Abstract
While it is known that precise dental epithelial-mesenchymal (DE-DM) cell interactions provide critical functions in tooth development, reliable methods to establish proper DE-DM cell interactions for tooth regeneration have yet to be established. To address this challenge, and to generate bioengineered teeth of predetermined size and shape, in this study, we characterize three-dimensional (3D) pre-fabricated DE-DM cell constructs. Human dental pulp cell seeded Collagen gel layers were co-cultured with porcine DE cells suspended in Growth Factor Reduced (GFR) Matrigel. The resulting 3D DE-DM cell layers were cultured in vitro, or implanted and grown subcutaneously in vivo in nude rats. Molecular, histological and immunohistochemical (IHC) analyses of harvested implants revealed organized DE-DM cell interactions, the induced expression of dental tissue-specific markers Amelogenin (AM) and Dentin Sialophosphoprotein (DSPP), and basement membrane markers Laminin 5 and collagen IV, and irregular mineralized tissue formation after 4 weeks. We anticipate that these studies will facilitate the eventual establishment of reliable methods to elaborate dental tissues, and full sized teeth of specified sized and shape.
Keywords: Dental epithelial and mesenchymal cell co-culture, tooth tissue engineering, regenerative medicine, postnatal tooth cells, Matrigel, Collagen I
1. Introduction
A major advantage of tissue engineering is the ability to use autologous cells, which avoid severe immune rejection responses. Recent advances in tissue engineering techniques have resulted in the successful in vitro regeneration of liver[1], skin[2], bone[3], cartilage[4] and muscle[5] tissues. Some regenerated tissues, including skin and bone, have gained the permission for clinical trials or even granted U.S. FDA marketing approval for treatment [6].
Recently, significant efforts have focused on dental tissue regeneration. Tooth loss, caused by caries, trauma, periodontal disease, and genetically inherited disease, is considered to be a major health issue. The ultimate treatment for tooth loss is whole tooth regeneration. Although the size and shape of certain regenerated tissues, such as skin, bone, or cartilage, can be controlled by the scaffold material used [6], the size and shape of regenerated dental tissues has proven to be fairly difficult to control[7]. A potential solution for guided dental tissue engineering is to reproduce the natural developmental process of tooth formation by facilitating the early sequential and reciprocal dental epithelial-mesenchymal cell interactions that provide critical functions in tooth development[8], and also control tooth morphogenesis[9, 10].
Attempts to establish proper dental epithelial and mesenchymal (DE-DM) cell interactions have been ongoing for many decades now. Our previous published results illustrated that dental cells obtained from dissociated porcine or rat tooth buds were capable of generating multiple, small, organized tooth crowns [11–13]. In those studies, cultured DE and DM cells were combined, seeded onto biodegradable polyester scaffolds, transplanted, and grown in the omentum of immunodeficient rat hosts. These studies showed that instead of forming one tooth of size and shape similar to the scaffold, small tooth crowns were formed throughout the implant. Also in these structured, tooth root development was only rudimentary. Similarly, multiple teeth of abnormal morphology were generated when mixed populations of DE and DM pig tooth bud cells were seeded onto collagen sponge scaffolds, which also exhibited tooth root like structures [14]. Single tooth regeneration was achieved by using a re-aggregation system, where enamel organ, or embryonic dental epithelial cells harvested from tooth buds, was combined with dental or non-dental mesenchymal stem cells, and transplanted and grown in the omentum or jaw bone of host animals. In these experiments, individual, albeit small, teeth were generated, which exhibited mature tooth structures [15–17]. A more recent report demonstrated the formation of functional teeth generated from transplanted tooth buds formed from re-aggregated E14.5 mouse tooth bud cells [18]. These promising results suggested the possibility of functional tooth regeneration. However, it is notable that all of the successful single tooth regeneration experiments used cells harvested from early stage, embryonic tooth buds. It will be difficult, if not impossible, to reliably obtain suitable autologous human embryonic dental cells for tooth regeneration efforts.
The current challenge therefore remains to devise reliable methods to regenerate teeth of predetermined shape and size, using cells derived from postnatal tissues. The purpose of this study is to establish and characterize reliable 3D construct methods to generate organized post-natal dental epithelial-mesenchymal cell layers, for the subsequent elaboration of soft and mineralized dental tissues of specified size and shape. We anticipate that these studies will provide a template for future human bioengineered tooth formation, using post-natal human derived dental cells.
2. Materials and Methods
2.1 Cell isolation, fluorescent cell tracker labeling, and co-culture
Dental mesenchymal cells were isolated from dental pulp obtained from a wisdom tooth extracted from a 16 year old Caucasian male. Dental pulp was minced and enzymatically digested using 0.3 mg/mL collagenase type I and 0.4 mg/mL dispase, and filtered through a 40 μm cell sieve. The resulting single cell suspensions were expanded by culturing in mesenchymal medium (DMEM/F12 medium with 10% FBS, 1% GlutaMAX, 50 μg/ml ascorbic acid, and 1% penicillin G/streptomycin/amphotericin B), and cryopreserved until use. Dental epithelial cells were isolated from pig tooth buds harvested from the mandible of 6-month-old minipigs. Porcine DE cells were obtained as follows. Porcine tooth enamel and pulp organs were separated surgically, and the enamel organ was minced into small pieces, digested in HBSS containing 0.4 mg/mL collagenase type I and 0.2 mg/mL dispase, triturated mechanically, and filtered to obtain single cell suspensions. Porcine DE cell suspensions were cultured in epithelial medium (LHC-8 medium, supplemented with 10% FBS, 0.5 μg/mL epinephrine, and 1% penicillin/streptomycin/amphotericin), and the expanded DE cells were cryopreserved until use.
