.Adage T, Scheurink AJ, de Boer SF, de Vries K, Konsman JP, Kuipers F, Adan RA, Baskin DG, Schwartz MW, van Dijk G. Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats. J Neurosci. 2001;21:3639–3645. doi: 10.1523/JNEUROSCI.21-10-03639.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, Friedman JM. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic T, Purpera MN, Banfield BW, Berthoud HR, Morrison CD. Innervation of skeletal muscle by leptin receptor-containing neurons. Brain Res. 2010;1345:146–155. doi: 10.1016/j.brainres.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967;46:1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 7.Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banting FG, Best CH. Pancreatic extracts. 1922. J Lab Clin Med. 1990;115:254–272. [PubMed] [Google Scholar]
- 9.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte D, Jr, Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47:353–362. doi: 10.1177/002215549904700309. [DOI] [PubMed] [Google Scholar]
- 11.Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM. Insulin and insulin-like growth factors in the CNS. Trends Neurosci. 1988;11:107–111. doi: 10.1016/0166-2236(88)90155-5. [DOI] [PubMed] [Google Scholar]
- 12.Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 13.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metabolism. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 15.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Avraham S, Harman-Boehm I, Schwarzfuchs D, Shai I. Dietary strategies for patients with type 2 diabetes in the era of multi-approaches; review and results from the Dietary Intervention Randomized Controlled Trial (DIRECT) Diabetes Res Clin Pract. 2009;86(Suppl 1):S41–48. doi: 10.1016/S0168-8227(09)70008-7. [DOI] [PubMed] [Google Scholar]
- 17.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 18.Bernard C. Lecons de physiologie experimentale appliqués à là medecine. Baillere et Fils; Paris: 1854. [Google Scholar]
- 19.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 22.Bjorbaek C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 23.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 24.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brainstem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004 doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 26.Boni-Schnetzler M, Schmid C, Mary JL, Zimmerli B, Meier PJ, Zapf J, Schwander J, Froesch ER. Insulin regulates the expression of the insulin-like growth factor binding protein 2 mRNA in rat hepatocytes. Mol Endocrinol. 1990;4:1320–1326. doi: 10.1210/mend-4-9-1320. [DOI] [PubMed] [Google Scholar]
- 27.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 28.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burcelin R, Kamohara S, Li J, Tannenbaum GS, Charron MJ, Friedman JM. Acute intravenous leptin infusion increases glucose turnover but not skeletal muscle glucose uptake in ob/ob mice. Diabetes. 1999;48:1264–1269. doi: 10.2337/diabetes.48.6.1264. [DOI] [PubMed] [Google Scholar]
- 30.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 31.Caspi L, Wang PY, Lam TK. A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab. 2007;6:99–104. doi: 10.1016/j.cmet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Cherrington AD, Moore MC, Sindelar DK, Edgerton DS. Insulin action on the liver in vivo. Biochem Soc Trans. 2007;35:1171–1174. doi: 10.1042/BST0351171. [DOI] [PubMed] [Google Scholar]
- 33.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 34.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 35.Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford ML, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 37.Coleman DL. Obese and diabetes: Two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 38.Colombo C, Cutson JJ, Yamauchi T, Vinson C, Kadowaki T, Gavrilova O, Reitman ML. Transplantation of adipose tissue lacking leptin is unable to reverse the metabolic abnormalities associated with lipoatrophy. Diabetes. 2002;51:2727–2733. doi: 10.2337/diabetes.51.9.2727. [DOI] [PubMed] [Google Scholar]
- 39.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 40.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwing T, Chua SC., Jr The hypothalamic arcuate nucleus: A key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metabolism. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 42.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 43.da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1275–1282. doi: 10.1152/ajpregu.00187.2006. [DOI] [PubMed] [Google Scholar]
- 44.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 46.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Dobbs R, Sakurai H, Sasaki H, Faloona G, Valverde I, Baetens D, Orci L, Unger R. Glucagon: role in the hyperglycemia of diabetes mellitus. Science. 1975;187:544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 48.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene [see comments] Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 49.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 51.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 52.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 53.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. 1998;395:535–547. [PubMed] [Google Scholar]
