Abstract
Introduction
ZM336372 is small molecule tyrosine kinase modulator. It has been shown to inhibit glycogen synthase kinase-3β (GSK-3β) through phosphorylation of GSK-3β at Ser 9. GSK-3β has previously been shown to mediate cell survival in pancreatic cancer cells. Here we determine the effects of ZM336372 on GSK-3β phosphorylation, apoptosis, and growth in pancreatic adenocarcinoma cell lines.
Methods
Panc-1 and MiaPaCa-2 cells were treated with ZM336372 or lithium chloride (LiCl) and compared to solvent control. The effects on proliferation for each cell line were determined using the MTT assay. Western blot analysis was performed to examine the effects of treatment on the phosphorylation of GSK-3β. In addition, western blot was utilized to examine the cleavage of PARP, a marker of apoptosis.
Results
A dose-dependent increase in phosphorylation of GSK-3β was observed after treatment with both ZM336372 and LiCl. Growth inhibition due to treatment with ZM336372 and LiCl also occurred in a dose-dependent fashion. An increase in cleaved PARP was demonstrated after treatment with both agents, as was seen previously with GSK-3β inhibition in pancreatic adenocarcinoma cells.
Conclusion
This is the first description of growth inhibition and apoptosis in pancreatic cancer cells related to GSK-3β inhibition through treatment with ZM336372.
Keywords: ZM336372, lithium, GSK-3β, pancreatic cancer, apoptosis
Introduction
Glycogen synthase kinase-3β (GSK-3β) is a multi-functional serine/threonine kinase that regulates numerous cellular functions, including glycogen metabolism, apoptosis, cell cycle regulation, and neuronal functions among others [1,2,3]. In addition, GSK-3β has been shown to regulate cell survival and proliferation in pancreatic cancer cells [4]. The GSK-3β pharmacologic inhibitors, AR-A014418 and SB-216763, and depletion of GSK-3β by siRNA in pancreatic cancer cells have resulted in suppression of cellular proliferation [4]. Furthermore, it has been suggested that GSK-3β mediates its anti-proliferative effects through its interaction with nuclear factor-κB (NF-κB) [4]. Previous studies examining the pharmacologic inhibition of GSK-3β have targeted the ATP-binding site. GSK-3β is also inhibited through phosphorylation at the Ser9 position [5].
LiCl, which is commonly used to treat mood disorders, has been shown to inhibit GSK-3β. This GSK-3β inhibition secondary to LiCl has been shown to occur via two mechanisms [6]. LiCl can directly inhibit GSK-3β by reversibly competing with Mg2+ and can also inhibit GSK-3β through Ser9 phosphorylation [7,8]. LiCl has been shown to cause GSK-3β inhibition and growth suppression in neuroendocrine tumors [9].
ZM336372 is a small molecule kinase modulator whose effects on GSK-3β have not been investigated before in pancreatic cancer cells. ZM336732 has previously been shown to have anti-neoplastic effects in neuroendocrine and hepatocellular carcinoma cell lines [10,11].
The aim of this study is to determine if ZM336372 is able to inhibit GSK-3β activity in pancreatic cancer cells through Ser9 phosphorylation and determine the potential for ZM336372 as a new chemotherapeutic agent for pancreatic adenocarcinoma by measuring its effects on cellular proliferation and apoptosis.
Methods
Cell Culture
Panc-1, and MiaPaCa-2 cell lines (American Type Culture Collection, Manassas, VA) were incubated in a humified 5% CO2 atmosphere at 37°C in Dulbeco's modified eagles medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, VT), 100 IU/mL of penicillin, and 100 pg/mL of streptomycin (Gibco).
Pharmacologic Treatment
ZM336372 (Tocris, Ellisville, MO) or LiCl (Sigma) were dissolved in 100% dimethyl sulfoxide, DMSO, (Sigma, St. Louis, MO) to the appropriate concentration and added directly to the culture media to treat the cells. For the duration of each experiment, the media and drug were exchanged every two days. Experimental conditions were kept constant for the ZM336372 and LiCl treated cells.