Cryopreserved human DM and porcine DE cells were thawed and expanded in vitro, and labeled with fluorescent dyes for subsequent tracking. DM cells were labeled with Cell Tracker Orange CMTMR (C2927, Molecular Probes, Invitrogen, Eugene, OR), and DE cells were labeled with Vybrant CFDA SE Cell Tracer (V12883, Molecular Probes), using standard protocols. The samples were checked under fluorescent microscope.
2.2 Immunofluorescent (IF) staining
Unlabeled human DM and porcine DE cell constructs were co-cultured in 1:1 mesenchymal:epithelial cell media on chamber slides for up to two weeks with media changes at 2 to 3 day intervals, and examined by immunofluorescent (IF) staining. Cells cultured on 8-well chamber slides were washed in PBS, fixed in 100% methanol, permeabilized in Triton X-100, and blocked in 5% BSA. Subsequently, the cells were incubated in primary antibody against Laminin 5 (1:200 dilution, sc-13587, Santa Cruz Biotechnology, Santa Cruz, CA) or Collagen IV (1:200 dilution, sc-52317, Santa Cruz Biotechnology, Inc.), washed, and incubated in secondary Rhodamine TRITC-labeled Donkey anti-mouse antibody (1:50 dilution, 715-025-151, Jackson ImmunoResearch Laboratories, Inc. West, Grove, PA). Additional slides were examined using primary antibodies for the epithelial cell marker anti-cytokeratin-14 (CK-14, 1:25 dilution, sc-53253, Santa Cruz Biotechnology, Inc.), and the mesenchymal cell marker anti-vimentin (VM, 1:1,600, kind gift of Dr. Eugenia Wang [19]), and then incubated in secondary antibodies containing Fluorescein FITC-labeled Donkey anti-mouse antibody (1:50 dilution, 715-095-151, Jackson ImmunoResearch Laboratories, Inc.) and Rhodamine TRITC-labeled Donkey anti-rabbit antibody (1:50 dilution, 711-025-152, Jackson ImmunoResearch Laboratories, Inc.). All slides were then washed with PBS, sealed with VECTASHIELD HardSet Mounting Medium with DAPI (H-1500, Vector Laboratories, Burlingame, CA), and examined under fluorescence using a ZeissAxiophot Imager microscope and digital camera imaging system.
2.3 Confocal laser scanning microscopy (CLSM)
Cell tracker CMTMR stained human DM cells were suspended in Collagen gel (#354236, BD Biosciences, Bedford, MA), and Vybrant™ CFDA SE Cell Tracer stained porcine DE cells were suspended in GFR Matrigel (#354230, BD Biosciences). The 3D cell-seeded constructs were plated side by side on a glass-bottomed petri-dish, gelled in a 37°C incubator for one hour, cultured in 1:1 mesenchymal:epithelial media for one week, and analyzed by CLSM (Olympus FluoView™ FV1000 Confocal Microscope, Center Valley, PA ).
2.4 Establishing three dimensional (3D) co-culture constructs for in vitro
To generate 3D constructs, 100 μl human DM cells in Collagen gel (5×103 cell/μL) were first plated in 24-well transwells. Next, two aliquots of 10 μl of porcine DE cells mixed with GFR Matrigel (5×103 cell/μl) were pipetted into the collagen-DM cell layer (Figure 3 A). The formed 3D constructs were placed into a 37°C incubator for one hour to gel, and cultured in vitro in 1:1 mesenchymal:epithelial cell media with osteogenic supplements (100 nM Dexamethasone, 10 mM beta Glycerol phosphate, and 50 μg/ml Ascorbic acid), and harvested at weekly intervals for four weeks. Controls included: 1) human DM cells with Collagen gel combined with Matrigel only; 2) porcine DE cells with Matrigel combined with Collagen gel only; 3) porcine DM cells with Collagen gel with porcine DE cells with Matrigel; and 4) unseeded Collagen gel with Matrigel. At least three replicate samples were prepared for each group at each time point.
Figure 3. DE-DM construct.
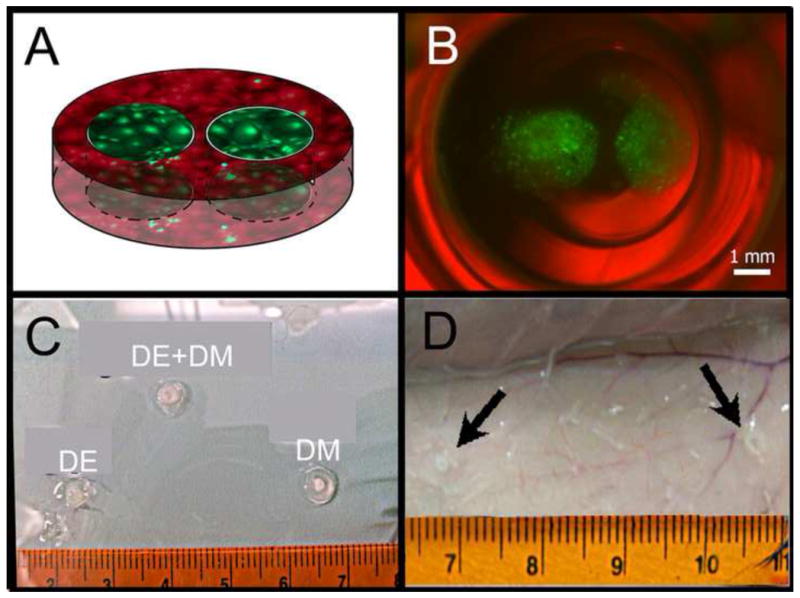
A) Schematic of DE-DM construct containing green labeled DE cells and red labeled DM cells. B) Light micrograph image of fluorescent DE-DM construct after 2 days in vitro culture. Merged bright field photo and fluorescent images: green DE cells in Matrigel; red DM cells in Collagen. C) Dental cell seeded constructs prior to implantation. DE-DM and DM only implants maintained their shape, while the DE collagen implant was very soft. D) Subcutaneous implant after 4 weeks in vivo (arrows).
