Abstract
One-dimensional nanostructures are ideal building blocks for functional nanoscale assembly. Peptide based nanofibers have great potential in building smart hierarchical structures due to their tunable structures at the single residue level and their ability to reconfigure themselves in response to environmental stimuli. We observed that pre-adsorbed silk-elastin based protein polymers self-assemble into nanofibers through conformational changes on a mica substrate. Furthermore, we demonstrate that the rate of self-assembly was significantly enhanced by applying a nanomechanical stimulus using atomic force microscopy. The orientation of the newly grown nanofibers was mostly perpendicular to the scanning direction, implying that the new fiber assembly was locally activated with directional control. Our method provides a novel way to prepare nanofiber patterned substrates using a bottom-up approach.
Self-assembly is one of the most important mechanisms that nature exploits to build complex structures. Numerous research groups are focusing on controlling the self-assembly process to develop nanostructures of periodic patterns.1–2 Spontaneous organization often occurs in a hierarchical manner in a wide range of length scales, creating higher-order structures built from preassembled structures in a stepwise fashion.3 During this process, the preformed substrate often provides crucial cues in facilitating subsequent assembly steps.4–5 These cues consist of local geometric constraints and mostly non-covalent interactions such as hydrogen bonding, hydrophobic or electrostatic interactions. The formation of 1-dimensional peptide based self-assembled nanostructures on the substrate has been reported.6 However, controlling location and direction of the nanostructures on the substrate to create well-defined 1-dimensional nanoscale patterns remains challenging. Here we report on the application of nanomechanical forces in a local manner to initiate the self-assembly of protein polymer based nanofibers at a specific location with control of their growth direction, without using any local geometric constraints such as preformed surface patterns. This method may provide a simple way to prepare well-ordered nanofiber substrates, which can serve as building templates for functional nanoscale devices.
Self-assembling peptides have received significant attention in nanotechnology for bottom-up fabrication7 and biomimetic structure.8–9 Silk-elastin-like protein polymers (SELPs) are genetically engineered block copolymers made of silk-like blocks (Gly-Ala-Gly-Ala-Gly-Ser) from Bombyx mori (silkworm) and elastin-like blocks (Gly-Val-Gly-Val-Pro) from mammalian elastin. The repeating unit of SELP-815K, contains eight silk (S) units, fifteen elastin (E) units, and one lysine (K) modified elastin unit. The molecular weight is 65,374 Da and its complete amino acid sequence is MDPVVLQRRDWENPGVTQLNRLAAHPPFA SDPM[GAGS(GAGAGS)2(GVGVP)4GKGVP(GVGVP)11(GAGAGS)5GAGA]6MDPGRYQDLRSHHHHHH.10
The structure of SELPs can be controlled at the single amino acid residue level, allowing for a strategic introduction of various functional motifs for hierarchical self-assembly, stimuli-sensitivity, and biorecognition. At body temperature, SELP forms a hydrogel which is advantageous in injectable biomaterials for drug delivery11, and tissue engineering applications.12
In our previous studies, we observed that SELPs self-assemble into nanofibers on specific substrates.13 To further investigate the mechanisms of surface facilitated self-assembly, 5 µg/ml of SELP-815K (Protein Polymer Technologies, Inc., San Diego, CA) in distilled water was dropped on a freshly cleaved 8×8 mm2 mica surface (Ted Pella, Inc., Redding, CA). The samples were incubated for 30 minutes in a humidity chamber at room temperature and then washed with distilled water three times. Atomic force microscopy (AFM) images were obtained with a Molecular Force Probe-3D instrument (MFP-3D, Asylum Research Inc., Santa Barbara, CA) at a scanning speed of 5 µm/s in tapping mode. An MLCT probe (Veeco, Santa Barbara, CA) with a spring constant ranging from 0.03 to 0.06 N/m was used.
The AFM images of the same area at different time intervals illustrate that SELP-815K formed nuclei at various locations and slowly self-assembled to form a nanofibrous structure (Figure 1). This is quite surprising since the sample was washed thoroughly with water after incubation, which means that there should be no supply of polymers from the bulk phase for further growth. This observation strongly suggests that nuclei formation and nanofiber self-assembly occurred through conformational changes of the pre-adsorbed SELP-815K on the mica surface.