Cellular Proliferation Assay
MTT reagent, 3,4-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliμM bromide, (Sigma) was reconstituted in 1 × PBS to a final concentration of 5 mg/ml. The culture media was replaced with phenol free media prior to MTT reagent addition equaling one tenth the original culture volume. Cultures were then incubated for 3 hours at 37°C. The medium was then removed and the dye was solubilized with 200 μL of 0.1 N HCl in isopropanol. The absorbance was then measured at a wavelength of 540 nm with background subtraction at 630-690 nm.
Western Blot Analysis
Cellular extracts were prepared as described previously [10]. 40 μg of cellular extract from ZM336372 treated cells and controls were loaded onto precast 10% polyacrylamide gels (Pierce). The gels were run at 100 volts for 60 minutes and then transferred to PVDF Immobilon-P membranes (Millipore, Bedford, MA) at 40 volts for 90 minutes. The membranes were blocked with a 5% milk solution for one hour and the primary antibodies, GSK-3β, pGSK-3β, cPARP (Cell Signaling Technology, Beverly, MA), were incubated overnight in bovine serum albumin (Sigma) at a 1:1000 ratio. Following incubation with the primary antibody, membranes were washed in TBS-T wash buffer (Tris buffered saline, 0.05% Tween 20). Next, goat anti-rabbit horseradish peroxidase labeled antibody (Pierce) was added at ratio of 1:7000 in milk solution and incubated for 1-2 hours. The membranes were then washed again with TBS-T. SuperSignal West Pico Chemiluminescent Substrate (Pierce) was added according to manufacturer's instructions and incubated for five minutes. Following removal of the substrate the membranes were placed in plastic sleeves and exposed to film. Anti-G3PDH antibody (Pierce) was utilized as a loading control at a ratio of 1:7500.
Results
ZM336372 Causes Growth Inhibition in Pancreatic Cancer Cells
ZM336372 has been shown to posses antineoplastic properties previously. Here we utilize the MTT assay to determine if there is a difference in cellular proliferation in response to treatment with ZM336372 in pancreatic adenocarcinoma cells. (Figure 1A and 1B). The two pancreatic cancer cell lines displayed a decrease in growth only after treatment with the 50 μM concentration of ZM336372. There was little observable difference between the control cells and the 25 μM concentration in the Panc-1 and MiaPaCa-2 cell lines. Complete growth suppression was seen using the 100 μM concentration of ZM336372.
Figure 1.
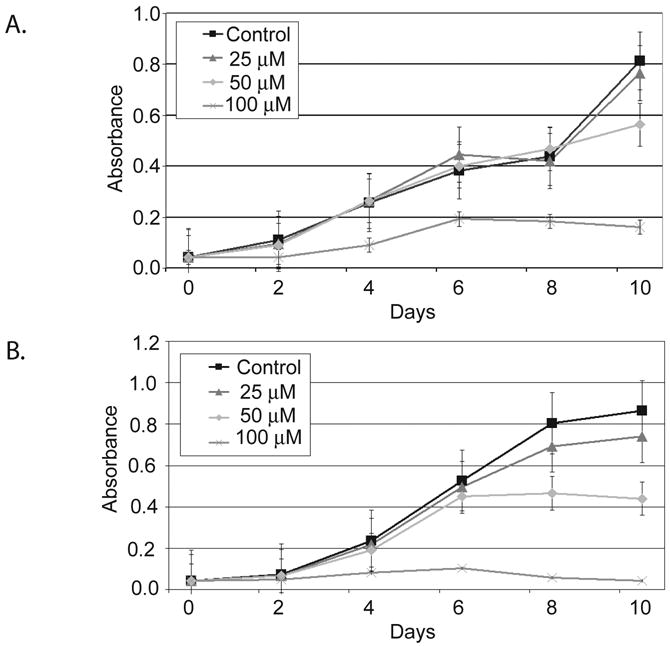
MTT assay of pancreatic adenocarcinoma cells treated with ZM336372 every other day for 8 days. A. MiaPaCa-2 cells treated with ZM336372 demonstrated complete growth suppression at a concentration of 100μM. No significant decrease in growth was observed at the 25μM concentration. B. Panc-1 cells also displayed complete growth suppression at the 100μM concentration of ZM336372. However, no change in growth was observed with the 25μM concentration.