2.5 In vivo transplantation of 3D co-culture constructs
All animal experiments were performed under the guidance and approval of the Animal Care and Use Committee of Tufts University. The 3D constructs, fabricated as described for in vitro study, were cultured for 1 week in 1:1 mesenchymal:epithelial media with osteogenic supplements, and transplanted subcutaneously into four-week old nude rat hosts (NIH-Foxn1, Charles River, Wilmington, MA). Briefly, animals were anesthetized with Isoflurane, which was administered by an anesthesia machine with a precision vaporizer. Samples (n=3) were collected at one and four weeks. For controls, human DM cells in Collagen gel combined with Matrigel alone, and porcine DE cells in Matrigel combined with Collagen gel alone, were treated and transplanted parallel.
2.6 Histological and immunohistochemical (IHC) analyses
In vitro and in vivo cultured 3D DE-DM cell constructs were fixed in 10% formalin solution (SF98-4, Fisher, PA, USA), dehydrated in an ethanol series, embedded in paraffin, and sectioned at 6 μm intervals. Sections were analyzed by Hematoxylin& Eosin (H&E) staining, and IHC staining was performed on selected sections using tissue specific markers including: dentin expressed dentin DSPP (1:4,000 dilution, kind gift of Dr. Larry Fisher); enamel expressed AM (1:8,000 dilution, kind gift of Dr. Jim Simmer); the epithelial cell marker CK-14; mesenchymal cell marker vimentin; basement membrane markers Laminin5 (1:200 dilution, sc-13587, Santa Cruz biotechnology) and Collagen IV (1:200 dilution, sc-52317, Santa Cruz biotechnology), and the Anti-human mitochondria antibody (1:50 dilution, MAB1273, Millipore Corporation, Billerica, MA). All sections were quenched with hydrogen peroxide, unmasked using hyaluronidase, blocked in 10% normal Donkey serum, incubated with primary antibodies for 1 hour washed, incubated with biotinylated secondary antibody, stained using the Vectastain ABC staining kit (Vector Laboratories), developed in 3,3-diaminobenzidine (DAB), and counterstained with fast green. Processed sections were dehydrated through a graded ethanol series, sealed with Permount (SP15-100, Fisher), and analyzed using Zeiss Axiophot Imager and M2Bio microscopes. Photos were taken using a digital Zeiss Axiocam camera.
2.7 Von Kossa staining
Von Kossa staining for calcified tissues was performed on paraffin sectioned in vivo cultured samples. Briefly, sections were deparaffinized, hydrated, incubated in 5% silver nitrate solution, washed in 5% sodium carbonate, and counterstained with nuclear fast red. The stained samples were then sealed and analyzed as described for IHC samples.
3. Results
3.1 Cell isolation, fluorescent cell tracking, co-culture, and IF staining
DE cells isolated from 6 month old pig tooth buds exhibited a typical cobble-stone like morphology (Figure 1B), and DM cells isolated from a human 3rd molar pulp showed a typical spindle shaped fibroblast morphology (Figure 1E). Both types of cells retained their characteristic morphologies after being stained by fluorescent cell tracer dyes (Figure 1C, F). Analyses under fluorescent microscopy confirmed that 3D cultured DE and DM cell constructs maintained their normal morphology and proliferation rate (Figure 1G). The expression of a basement membrane marker Laminin 5 and Collagen IV (Figure 1H, I) was detected in the co-culture system, suggesting the formation of DE-DM cell interface in cultured 3D constructs.
Figure 1. Dental epithelial (DE) and dental mesenchymal (DM) cells.

A) Harvested porcine enamel organs exhibited highly vascularized tissues and mineralized tooth cusps. B) Cultured pig DE cells exhibited typical cobble-stone like morphology. C) Vybrant™ CFDA SE Cell Tracer labeled DE cells. D) Human 3rd molar tooth, split in half. E) Cultured human DM pulp cells exhibited typical fibroblast morphology. F) Cell Tracker Orange CMTMR labeled DM cells. Immunofluorescent (IF) co-cultured porcine DE and human DM cells. G) IF staining of DE expressing CK-14 (green) and DM expressing Vimentum (red). IF of (H) Laminin (red), (I) Collagen IV (red) and (G, H, I) DAPI stained nuclei (blue). Scale bar=200 μm (B, C, E, F), and =20 μm (G–I).
3.2 Confocal laser scanning microscopy (CLSM)
After 7 days of in vitro 3D cell culture in glass bottomed petri dishes, porcine DE cells in Matrigel formed nodular-like structures, while human DM cells in Collagen appeared evenly distributed throughout the cell layer (Figure 2A). CLSM analyses revealed the close interactions between porcine DE and human DM cells, and the presence of distinctly polarized DE but not polarized DM cells along the DE-DM cell interface (Figure 2B, C).
