Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder with defining clinical features that include kinetic tremor, gait ataxia, and parkinsonism, with associated features spanning medical, cognitive, and psychiatric clinical domains. The emerging model for the pathogenesis of FXTAS is that of RNA toxicity as a consequence of the sequestration of RNA binding proteins by the expanded CGG-repeat element within the FMR1 message, thus compromising the normal functions of those proteins. A principal challenge at this point is to determine precisely which proteins are involved in FXTAS pathogenesis and how to prevent or reverse this process. A second challenge is to determine why there is incomplete penetrance of FXTAS among premutation carriers with identical CGG-repeat lengths, and what the protective factors are in some carriers. Finally, the discovery in premutation mice of early neurodevelopmental abnormalities, some occurring even during late embryogenesis, raises the question of whether FXTAS is the end-stage of a life-long process of neuronal dysregulation. If an extended pre-clinical phase precedes the development of FXTAS, there is great potential for therapeutic intervention, years or even decades before its clinical features are manifest.
Keywords: Fragile X syndrome, primary ovarian insufficiency, Alzheimer, Parkinson, neurodegeneration, neurodevelopment
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late adult-onset neurodegenerative disorder that affects, with age- and gender-specific penetrance, carriers of premutation alleles (55–200 CGG repeats) of the fragile X mental retardation 1 (FMR1) gene. Defining clinical features of FXTAS include progressive kinetic tremor; gait ataxia; and parkinsonism with associated, variable features including cognitive impairment and executive dysfunction, peripheral neuropathy, and dysautonomia. At the time of its discovery and initial report,1 premutation carriers were generally regarded as asymptomatic, with the features noted above ascribed to various other conditions,2 or to “normal aging”. The significance of the premutation allele was thought to be primarily its propensity to expand, upon maternal transmission, to a full mutation allele (>200 CGG repeats) in the child.
Two critical observations have helped to define FXTAS as a novel, premutation-associated disorder. Firstly, it was found that premutation alleles of the FMR1 gene are transcriptionally up-regulated (Figure 1), with some individuals producing more than eight times the normal levels of FMR1 mRNA.3,4 This observation both established that there is biochemical dysregulation (not just genetic instability) in premutation carriers—thus providing a context for clinical involvement—and gave rise to the current model for FXTAS pathogenesis, namely, RNA toxic gain-of-function of the expanded CGG-repeat mRNA.5,6 Secondly, because cases of FXTAS are largely limited to the premutation range (FMRP levels at or near normal levels), and are generally not found among those with full mutation alleles (FMRP substantially diminished), the mechanisms of FXTAS and the neurodevelopmental disorder, fragile X syndrome, must be entirely distinct—one involving elevated mRNA and the other involving a protein deficit.7 A related issue is that additional neurological and medical signs that may have been regarded as unrelated to the premutation (e.g., peripheral neuropathy, hypertension, autonomic dysfunction) can be established as associated (or non-associated) through their linkage to the premutation-expanded allele; this finding is particularly evident with the recent observation of medical problems experienced by female carriers with FXTAS.8,9
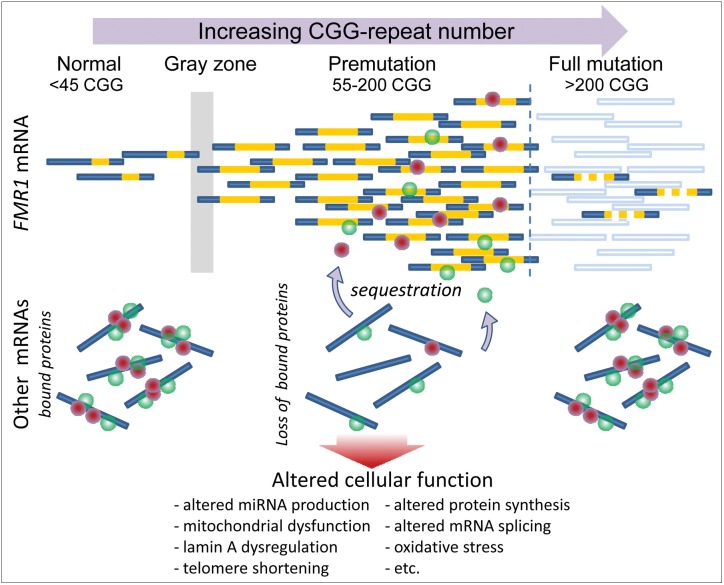
Figure 1. Overview of the Expression of the FMR1 Gene.