The growth inhibition of ZM336372 was cooberated with the treatment of pancreatic adenocarcinoma cells with LiCl treatment, a GSK-3β inhibitor. (Figure 2A and 2B). A dose-dependent decrease in growth was observed in both the MiaPaCa-2 and Panc-1 cell lines. At a concentration of 20 mM complete growth suppression was observed. No significant change in growth was noted with the 5 mM concentration, corresponding to low levels of pGSK-3β at this dose.
Figure 2.
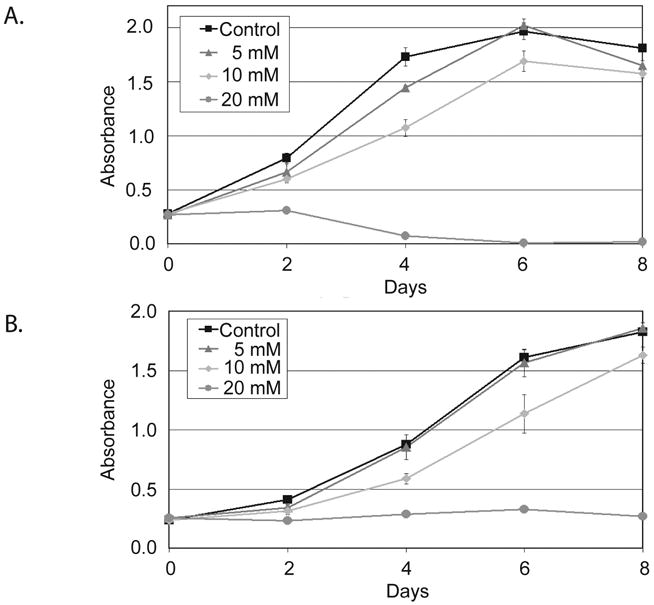
MTT assay of pancreatic adenocarcinoma cells treated with LiCl for 8 days. A. MiaPaCa-2 cells displayed a dose-dependent decrease in cellular proliferation. Complete growth inhibition was observed at a concentration of 20mM. B. LiCl treatment of Panc-1 cells resulted in no significant change in growth at the 5mM concentration. A dose-dependent decrease in growth was then seen with complete growth suppression at the 20mM concentration.
ZM336372 Causes Phosphorylation of GSK-3β at Ser9
The effect of ZM336372 on the level of phosphorylation of GSK-3β at Ser9 was examined. The Panc-1 and MiaPaCa-2 pancreatic cancer cell lines both show minimal pGSK-3β at baseline. Following two days of treatment with ZM336372 there is a significant increase in the level of pGSK-3β. (Figure 3A and 3B). No change in the level of the non-phosphorylated form of GSK-3β was observed.
Figure 3.
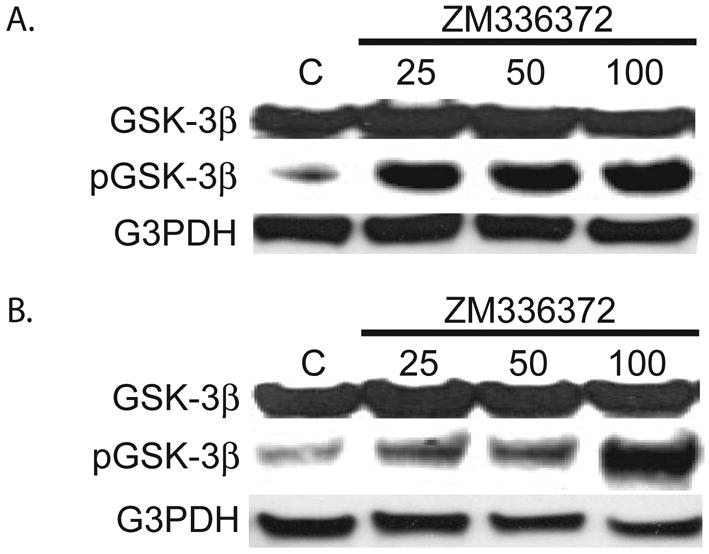
Mia PaCa-2 (A.) and Panc-1 (B.) cells treated with ZM336372 for 2 days were extracted. An increase in pGSK-3β was observed in cells treated with ZM336372. No change in GSK-3β was observed. G3PDH was used as a loading control.