Figure 2. DE-DM cell in vitro co-culture.
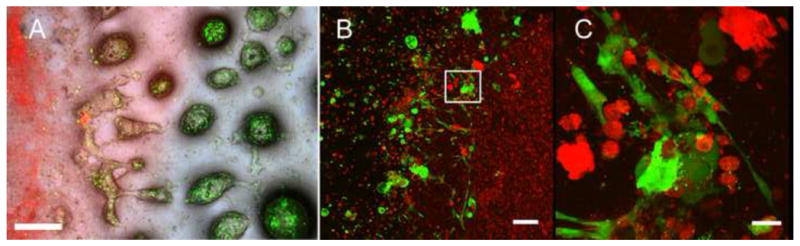
A) Labeled DE and DM cells still exhibited strong fluorescence after 7 days in vitro culture. Merged bright field fluorescent images: green DE cells in Matrigel; red DM cells in Collagen. B) Confocal imaging revealed polarized DE and DM cell interactions along the cell seeded gels interface. C) High magnification view of B (white box). Scale bar=500 μm (A), 100 μm (B), and 20 μm (C).
3.3 In vitro co-cultured 3D constructs
Cell tracker stained DE and DM cells both exhibited strong fluorescent signals after being seeded into Collagen and Matrigel materials (Figure 3B). Collagen constructs containing both DE and DM cells, and the constructs containing DM cells alone, retained their original shape. In contrast, Collagen gel constructs containing only DE cells became increasing fragile and hard to handle after one week in vitro culture (Figure 3C). H&E staining was used to confirm that DM cell constructs maintained their original size and shape up until four weeks in vitro culture, at which time both Collagen and Matrigel gels exhibited significant degradation. Significant contraction of the 3D gels was apparent in all constructs lacking DM cells.
DE cells generated rosette or tubular-like structures in Matrigel 3D cultures. In contrast, underlying DM cells retained their distinctive fibroblast-like morphology, and appeared oriented parallel to the bottom of the culture well (Figure 4, 5). Control porcine DE-porcine DM cell constructs exhibited similar morphologies as porcine DE-human DM cell constructs (Figure 4D, H, L, P). DE and DM cell polarization, which is essential for DE-DM differentiation into ameloblasts and odontoblasts, respectively, was only apparent in limited areas along the DE-DM cell interface (Figure 5A, B). Interestingly, significant Matrigel degradation was observed at the DE-DM interface coincident with the presence of polarized, early differentiation stage DE and DM cells. Higher magnification views confirmed that the tubular and rosette structures generated by porcine DE cells strongly resembled tubular gland-like structures (Figure 5C–F). For the controls without either type of cells, both types of materials were still be able to detect even after 4 weeks in vitro culture, only showed more degradation and exhibited very irregular shape and size (data no show).
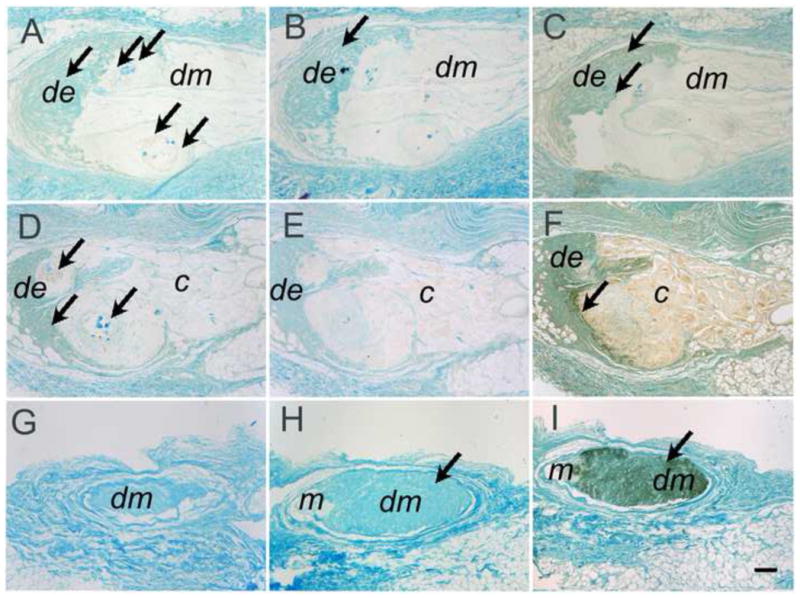
Figure 4. Staged Hematoxylin and eosin (H&E) stained in vitro cultured DE-DM constructs.