FMR1 mRNA levels increase with increasing CGG-repeat length (gold segments) throughout the premutation range, and undergo a transition to greatly diminished levels in the full mutation range because of hypermethylation of the FMR1 promoter region. In some instances, methylation mosaicism results in continued production of low-to-moderate levels of mRNA in the full mutation range. RNA toxicity in the premutation range is thought to arise through sequestration, by direct binding to the expanded CGG-repeat element within the FMR1 mRNA, of one or more RNA binding proteins that would normally be associated with other mRNAs. Sequestration in turn leads to loss of the normal function(s) of those proteins, which may include splice modulation and regulation of miRNA production, among other functions. Dysregulation of RNA processing is thought to lead to multiple forms of downstream cellular dysregulation.
However, the issues of RNA toxicity and premutation-association also raise significant challenges. In the first instance, how does the FMR1 mRNA cause toxicity and disease, and why does it not occur in all premutation carriers? In the second instance, how do we determine whether a given clinical feature is associated with, or prodromal to, FXTAS, as opposed to its more general association with the premutation, wherein it would have little predictive value for the eventual onset of FXTAS? Successful resolution of these questions is critically important to the development of accurate diagnoses and treatment for the spectrum of premutation-associated disorders, including not only FXTAS, but also fragile X-associated primary ovarian insufficiency (FXPOI) and neurodevelopmental involvement (attention deficit hyperactivity disorder [ADHD]; autism and autism spectrum disorders [ASD]; seizures and intellectual disability [ID]10). Indeed, these questions have much broader relevance to neurodegenerative disorders, since co-morbid features may reflect differing pathogenic mechanisms. This issue is difficult enough for principally monogenic disorders such as FXTAS, but is compounded for the more common, sporadic disorders, such as Parkinson's disease (PD) and Alzheimer's disease (AD).
Pathogenesis of FXTAS
As noted above, the fundamentally distinct nature of FMR1 expression in the premutation range (high mRNA, relatively preserved FMRP levels), and the full mutation range (reduced/absent mRNA, FMRP), led to the idea of an RNA-mediated pathogenesis of FXTAS, based on the “RNA toxicity” model for myotonic dystrophy (DM).11,12 For the latter disorder, one or more proteins, including muscleblind-like 1(MBNL1), is bound excessively (sequestered) by an expanded, non-coding C(C)UG repeat element. For MBNL1, specific domains of clinical involvement, e.g., myotonia and insulin resistance, arise from the altered splicing of specific mRNAs due to functional insufficiency of the sequestered protein. FXTAS pathogenesis is believed to operate through a similar mechanism, whereby the expanded CGG-repeat element in the 5′ non-coding portion of FMR1 mRNA sequesters protein(s) that affect diverse pathways, leading to clinical involvement (Figure 1). For FXTAS, the fundamental challenge at this point is two-fold; namely, to establish that the FMR1 mRNA is necessary and sufficient to produce all features of the neurodegenerative disorder and, predicated on the correctness of the model, to identify the specific proteins involved in mediating the RNA-triggered pathogenesis.
At present, several proteins have been identified that both interact with the CGG repeat and are potential mediators of downstream cellular dysregulation. Among these proteins are Sam68,13 a splicing modulator whose absence in the mouse leads to motor dysfunction;14,15 Pur α,16,17 an RNA-binding protein whose targeted disruption in mouse leads to tremor and gait disturbances, and early loss of viability;18 and hnRNP A2,17,19 a multifunctional heterogeneous nuclear RNA binding protein thought to be involved with mRNA metabolism and transport. For Sam68, there is evidence of functional impairment in the brains of individuals with FXTAS, with disease-specific alterations in splicing of ATP11B and SMN2 mRNAs. There is also recovery of control isoform ratios in cultured cells harboring expanded CGG-repeat alleles upon over-expression of exogenous Sam68 protein.13 Similarly, overexpression of Pur α protein in a CGG-repeat Drosophila model reverses a neurodegenerative (eye) phenotype,16,19 even though there has not, as yet, been any demonstration of the functional significance of Pur α in rescuing a CGG-repeat-induced neurodegenerative phenotype in a mammalian system.
More recently, Muslimov et al.20 presented evidence based on rat neuronal transfection experiments, that binding of hnRNP A2 to the expanded CGG-repeat RNA interferes with its binding to the non-coding RNA, BC1, which normally facilitates dendritic transport of mRNAs that are required for synaptic function. In addition, they showed that overexpression of hnRNP A2 partially reversed the impaired dendritic transport of an mRNA known to be targeted for dendritic transport. These intriguing observations point to a possible role for hnRNP A2 in the pathogenesis of FXTAS. However, many dendritically-transported mRNAs occur via mechanisms that are likely to be independent of BC1.21 Thus, to assess what role, if any, that hnRNA A2 and/or BC1 play in FXTAS, it will be essential to study their roles in vivo, e.g., with knock-in (KI) mice.