LiCl has previously been shown to inhibit GSK-3β. Here the effects of LiCl treatment on the phosphorylation of GSK-3β at Ser9 were investigated in pancreatic adenocarcinoma cells. After treatment of Panc-1 and MiaPaCa-2 cells with LiCl for two days, cellular extracts were isolated. A dose-dependent increase in pGSK-3β after treatment with LiCl was observed in both cell lines. (Figure 4A and 4B). As with ZM336372, no observable change in the level of non-phosphorylated GSK-3β was seen with LiCl treatment.
Figure 4.
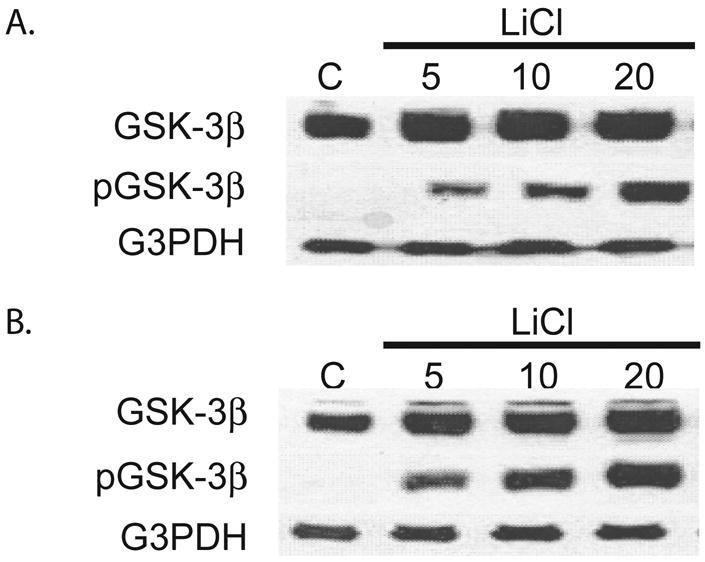
Treatment of MiaPaca-2 (A.) and Panc-1 (B.) cells with LiCl for 2 days was performed. Extracts show an increase in pGSK-3β in those cells treated with LiCl. No significant change in GSK-3β was observed.
ZM336372 Induces Cleavage of PARP, a Marker of Apoptosis
Previously it has been shown that GSK-3β inhibition leads to apoptosis through NF-κB [4]. Here we examined the cleavage of PARP to determine if ZM336372 treatment will induce apoptosis. PARP is a nuclear enzyme that binds DNA strand breaks and is a caspase substrate, which are activated in the early stages of apoptosis [12]. For this reason cPARP is used as a marker of apoptosis. Cleavage of PARP was observed after treatment with ZM336372 for 48 hours in MiaPaCa-2 and Panc-1 cell lines. (Figure 5A and B). Minimal cleavage of PARP was observed at baseline. LiCl also induced cleavage of PARP in the MiaPaCa-2 cell line (figure 5C) after 2 days of treatment. These results correlate with the MTT assays for the various treatments. Cleavage of PARP is seen at concentrations where growth inhibition is observed.
Figure 5.