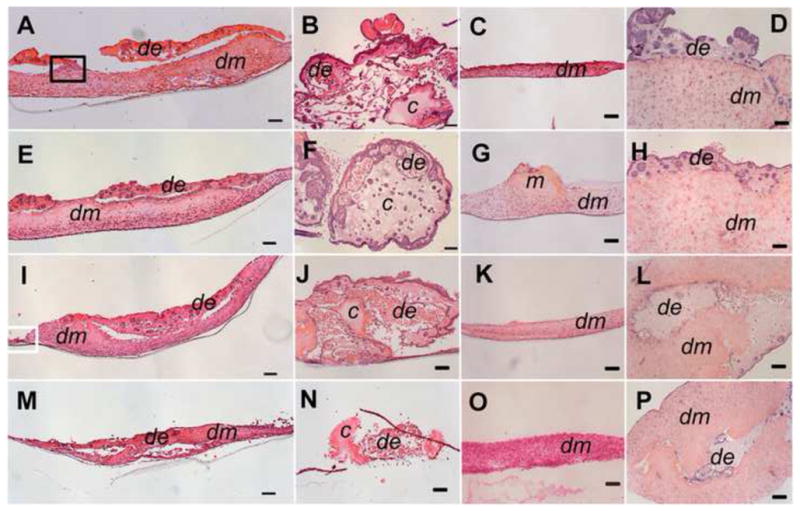
Porcine DE and human DM cell construct (A, E, I, M). Porcine DE cell construct (B, F, J, N). Human DM cell construct (C, G, K, O). Porcine DE and porcine DM cell constructs (D, H, L, P). 1 wk (A–D), 2 wk (E–H), 3 wk ((I–L), and 4 wk (M–P) in vitro cultured constructs. In all constructs, DE cells formed rosette and tubular like structures (arrows). Matrigel and collagen gels showed significant degradation by 4 wks in vitro. Abbreviations: de: DE cells with Matrigel; dm: DM cells with Collagen gel; m: Matrigel without DE cells; c: Collagen gel without DM cells. Scale bar=100 μm.
Figure 5. High magnification images of in vitro cultured DE-DM structures.

A) High magnification view of black boxed area in Figure 4A. B) High magnification view of white boxed area of Figure 4I. Short columnar DE cells were found in the area with severe material degradation. H&E stained (C, E) and polarized light (D, F) images of rosette (C, D) and tubular (D, F) DE cell construct structures. Scale bar=20 μm (A, B) or 10 μm (C–F).
Strong cell tracker label signals were detected even after two weeks of in vitro culture (Figure 6B). Red fluorescing DM cells appeared to be restricted to the Collagen layers, while the green fluorescing DE cells appeared restricted to the Matrigel layers (Figure 6B). This observation correlated well with IHC results using the epithelial cell marker CK-14, and the mesenchymal cell marker Vimentin (Figure 6E, F). DSPP immuno-reactivity was detected in both DE and DM layers (Figure 6C), while in contrast, AM positive cells were only identified in the rosette or tubular structures formed in the DE layer (Figure 6D). Laminin 5 and Collagen IV exhibited overlapping expression patterns, with positive signals for both antibodies detectable in the Matrigel layer surrounding the rosette or tubular structures, and with stronger signals detectable along the DE-DM cell layer interface. No calcified nodules or tooth like structures were detected in any of the experimental or control samples.
Figure 6. IHC analyses of 2 wk in vitro cultured DE-DM structures revealed cell differentiation and basal membrane formation.
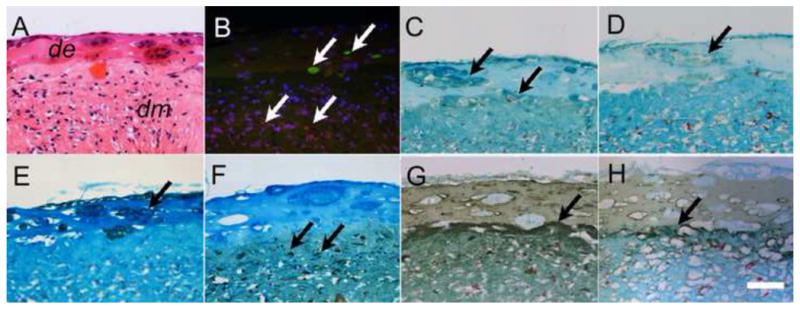
A) H&E stained DE-DM layer interface. B) Serial sectioned construct revealed fluorescent stained DE (green) and DM (red) cells. C) DSPP expression in both DE rosettes and DM layer. D) AM expression was detected only in DE cell layer. E) CK-14 positive DE cell layer. (F) Vimentum was detected only in the DM cell layer (F). Strong Laminin (G) and collagen IV (H) expression was detected at the DE-DM border. In all panels, arrows indicate positive expression. Scale bar=20 μm.
3.4 Co-cultured 3D constructs under in vivo culture
After one week in vivo culture, round shaped nodules were detected in the DE-DM constructs (Figure 7A), but not in the DE alone (Figure 7B), or DM alone (Figure 7C) constructs. After 4 weeks in vivo growth, similar but larger mineralized tissue nodules were found in both DE-DM constructs (Figure 7D, 8A) and in DE alone constructs (Figure 7E), but not in DM alone constructs (Figure 7E). Von Kossa staining was used to confirm that the nodules were calcified (Figure 7I, 8B). In addition, several layers of mesenchymal-like cells with well-organized Collagen fibers were detected surrounding the calcified nodule cores (Figure 7G, H). Only limited amount of cells were detected in the cell-free constructs, indicating lack of host tissue ingrowth (Figure 7B, C, E, F). A few cells surrounding the calcified nodules were positive for the Anti-human specific Mitochondria marker (Figure 8C, D), which did not cross-react to pig or rat cells (data not shown).
Figure 7. 1 and 4 wk in vivo cultured DE-DM cell constructs.

H&E indicated the presence of calcified tissue in DE-DM constructs after 1 wk in vivo culture (A), but not in DE (B), or DM cell only (C) constructs. Greater numbers of calcified nodules were observed in both DE-DM cell constructs (D) and DE cell only constructs (E) after 4 wk in vivo culture. DM cell alone constructs (F) exhibited no evidence of calcified tissue formation. G) High magnification view of the black boxed area in Panel E viewed under light (G) and polarized light (H), and von Kossa stained. Abbreviations: de, DE cells with Matrigel; dm, DM cells with Collagen gel; m, Matrigel without DE cells; c, Collagen gel without DM cells. Scale bar=100 μm (A–F), and 20 μm (G–I).