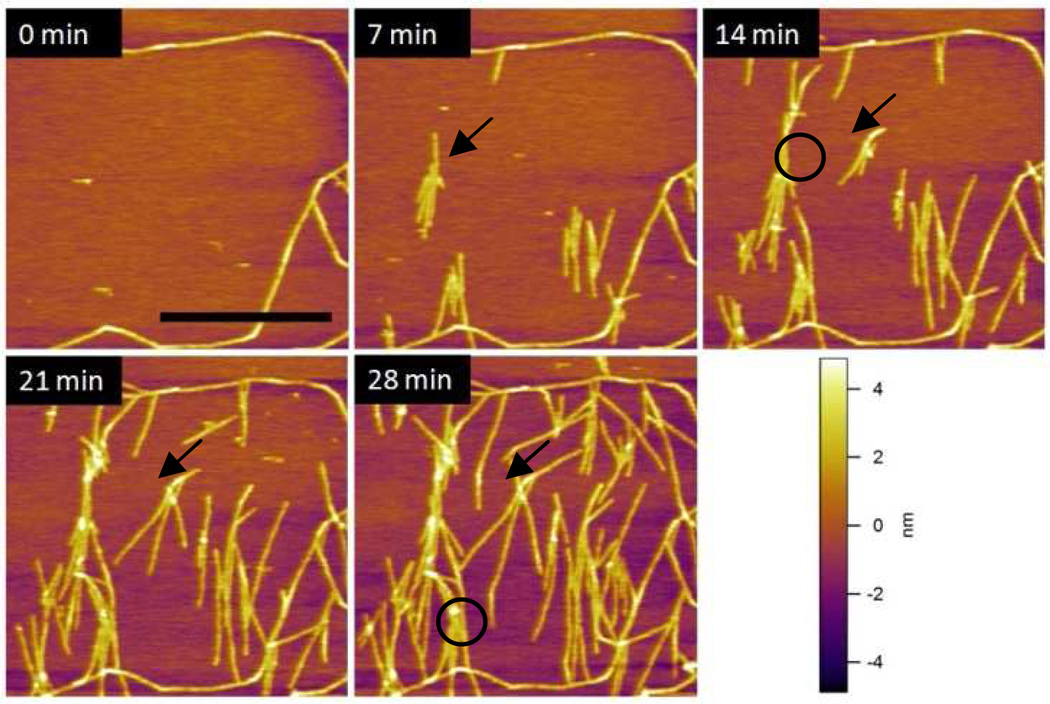
Figure 1.
AFM images (2µm × 2µm) of SELP-815K nanofibers obtained in tapping mode AFM at different time intervals. The scale bar corresponds to 1 µm. The setpoint/free amplitude was 380 mV/400 mV, for which the calculated maximum tapping pressure was 110 MPa.
After 30 min incubation and washing, nanofibers were usually sparsely formed throughout the mica surface but the area imaged at 0 min was chosen so that there were a few potential nucleation sites without many nanofibers. The image taken after 7 min shows that nucleation sites were formed on the mica and grew into nanofibrous structures at random locations. One of the new nucleation sites was identified with an arrow in this image. New nanofibers grew from this site as shown inside the circled area in the 14 min image. Not only were new nanofibers formed from the new nucleation sites, but the existing fibers also kept growing as indicated by the arrows in the 14, 21, and 28 min images. In the 28 min image, the circle shows the junction formed by physical blocking of one end of the growing nanofiber. This type of junction is observed in many different locations indicating the important role of the surface on the self-assembly process.