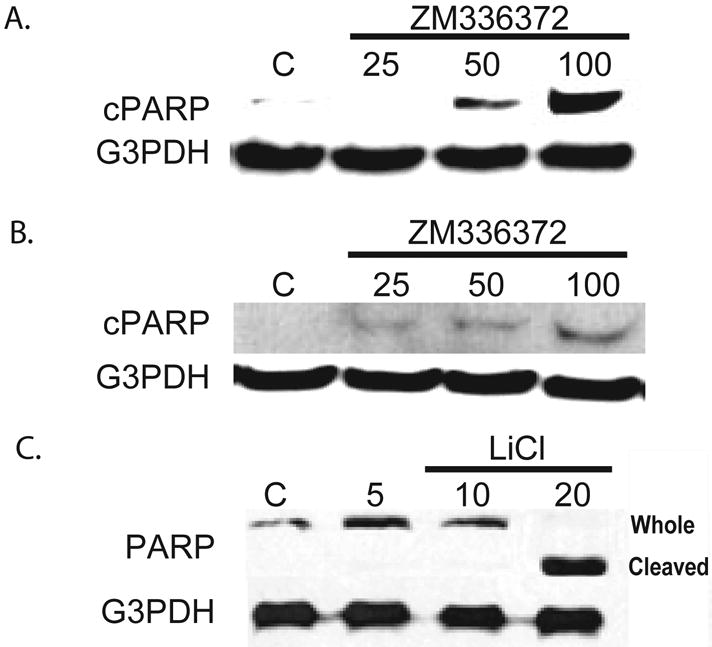
Growth inhibition is accompanied by cleavage of PARP, a marker of apoptosis. A. MiaPaCa-2 cells were treated with ZM336372 for 48 hours. A dose-dependent increase in cleaved PARP (cPARP) was observed. No observable cPARP at the 25μM concentration. B. Panc-1 cells treated with ZM336372 for 48 hours also resulted in PARP cleavage. Minimal cPARP was observed at the 25μM concentration and increases in a dose-dependent manner. A and B were performed using a specific cleaved PARP antibody. C. LiCl treatment of MiaPaCa-2 cells was performed. PARP cleavage occurred with the 20mM concentration. This correlates with the MTT growth assay.
Discussion
Pancreatic adenocarcinoma is a significant health problem, with more than 30,000 cases diagnosed in the United States each year and almost as many deaths. Although gemcitabine and recently approved erlotinib have shown to improve survival times by a few weeks, the prognosis remains poor [13]. New therapies, and more importantly, a better understanding of the cellular mechanisms of this disease are desperately needed. We, therefore, have investigated the effects of GSK-3β manipulation on two human pancreatic cancer cell lines.
Here we describe GSK-3β inhibition by ZM336372 through phosphorylation of GSK-3β at Ser9 in pancreatic cancer cells. In addition, growth suppression and induction of a marker of apoptosis were observed. This corroborates with the previously reported results with the GSK-3β ATP-binding site inhibitors and siRNA [4]. ZM336372 was originally discovered as a raf-1 inhibitor [14], however we have found it to activate raf-1 in cell culture [10,11]. This agent has been shown to decrease cellular growth in numerous cell types, including neuroendocrine tumor, hepatocellular carcinoma and now pancreatic adenocarcinoma cells [10,11,15]. Recently in addition to raf-1 activation, ZM33672 has also been shown to inhibit GSK-3β [15]. Further investigation has been performed to determine the significance of these ZM336372 induced kinase phosphorylations. We have shown in neuroendocrine cell lines that down-regulation of neuroendocrine markers is independent of the raf-1/MEK/ERK pathway [15]. Most importantly, the GSK-3β inhibition by ZM336372 was shown to be required for neuroendocrine phenotype reduction [15]. This suggests that the GSK-3β inhibition has greater significance in relation to the suppression of cellular proliferation due to ZM336372 treatment.
In pancreatic cancer cells, GSK-3β mediates regulation of NF-κB, controlling cellular survival and proliferation [4,16,17,18]. NF-κB is a ubiquitously expressed transcription factor that is associated with a wide spectrum of cellular functions, including mediation of cell cycle progression, adaptations to stress, response to inflammation, and regulation of apoptosis [19]. In pancreatic cancer cell lines, GSK-3β inhibition leads to decreased expression of NF-κB target genes involved in cellular proliferation and survival [4]. In addition, a dose dependent decrease in cell proliferation of pancreatic cancer cell lines have been observed with GSK-3P RNA interference and the GSK-3P inhibitors, AR-AO14418 and SB216763, which inhibit GSK-3P via targeting the ATPase binding site [4,20,21]. GSK-3P acts distal to the IKK, as the growth suppression of GSK-3P inhibition were able to be rescued by p65/p50 over expression, and not constitutively active mutant IKK [4]. Interestingly, the NF-κB pathway has been shown to mediate cellular survival through anti-apoptotic mechanisms and is associated with chemotherapy resistance in pancreatic cancer cell lines [22,23]. Therefore, inhibition of NF-κB activity could result in apoptosis and also sensitize pancreatic cancer cells to chemotherapeutic agents.