Figure 8. Human dental pulp cells and mineralized tissues in 4 wk DM in vivo constructs.
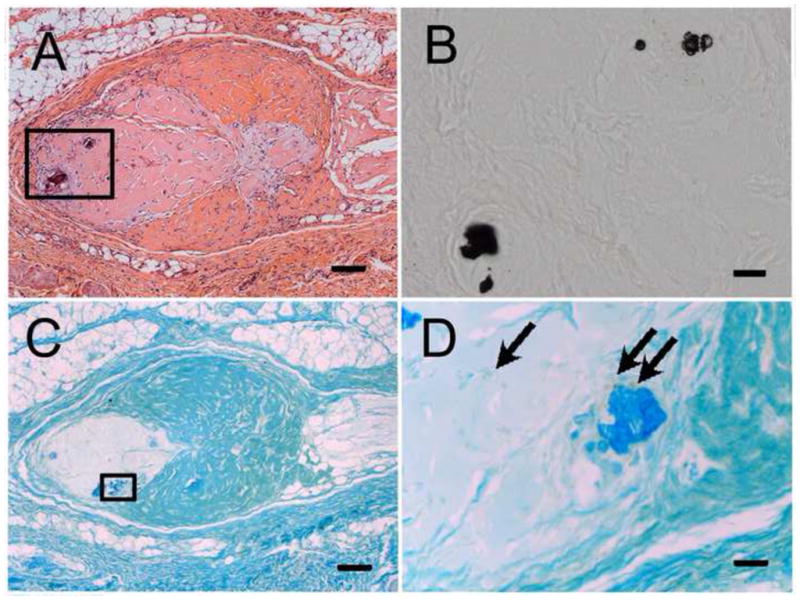
A) H&E stained DE-DM cell construct revealed mineralized tissue formation. B) High magnification view of Von Kossa stained area boxed in Panel A. (C) Human dental pulp cells expressing human mitochondrial protein were located adjacent to calcified tissues (D, arrows). Scale bar = 100 μm (A, C), and 20 μm (B, D).
AM positive cells were observed in all DE cell containing constructs whether or not DM cells were present, and around the calcified tissue nodules in the DM cell seeded constructs (Figure 9A, D), but not in implants without DE cells (Figure 9G). Limited DSPP expression was only found in the implants containing both DE and DM cells (Figure 9B), but not in implants containing only one dental cell type (Figure 9E, H). No DSPP positive cells were detected around the calcified nodule tissues. Positive staining for collagen IV was detectable in all cell-containing implants.
Figure 9. IHC analyses of 4 wk in vivo cultured DE-DM constructs.
AM positive expression was observed in DE cell layers of DE-DM (A) and DE only (D) constructs, adjacent to calcified tissues, while DSPP expression was observed in implant tissues located at a distance from calcified tissues (B, E, H). Collagen IV was detected in all cell seeded constructs (C, F, I). Black arrows indicate positive staining, while white arrows indicate calcified tissue formation. Scale bar = 100 μm.
4. Discussion
The existence of dental stem cells in the dental pulp of both permanent teeth[20] and deciduous teeth [21] supports the possibility of using these cells for autologous dental tissue regeneration in humans. Human dental pulp stem cells (DPSCs) have been demonstrated to form hard tissue both in vitro and in vivo [20–22]. Moreover, DPSCs were shown to exhibit multi-lineage differentiation potential, including highly angiogenic and neurogenic potential, which could be used to support the formation of functional dental pulp tissues [23, 24]. With respect to whole tooth regeneration, dental epithelial cells, required for enamel formation, are also required to induce the differentiation and maturation of dental mesenchymal cells, as mediated by close contact through their basement membranes [25]. In turn, dental mesenchymal cells regulate dental epithelial cells mainly through the secretion of soluble factors [26]. It is widely accepted that tooth formation is initiated by the dental epithelium, after which the odontogenic potential then shifts to the dental mesenchyme [27, 28]. Natural rat tooth buds, retaining their original DE-DM cell interactions, maintained their natural morphology and continued to develop after being autologously transplanted into the jaw bone [29]. In contrast, no tooth-like structures formed when only intact dental pulp, lacking the dental epithelial cell layer, was transplanted and grown beneath the kidney capsule of athymic mice [24, 30]. Furthermore, published reports have shown that tooth morphology is controlled by mesenchyme-derived tissue [31, 32].
Molecular mechanisms regulating DE and DM cell interactions remain poorly understood. Epithelial-Mesenchymal (EP-MS) cell interactions control the development of all ectodermal organs, including teeth [8, 33], hair follicles [34], and salivary glands [35]. A better understanding of the mechanisms regulating EP-MS interactions in tooth development will not only help to understand tooth regeneration, but can also benefit other epidermal organ regeneration efforts.