- 54.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 55.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276:R1545–1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 56.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Ernsberger P, Koletsky RJ, Friedman JE. Molecular pathology in the obese spontaneous hypertensive Koletsky rat: a model of syndrome X. Ann N Y Acad Sci. 1999;892:272–288. doi: 10.1111/j.1749-6632.1999.tb07801.x. [DOI] [PubMed] [Google Scholar]
- 58.Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Munzberg H, Hampel B, Alber J, Kloppenburg P, Bruning JC, Wunderlich FT. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 60.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 61.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 62.Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–1239. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 63.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gavrilova O, Marcus-Samuels B, Leon LR, Vinson C, Reitman ML. Leptin and diabetes in lipoatrophic mice. Nature. 403:850. doi: 10.1038/35002663. discussion 850-851, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG, Jr, Rhodes CJ, Schwartz MW. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3:67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 66.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009 doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.German JP, Wisse BE, Thaler JP, Matsen ME, Taborsky GJ, Jr, Schwartz MW, Morton GJ. American Diabetes Association Annual Scientific Sessions. Orlando, FL: 2010. Leptin action in the brain normalizes diabetic yyperglycemia via insulin-independent mechanisms. [Google Scholar]
- 68.German JP, Wisse BE, Thaler JP, Oh-I S, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky GJ, Jr, Schwartz MW, Morton GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626–1634. doi: 10.2337/db09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 70.Gong L, Yao F, Hockman K, Heng HH, Morton GJ, Takeda K, Akira S, Low MJ, Rubinstein M, MacKenzie RG. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology. 2008;149:3346–3354. doi: 10.1210/en.2007-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 72.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 73.Gutierrez-Juarez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004;279:49704–49715. doi: 10.1074/jbc.M408665200. [DOI] [PubMed] [Google Scholar]
- 74.Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 76.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 77.Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706. [DOI] [PubMed] [Google Scholar]
- 78.Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahren B. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274:R1482–1491. doi: 10.1152/ajpregu.1998.274.5.R1482. [DOI] [PubMed] [Google Scholar]
- 79.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 80.Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. Jama. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 82.Hidaka S, Yoshimatsu H, Kondou S, Tsuruta Y, Oka K, Noguchi H, Okamoto K, Sakino H, Teshima Y, Okeda T, Sakata T. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. Faseb J. 2002;16:509–518. doi: 10.1096/fj.01-0164com. [DOI] [PubMed] [Google Scholar]
- 83.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 86.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 88.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 90.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 91.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 92.Kennedy G. The role of depot fat in the hypothalamic control of food intake in the rat. Procedings of the Royal Society of London. 1953;140:578–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 93.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest. 2004;114:917–927. doi: 10.1172/JCI21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med. 2010;16:392–395. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]
- 99.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 100.Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 101.Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, Louis GW, Leinninger GM, Bertuzzi S, Seeley RJ, Robinson GW, Myers MG, Hennighausen L. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One. 2008;3:e1639. doi: 10.1371/journal.pone.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leedom LJ, Meehan WP. The psychoneuroendocrinology of diabetes mellitus in rodents. Psychoneuroendocrinology. 1989;14:275–294. doi: 10.1016/0306-4530(89)90030-9. [DOI] [PubMed] [Google Scholar]