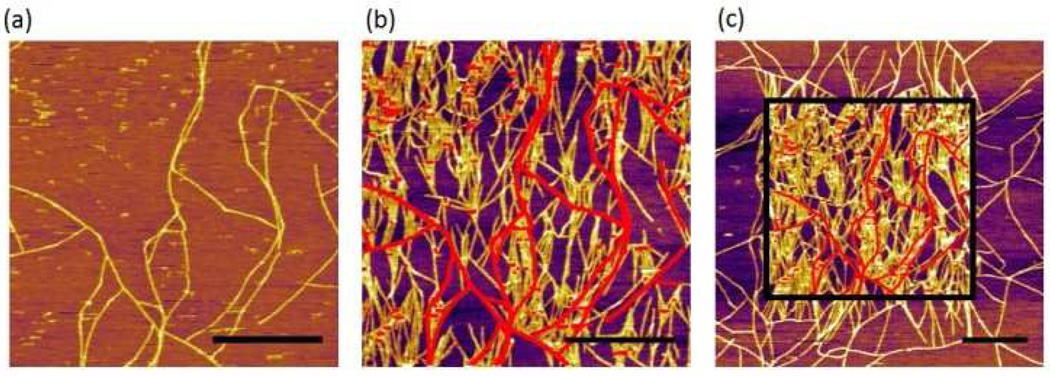
In other areas with some preformed nanofibers, we observed a dramatic effect of the AFM tapping mode imaging on the nanofiber assembly. Figure 2a is the first scanned image using AFM which shows some nanofibers formed during incubation. When the same area was scanned again after 6 min, the density of nanofibers significantly increased (Figure 2b). The red lines in Figure 2b represent the nanofibers imaged in the first scan. The drastic effect of the AFM scan on nanofiber growth is clearly visible in the zoomed out image in Figure 2c where sharp contrast in nanofiber density was clearly observed between the area scanned once (outside the square) and the area scanned twice (inside the square). This remarkable difference clearly indicates the strong effect of the AFM tapping force on the self-assembly of nanofibers. Furthermore, the newly grown nanofibers, as a result of AFM scanning, seemed to have a strong preference in their growth direction. We used the OrientationJ plug-in in ImageJ software14 to analyze the orientation of each nanofiber with respect to the AFM scanning direction (horizontal in the AFM images) (Figure 3a). The orientation of the nanofibers formed during incubation (without any mechanical stimulus) was evenly distributed between 0° and 90°, indicating that the crystal plane of the mica had no effect on their growth direction.15 In sharp contrast, 80% of the newly grown fibers after nanomechanical stimulus by AFM showed preferred orientation between 60° and 90°. This suggests preferential growth perpendicular to the AFM scanning direction, and confirms the effect of tapping mode imaging on self-assembly.
Figure 2.
AFM images of SELP815K nanofibers obtained in tapping mode. The setpoint/free amplitude was 760 mV/800 mV, for which the calculated maximum tapping pressure was 213 MPa. (a) First scanned image, (b) second scanned image of the same area, (c) zoomed out image that includes the second scanned area. The scale bars correspond to 1 µm and the red lines in images (b) and (c) represents the preformed nanofibers in the image (a).
Figure 3.
(a)Histogram of nanofiber orientation distribution with and without nanomechanical stimulus by AFM, (b)Percent increase of nanofiber coverage measured under various tapping pressures as a function of time (n=5). The coverages under a specific pressure were obtained by imaging the same area repeatedly at different time points. The coverages with no tapping stimulus were obtained from first scanned images at specific time points.
To investigate the novel effect of nanomechanical stimulus on self-assembly further, the ratio of the amplitude setpoint and the free amplitude in tapping mode AFM were varied. In this mode of imaging, the AFM tip intermittently contacts the surface, exerting a momentary impact.16–17 By varying the setpoint amplitude and the free amplitude, the tapping force level was adjusted systematically. To quantify the tapping force exerted on the surface, the tapping mode imaging process was simulated using a 2-eigenmode cantilever model. The calculated force level was normalized by the nominal radius of the AFM tip to obtain an approximate pressure in each condition. The coverage of nanofibers under calculated tapping pressures varying from 18.8 MPa to 213 MPa was then measured at five different time points (Figure 3). Overall, coverage increased as a function of time under the range of pressures tested. The rate of percent coverage change was increased from ~ 0.2 %/min at 18.8 MPa to ~2 %/min at 213 MPa. This observation clearly highlights the effect of local nanomechanical pressure on enhancing the self-assembly of SELP-815K nanofibers.
The coverage of nanofibers increased because both the nucleation and elongation rates increased in the presence of the tapping pressure. Since both processes involve the conformational rearrangement of SELP-815K on the mica surface, the tapping pressures must have lowered the activation energy involved, thereby accelerating the rate of nanofiber self-assembly. The effect of mechanical forces on nucleation rate has been reported. In amyloid fibers, mechanical agitation broke the existing nanofibers into smaller pieces, creating new nucleation sites for growth.18 In our case, new nucleation sites were created through nanomechanical force rather than from existing nanofibers, implying that the location of nucleation can be controlled. Moreover, the growth direction of the nanofibers demonstrated a strong correlation with the AFM scanning direction, suggesting that a new nanofiber with a desired direction may be self-assembled with the guidance of the directional tapping pressure.