To date, the most successful models for tooth regeneration are: 1) the dissociated cell-scaffold model; 2) the combined enamel organ-derived epithelial cell layer and pulp organ-derived mesenchymal cell model; and 3) the collagen gel drop model. The dissociated cell-scaffold method seeds mixed populations of dental epithelial and mesenchymal cells, either pre-mixed or sequential added to the scaffold, onto 3-D scaffolds [16, 17, 36, 37]. Using this approach, multiple teeth formed in one construct, and were much smaller than naturally formed teeth. The second method, which has been studied for decades, combines an intact tooth bud enamel organ with dissociated dental mesenchymal cells [16, 17]. Although the teeth that formed using this model appear quite similar to naturally formed teeth, it is not a practical approach for human tooth regeneration, due to the fact that is it difficult, if not impossible, to obtain autologous human enamel organ tissues. The third method, the collagen gel drop model, was accomplished by injecting highly concentrated, dissociated DE and DM cells harvested from E14.5 tooth buds, into adjacent regions within a collagen gel drop [13, 16]. Although this model was successful using embryonic stage tooth bud cells, no positive results have been reported for similar approach using postnatal dental cells. Human dental epithelial cells are difficult to obtain due to the fact that dental epithelial cells have largely undergone apoptosis prior to tooth eruption. We have found that the growth characteristics of post natal porcine dental mesenchymal cells are very similar to those of human third molar tooth pulp cells. Based on the similar characteristics of human and porcine tooth development, porcine DE cells were used in this study. Our results showed that hetero-species co-cultured porcine DE and human DM cells established close interactions with each other, and supported their differentiation into mature DE and DM cell types. DE and DM cell polarization, critical for cell differentiation and maturation, is considered to be an important indicator of dental cell differentiation [38]. A growing body of research suggests that DE-DM cell adhesion, mediated through cell-cell or cell-extracellular matrix (ECM) interactions, plays an important role in cell polarization [39, 40], although it is not clear how dental cell polarization is induced and/or maintained. In this study, DE-DM cell polarization was observed along the DE-DM interface, but only at the regions with severe Matrigel degradation.
BD Matrigel™ Matrix, a soluble basement membrane extract of the Engelbreth-Holm-Swarm (EHS) tumor [41], contains all of the main components of the basement membrane, including laminin 5, collagen IV, entactin, and heparan sulfate proteoglycans[42]. A Collagen-Matrigel gel construct was successfully used to study salivary gland regeneration [43, 44], where tubular gland-like structures were observed in vitro cultured DE cell constructs.
The results presented here show that the calcified nodules formed by DE cell containing constructs did not resemble natural dental epithelial cell derived enamel tissue, but rather resembled calcifications observed in dentinogenic ghost tumor cells [45, 46], which are characterized as ameloblastoma-like epithelial cell islands. The fact that human cells were detected around the observed calcified nodules, and that mineralized nodules only formed in DE containing constructs, indicates that human DM cells were not required for the observed mineralized nodule formation in this model.
Alternative scaffolds to Matrigel may be indicated by the following concerns. Published reports have shown that BD Matrigel™ supports the long-term propagation and maintenance of human Embryonic Stem (hES) cells in an undifferentiated state, without requiring the use of cell feeder layers [47, 48]. Matrigel cultured hES cells were shown to maintain normal karyotype and stable proliferation rate, to express characteristic hES cell markers, and importantly to retain normal differentiation potential. These results suggest that Matrigel may therefore not be the best 3D matrix for inducing dental epithelial cell differentiation into enamel forming ameloblast cells. Another concern is that since Matrigel is extracted from EHS tumor, it is possible that Matrigel might induce normal cells to adopt malignant phenotypes. Together, these results suggest that alternative scaffolds may better facilitate DE-EM cell layer interactions, despite the fact that Matrigel does appear to support the maintenance, proliferation, and differentiation of DE cells in this model system.
5. Conclusion
Our results show that co-cultured 3D DE-DM constructs maintained their predetermined shape and size. Histological and IHC analyses suggested that DE and DM cells expressed proper dental tissue markers under both in vitro and in vivo culture conditions. However, only limited amount of irregular calcified tissues were observed in the implants containing DE cells, but not in the DM cell alone containing implants. Furthermore, the formed calcified tissues did not resemble that of naturally formed dental hard tissues. It is possible that the relatively slow degradation rates of Matrigel and Collagen restricted the direct contact of DM and DE cells, and in turn hampering the formation of organized dental cell layers, and mineralized dental tissue formation.
Acknowledgments
This work was supported by NIH/NIDCR grants DE016132-06 and D0066058 (PCY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kung JW, Currie IS, Forbes SJ, Ross JA. Liver development, regeneration, and carcinogenesis. J Biomed Biotechnol. 2010;2010:984248. doi: 10.1155/2010/984248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egles C, Garlick JA, Shamis Y. Three-dimensional human tissue models of wounded skin. Methods Mol Biol. 2010;585:345–359. doi: 10.1007/978-1-60761-380-0_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handschel J, Naujoks C, Langenbach F, Berr K, Depprich R, Ommerborn M, et al. Comparison of ectopic bone formation of embryonic stem cells and cord blood stem cells in vivo. Tissue Eng Part A. 2010 doi: 10.1089/ten.TEA.2009.0546. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Klein J. Chemistry. Repair or replacement--a joint perspective. Science. 2009;323(5910):47–48. doi: 10.1126/science.1166753. [DOI] [PubMed] [Google Scholar]