- 103.Li M, Li Z, Morris DL, Rui L. Identification of SH2B2beta as an inhibitor for SH2B1- and SH2B2alpha-promoted Janus kinase-2 activation and insulin signaling. Endocrinology. 2007;148:1615–1621. doi: 10.1210/en.2006-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282:E1084–1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- 105.Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–916. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu L, Karkanias GB, Morales JC, Hawkins M, Barzilai N, Wang J, Rossetti L. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem. 1998;273:31160–31167. doi: 10.1074/jbc.273.47.31160. [DOI] [PubMed] [Google Scholar]
- 107.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 108.Maghnie M. Diabetes insipidus. Horm Res. 2003;59(Suppl 1):42–54. doi: 10.1159/000067844. [DOI] [PubMed] [Google Scholar]
- 109.Marks JL, Waite K. Intracerebroventricular neuropeptide Y acutely influences glucose metabolism and insulin sensitivity in the rat. J Neuroendocrinol. 1997;9:99–103. doi: 10.1046/j.1365-2826.1997.00554.x. [DOI] [PubMed] [Google Scholar]
- 110.Matsuoka N, Ogawa Y, Hosoda K, Matsuda J, Masuzaki H, Miyawaki T, Azuma N, Natsui K, Nishimura H, Yoshimasa Y, Nishi S, Thompson DB, Nakao K. Human leptin receptor gene in obese Japanese subjects: evidence against either obesity-causing mutations or association of sequence variants with obesity. Diabetologia. 1997;40:1204–1210. doi: 10.1007/s001250050808. [DOI] [PubMed] [Google Scholar]
- 111.McCrimmon R. The mechanisms that underlie glucose sensing during hypoglycaemia in diabetes. Diabet Med. 2008;25:513–522. doi: 10.1111/j.1464-5491.2008.02376.x. [DOI] [PubMed] [Google Scholar]
- 112.Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Bruning JC. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 114.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 115.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 116.Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 118.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 119.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 120.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 123.Muller WA, Faloona GR, Unger RH. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971;50:1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 125.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 126.Mutze J, Roth J, Gerstberger R, Hubschle T. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett. 2007;417:286–291. doi: 10.1016/j.neulet.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 127.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol Metab. 2010 doi: 10.1016/j.tem.2010.08.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: A key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 130.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 131.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 133.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 134.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 136.Okamoto H, Nakae J, Kitamura T, Park BC, Dragatsis I, Accili D. Transgenic rescue of insulin receptor-deficient mice. J Clin Invest. 2004;114:214–223. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 138.Ooi GT, Tseng LY, Rechler MM. Post-transcriptional regulation of insulin-like growth factor binding protein-2 mRNA in diabetic rat liver. Biochem Biophys Res Commun. 1992;189:1031–1037. doi: 10.1016/0006-291x(92)92307-j. [DOI] [PubMed] [Google Scholar]
- 139.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 140.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 141.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 142.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 145.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 146.Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- 147.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metabolism. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 148.Posey K, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Jr, Xu AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59:894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Robertson S, Ishida-Takahashi R, Tawara I, Hu J, Patterson CM, Jones JC, Kulkarni RN, Myers MG., Jr Insufficiency of Janus kinase 2-autonomous leptin receptor signals for most physiologic leptin actions. Diabetes. 2010;59:782–790. doi: 10.2337/db09-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M, Wu J, Wang J. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272:27758–27763. doi: 10.1074/jbc.272.44.27758. [DOI] [PubMed] [Google Scholar]
- 153.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 154.Ruderman NB, Saha AK. Metabolic syndrome: adenosine monophosphate-activated protein kinase and malonyl coenzyme A. Obesity (Silver Spring) 2006;14(Suppl 1):25S–33S. doi: 10.1038/oby.2006.279. [DOI] [PubMed] [Google Scholar]
- 155.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 156.Runge CF. Economic consequences of the obese. Diabetes. 2007;56:2668–2672. doi: 10.2337/db07-0633. [DOI] [PubMed] [Google Scholar]