The self-assembly of the nanofiber without a supply from the bulk phase indicates that the concentration of the pre-adsorbed SELP-815K on the mica surface is likely in a supersaturated state. This 2-dimensional, densely packed state, is formed due to strong coulombic interactions between the negatively charged mica surface and positively charged SELP (pI=10.12) in distilled water, possibly through the fly-casting mechanism.19 Once SELPs form a densely packed 2-dimensional layer, they hardly self-assemble into nanofibers without mechanical stimulus. The local pressure exerted by the AFM tip during imaging dramatically enhances the rate of rearrangement of the SELP polymers to self-assemble into nanofibers. This rearrangement can be further enhanced by water expulsion from the SELP polymers under tapping pressure, assisting self-assembly.20 This molecular level mechanism can result in increase of both nucleation rate and elongation rate, as observed in our system.
In conclusion, we demonstrated that the local tapping pressure exerted by an AFM tip can initiate and promote the self-assembly of SELP nanofibers on a mica surface in a force dependent manner. The growth direction of the nanofiber was largely perpendicular to the scanning direction, indicating that this novel method could be applied to create 1-dimensional nanostructure patterned surfaces using nanomechanical stimuli.
Supplementary Material
Acknowledgment
We gratefully acknowledge financial support from Maryland Nano-Bio Fund (JS), the U.S. National Science Foundation, award No. CMMI-0800471 (SDS), and NIH Grant 2R01CA107621 (HG). We also acknowledge the support of the Maryland NanoCenter. We thank Prof. Gregory Payne for helpful comments on the manuscript and Sylvia Kang for analysis of nanofiber orientation.
Footnotes
Supporting Information Available: Detailed simulation description. This material is available free of charge via the internet at http://pubs.acs.org/
References
- 1.Kudernac T, Lei S, Elemans JAAW, De Feyter S. Chemical Society Reviews. 2009;38:402. doi: 10.1039/b708902n. [DOI] [PubMed] [Google Scholar]
- 2.Jiang FZ, Horber H, Howard J, Muller DJ. J. Struct. Biol. 2004;148:268. doi: 10.1016/j.jsb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J. Self-assembled nanostructures. Springer; 2003. [Google Scholar]
- 4.Brown CL, Aksay IA, Saville DA, Hecht MH. J Am Chem Soc. 2002;124:6846. doi: 10.1021/ja0261271. [DOI] [PubMed] [Google Scholar]
- 5.Loo RW, Goh MC. Langmuir. 2008;24:13276. doi: 10.1021/la803041v. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Fung SY, Pritzker M, Chen P. J Am Chem Soc. 2007;129:12200. doi: 10.1021/ja073168u. [DOI] [PubMed] [Google Scholar]
- 7.Scheibel T, Parthasarathy R, Sawicki G, Lin XM, Jaeger H, Lindquist SL. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4527. doi: 10.1073/pnas.0431081100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarikaya M, Tamerler C, Jen AKY, Schulten K, Baneyx F. Nat Mater. 2003;2:577. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S. Nat Biotech. 2003;21:1171. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 10.Dandu R, Cresce AV, Briber R, Dowell P, Cappello J, Ghandehari H. Polymer. 2009;50:366. [Google Scholar]
- 11.Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Journal of Controlled Release. 2009;140:256. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haider M, Cappello J, Ghandehari H, Leong KW. Pharmaceutical Research. 2008;25:692. doi: 10.1007/s11095-007-9282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang W, Kim BH, Dandu R, Cappello J, Ghandehari H, Seog J. Langmuir. 2009;25:12682. doi: 10.1021/la9015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abramoff M, Magelhaes P, Ram S. Biophotonics international. 2004;11:36. [Google Scholar]
- 15.Sun M, Stetco A, Merschrod EF. Langmuir. 2008;24:5418. doi: 10.1021/la703292h. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Melcher J, Basak S, Reifenberger R, Raman A. Physical Review Letters. 2009;102 doi: 10.1103/PhysRevLett.102.060801. [DOI] [PubMed] [Google Scholar]
- 17.Solares SD, Crone JC. The Journal of Physical Chemistry C. 2007;111:10029. doi: 10.1021/jp070067+. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Fung SY, Pritzker M, Chen P. Angew. Chem.-Int. Edit. 2008;47:4397. doi: 10.1002/anie.200705404. [DOI] [PubMed] [Google Scholar]
- 19.Levy Y, Onuchic JN, Wolynes PG. J Am Chem Soc. 2007;129:738. doi: 10.1021/ja065531n. [DOI] [PubMed] [Google Scholar]
- 20.Levy Y, Onuchic JN. Annu Rev Biophys Biomol Struct. 2006;35:389. doi: 10.1146/annurev.biophys.35.040405.102134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.