- 5.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322(5907):1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 6.Lee MH, Arcidiacono JA, Bilek AM, Wille JJ, Hamill CA, Wonnacott KM, et al. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng Part B. 2010;16(1):41–54. doi: 10.1089/ten.TEB.2009.0449. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Shi J, Jin Y. Current approaches and challenges in making a bio-tooth. Tissue Eng Part B Rev. 2008;14(3):307–319. doi: 10.1089/ten.teb.2008.0165. [DOI] [PubMed] [Google Scholar]
- 8.Slavkin HC, Snead ML, Zeichner-David M, Jaskoll TF, Smith BT. Concepts of epithelial-mesenchymal interactions during development: tooth and lung organogenesis. J Cell Biochem. 26(2):117–125. doi: 10.1002/jcb.240260207. [DOI] [PubMed] [Google Scholar]
- 9.Dassule HR, McMahon AP. Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol. 1998;202(2):215–227. doi: 10.1006/dbio.1998.8992. [DOI] [PubMed] [Google Scholar]
- 10.Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92(1):19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 11.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81(10):695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 12.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83(7):523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 13.Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11(9–10):1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 14.Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27(17):3238–3248. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 15.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H, Kim EJ, Cho SW, Jung HS. Analysis of tooth formation by reaggregated dental mesenchyme from mouse embryo. J Electron Microsc (Tokyo) 2003;52(6):559–566. doi: 10.1093/jmicro/52.6.559. [DOI] [PubMed] [Google Scholar]
- 17.Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83(7):518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106(32):13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang E, Cairncross JG, Liem RK. Identification of glial filament protein and vimentin in the same intermediate filament system in human glioma cells. Proc Natl Acad Sci U S A. 1984;81(7):2102–2106. doi: 10.1073/pnas.81.7.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Walboomers XF, van Osch GJ, van den Dolder J, Jansen JA. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A. 2008;14(2):285–294. doi: 10.1089/tea.2007.0146. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20(5–6):435–440. doi: 10.1016/j.cytogfr.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12(10):2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 25.Thesleff I, Lehtonen E, Saxen L. Basement membrane formation in transfilter tooth culture and its relation to odontoblast differentiation. Differentiation. 1978;10(2):71–79. doi: 10.1111/j.1432-0436.1978.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 26.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118(Pt 3):485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slavkin HC, Cummings E, Bringas P, Honig LS. Epithelial-derived basal lamina regulation of mesenchymal cell differentiation. Prog Clin Biol Res. 1982;85(Pt B):249–259. [PubMed] [Google Scholar]
- 28.Tompkins K. Molecular mechanisms of cytodifferentiation in mammalian tooth development. Connect Tissue Res. 2006;47(3):111–118. doi: 10.1080/03008200600727756. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro HH, Johnson DD. Homotransplantation of developing tooth buds in the rat. Ann N Y Acad Sci. 1958;73(3):576–583. doi: 10.1111/j.1749-6632.1959.tb40835.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuo MY, Lan WH, Lin SK, Tsai KS, Hahn LJ. Collagen gene expression in human dental pulp cell cultures. Arch Oral Biol. 1992;37(11):945–952. doi: 10.1016/0003-9969(92)90066-h. [DOI] [PubMed] [Google Scholar]
- 31.Kollar EJ, Baird GR. The influence of the dental papilla on the development of tooth shape in embryonic mouse tooth germs. J Embryol Exp Morphol. 1969;21(1):131–148. [PubMed] [Google Scholar]
- 32.Kollar EJ, Baird GR. Tissue interactions in embryonic mouse tooth germs. II. The inductive role of the dental papilla. J Embryol Exp Morphol. 1970;24(1):173–186. [PubMed] [Google Scholar]
- 33.D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, et al. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126(13):2911–2920. doi: 10.1242/dev.126.13.2911. [DOI] [PubMed] [Google Scholar]
- 34.Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129(10):2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- 35.Cutler LS, Gremski W. Epithelial-mesenchymal interactions in the development of salivary glands. Crit Rev Oral Biol Med. 1991;2(1):1–12. doi: 10.1177/10454411910020010101. [DOI] [PubMed] [Google Scholar]
- 36.Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007;28(4):680–689. doi: 10.1016/j.biomaterials.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 37.Cho SW, Lee HA, Cai J, Lee MJ, Kim JY, Ohshima H, et al. The primary enamel knot determines the position of the first buccal cusp in developing mice molars. Differentiation. 2007;75(5):441–451. doi: 10.1111/j.1432-0436.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen B, Goodman E, Lu Z, Bandyopadhyay A, Magraw C, He T, et al. Function of beta1 integrin in oral epithelia and tooth bud morphogenesis. J Dent Res. 2009;88(6):539–544. doi: 10.1177/0022034509338008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogita H, Takai Y. Nectins and nectin-like molecules: roles in cell adhesion, polarization, movement, and proliferation. IUBMB Life. 2006;58(5–6):334–343. doi: 10.1080/15216540600719622. [DOI] [PubMed] [Google Scholar]
- 40.Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94(8):655–667. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 42.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Sun L, Maffini MV, Soto A, Sonnenschein C, Kaplan DL. A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function. Biomaterials. 2010;31(14):3920–3929. doi: 10.1016/j.biomaterials.2010.01.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laoide BM, Gastinne I, Rougeon F. Tubular morphogenesis and mesenchymal interactions affect renin expression and secretion in SIMS mouse submandibular cells. Exp Cell Res. 1999;248(1):172–185. doi: 10.1006/excr.1999.4404. [DOI] [PubMed] [Google Scholar]
- 45.Hirshberg A, Dayan D, Horowitz I. Dentinogenic ghost cell tumor. Int J Oral Maxillofac Surg. 1987;16(5):620–625. doi: 10.1016/s0901-5027(87)80117-0. [DOI] [PubMed] [Google Scholar]
- 46.Juneja M, George J. Dentinogenic ghost cell tumor: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):e17–22. doi: 10.1016/j.tripleo.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 48.Gerrard L, Zhao D, Clark AJ, Cui W. Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency. Stem Cells. 2005;23(1):124–133. doi: 10.1634/stemcells.2004-0102. [DOI] [PubMed] [Google Scholar]