- 157.Sanchez-Lasheras C, Christine Konner A, Bruning JC. Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte DJ, Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalmic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 159.Schwartz MW, Niswender KD. Adiposity signaling and biological defense against weight gain: absence of protection or central hormone resistance? J Clin Endocrinol Metab. 2004;89:5889–5897. doi: 10.1210/jc.2004-0906. [DOI] [PubMed] [Google Scholar]
- 160.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 161.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 162.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 163.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 164.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 165.Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab. 2008;294:E630–639. doi: 10.1152/ajpendo.00704.2007. [DOI] [PubMed] [Google Scholar]
- 166.Shimazu T. Central nervous system regulation of liver and adipose tissue metabolism. Diabetologia. 1981;20(Suppl):343–356. [PubMed] [Google Scholar]
- 167.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev. 1987;3:185–206. doi: 10.1002/dmr.5610030109. [DOI] [PubMed] [Google Scholar]
- 168.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 169.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 172.Simha V, Garg A. Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol. 2006;17:162–169. doi: 10.1097/01.mol.0000217898.52197.18. [DOI] [PubMed] [Google Scholar]
- 173.Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes. 1999;48:1275–1280. doi: 10.2337/diabetes.48.6.1275. [DOI] [PubMed] [Google Scholar]
- 174.Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44:147–151. doi: 10.2337/diab.44.2.147. [DOI] [PubMed] [Google Scholar]
- 175.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 176.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 177.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 178.Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- 179.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 180.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 181.Takaya K, Ogawa Y, Hiraoka J, Hosoda K, Yamori Y, Nakao K, Koletsky RJ. Nonsense mutation of leptin receptor in the obese spontaneously hypertensive Koletsky rat. Nat Genet. 1996;14:130–131. doi: 10.1038/ng1096-130. [DOI] [PubMed] [Google Scholar]
- 182.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 183.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Morlarty A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 184.Thaler JP, Schwartz MW. Inflammation and Obesity Pathogenesis: The Hypothalamus Heats Up. Endocrinology. 2010 doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58:2757–2765. doi: 10.2337/db09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 187.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.van den Hoek AM, Voshol PJ, Karnekamp BN, Buijs RM, Romijn JA, Havekes LM, Pijl H. Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes. 2004;53:2529–2534. doi: 10.2337/diabetes.53.10.2529. [DOI] [PubMed] [Google Scholar]
- 189.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 190.Wang J, Wernette CM, Judd RL, Huggins KW, White BD. Guanethidine treatment does not block the ability of central leptin administration to decrease blood glucose concentrations in streptozotocin-induced diabetic rats. J Endocrinol. 2008;198:541–548. doi: 10.1677/JOE-08-0135. [DOI] [PubMed] [Google Scholar]
- 191.Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Weigle DS, Bukowski TR, Foster DC, Holderman S, Kramer JM, Lasser G, Lofton-Day CE, Prunkard DE, Raymond C, Kuijper JL. Recombinant ob protein reduces feeding and body weight in the ob/ob mouse. J Clin Invest. 1995;96:2065–2070. doi: 10.1172/JCI118254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.White ME, Leaman DW, Ramsay TG, Kampman KA, Ernst CW, Osborne JM. Insulin-like growth-factor binding protein (IGFBP) serum levels and hepatic IGFBP-2 and -3 mRNA expression in diabetic and insulin-treated swine (Sus scrofa) Comp Biochem Physiol B. 1993;106:341–347. doi: 10.1016/0305-0491(93)90311-r. [DOI] [PubMed] [Google Scholar]
- 195.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O'Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Williams KW, Scott MM, Elmquist JK. From observation to experimentation: leptin action in the mediobasal hypothalamus. Am J Clin Nutr. 2009;89:985S–990S. doi: 10.3945/ajcn.2008.26788D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Won JC, Jang PG, Namkoong C, Koh EH, Kim SK, Park JY, Lee KU, Kim MS. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 200.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 201.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 203.Wyse BM, Dulin WE. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia. 1970;6:268–273. doi: 10.1007/BF01212237. [DOI] [PubMed] [Google Scholar]
- 204.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 206.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang CS, Lam CK, Chari M, Cheung GW, Kokorovic A, Gao S, Leclerc I, Rutter GA, Lam TK. Hypothalamic AMPK Regulates Glucose Production. Diabetes. 2010 doi: 10.2337/db10-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]