Further analysis into these and other GSK-3P inhibitors is warranted in order to determine their ability to be used as anti-neoplastic agents. Specifically, if NF-κB inhibition sensitizes pancreatic cancer cells to chemotherapy, then combination chemotherapeutic regimens of ZM336372 with cytotoxic agents, such as gemcitabine, would be warranted. It is possible that these agents are performing these actions via other mechanisms than GSK-3P inhibition, therefore further mechanistic studies examining the downstream interactions of GSK-3P and NF-κB after ZM336372 treatment are required. This is the first description of ZM336372 as a possible agent for the treatment of pancreatic adenocarcinoma.
Acknowledgments
Grant Support: This work was supported in part by a Research Scholars Grant from the American Cancer Society, National Institutes of Health grants CA109053; the George H.A. Clowes, Jr., Memorial Research Career Development Award of the American College of Surgeons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meijer L, et al. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharm Sci. 2004;25:471–80. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Doble B, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sc. 2006;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frame S, Cohen P. GSK3 takes center stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ougolkov A, Fernandez-Zapico M, Savoy D, et al. Glycogen Synthase Kinase-3β participates in Nuclear Factor κB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65(6):2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 5.Kockeritz L, Patel S, Woodgett JR. Glycogen synthase kinase-3-An overview of an over-achieving protein kinase. Current Drug Targets. 2006;7:1377–88. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 6.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharm Sci. 2003;24:441–3. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 7.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 8.De Sarno P, et al. Regulation of Akt and glycogen synthase kinase-3β phosphorylation by sodium valproate and lithium. Neuropharmocology. 2002;43:1158–64. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–8. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 10.Van Gompel JJ, Kunnimalaiyaan M, Holen K, Chen H. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4:910–917. doi: 10.1158/1535-7163.MCT-04-0334. [DOI] [PubMed] [Google Scholar]
- 11.Deming D, Geiger P, Chen H, et al. ZM336372, a Raf-1 activator, causes suppression of proliferation in a human hepatocellular carcinoma cell line. J Gastrointest Surg. 2008;12(5):852–7. doi: 10.1007/s11605-008-0495-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann SH, Desnovers S, Ottaviano Y, et al. Specific proteolytic cleavage of poly(ADP-ribose)polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–85. [PubMed] [Google Scholar]
- 13.Eckel F, Schneider G, Schmid RM. Pancreatic cancer: a review of recent advances. Expert Opin Investig Drugs. 2006;15(11):1395–410. doi: 10.1517/13543784.15.11.1395. [DOI] [PubMed] [Google Scholar]
- 14.Hall-Jackson CA, Eyers PA, Cohen P, et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem Biol. 1999;6:559–68. doi: 10.1016/s1074-5521(99)80088-x. [DOI] [PubMed] [Google Scholar]
- 15.Kunnimalaiyaan M, Ndiaye M, Chen H. Neuroendocrine tumor cell growth inhibition by ZM336372 through alterations in multiple signaling pathways. Surgery. 2007;142:959–64. doi: 10.1016/j.surg.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlowski RZ, Baldwin AS. NF-κB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–9. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3β in TNF-α-induced NF-κB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest, Liver Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- 18.Ougolkov AV, Fernandez-Zapico ME, Bilim VN, et al. Aberrant nuclear accumulation of glycogen synthase kinase-3β in human pancreatic cancer: Association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;12:5074–81. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karin M. Nuclear Factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 20.Coghlan MP, Culbert AA, Cross DA, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 21.Bhat R, Xue Y, Berg S, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278(46):45937–45. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 22.Arlt A, Gehrz A, Muerkoster S, et al. Role of NF-κB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 23.Arlt A, Vorndamm J, Breitenbroich M, et al. Inhibiton of NF-κB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001;20:859–68. doi: 10.1038/sj.onc.1204168. [DOI] [PubMed] [Google Scholar]