Finally, Sellier et al.22 reported preliminary findings that the expanded CGG-repeat RNA sequesters DiGeorge syndrome critical region 8 (DGCR8) protein (haploinsufficient in DiGeorge syndrome/22qdel syndrome).23,24 DGCR8, along with the RNase III Drosha, constitutes the microRNA (miR) nuclear processing complex that cleaves primary miRNAs (pri-miRs residing within portions of larger RNA transcripts) into precursor miRNAs (pre-miRs) in the miR biogenesis pathway.25 The importance of the observation by Sellier et al.22 is that substantial miR dysregulation (low miRs and elevated levels of pri-miRs) is observed in the brains of FXTAS cases.
Although one or more of these proteins, when sequestered, may mediate the “toxicity” of the FMR1 mRNA, there is still little understanding as to the downstream consequences of their actions. The problem is particularly daunting for both the DGCR8 protein, where reductions of multiple miRs would be expected to influence the expression of hundreds of proteins and many pathways, and for hnRNP A2, which is likely to facilitate the transport of many mRNAs of diverse function. Thus, even though we know of specific aspects of cellular dysfunction, e.g., mitochondrial abnormalities,26,27 altered lamin A nuclear architecture,28 and reduced telomere length,29 we do not know how they are linked. The challenge is now to “connect the dots” between the basic sequestration mechanism and the downstream processes that lead to disease.
The broader implications of FXTAS to neurodegenerative disease
The spectrum of clinical involvement among individuals with FXTAS includes features of both parkinsonism30–32 and dementia,33,34 suggesting that studying the pathogenesis of FXTAS might shed light on the pathogenesis of both PD and AD, since there are likely to be shared mechanisms of neurodegeneration among the three disorders. Indeed, for idiopathic PD, there is growing evidence of a pathogenic synergism at the level of mitochondrial dysfunction. The involvement of mitochondrial dysfunction in both PD and AD is well-known.35–38 However, evidence of mitochondrial dysfunction has only recently become apparent for FXTAS, and more broadly among carriers of premutation FMR1 alleles.26,27,31,32
Since both FXTAS and PD are associated with altered mitochondrial function, this suggests that premutation alleles can contribute in two ways to the clinical burden of PD, and more broadly, parkinsonism, in society. Firstly, since parkinsonism is an associated feature of FXTAS, premutation alleles of the FMR1 gene would directly contribute to cases of parkinsonism, or the misdiagnosis of PD.2 Both mechanistically and clinically, such cases are distinct from PD and are therefore likely to respond poorly to therapeutic approaches for treating PD. However, given the much higher frequency of PD and atypical parkinsonism compared to premutation carrier frequency in the general population, misdiagnosis of FXTAS as PD would be of note as a failure to detect the former, and, therefore, carriers of premutation alleles.
Secondly, and perhaps more importantly, both gray zone (∼45–54 CGG repeats) and premutation alleles may contribute directly to the appearance and/or severity of PD,31 albeit by entirely separate mechanisms, if such mechanisms affect common features of neuronal viability, e.g., mitochondrial function.39 Such a confluence leads to the prediction that both the genetic and environmental factors that contribute to late-onset PD may contribute to the penetrance and severity of some cases of FXTAS, perhaps influencing why some premutation carriers develop FXTAS, where others, with an identical number of CGG repeats, do not develop this neurodegenerative disorder. Thus, as a general principle of disease pathogenesis, the example of a possible interplay between the premutation and PD, e.g., mitochondrial dysfunction, oxidative stress, etc., could also extend to the mitochondrial dysfunction in AD, to idiopathic autism26 and to other disorders that share both phenotypic domains and overlapping or convergent mechanisms.
Going back in time
One of the remarkable and unexpected outcomes of research on FXTAS, particularly from mouse studies, is finding that the premutation allele leads to early developmental abnormalities that are evident even in late embryonic development. In particular, embryonic premutation mice have both abnormal neuronal migration in the neocortex and altered neuronal differentiation.40 Furthermore, cultured hippocampal neurons from neonatal mice bearing premutation FMR1 alleles develop shorter and fewer dendritic branches than wildtype (WT) littermates,41 and although, the numbers of synaptic puncta do not differ between the premutation and WT mice, the premutation puncta are larger.
The premutation mice also manifest neurodevelopmental behavioral/cognitive deficits that probably reflect a much broader (and earlier) spectrum of involvement among premutation carriers than the late-adult-onset neurodegenerative disorder, FXTAS. Hunsaker et al.42 demonstrated that at 12 and 24 weeks of age, premutation mice did not recognize changes in the distance between two objects (metric task), but were able to detect transposition of the objects (topological task). However, at 48 weeks, this second ability was also lost. The authors' results suggest that altered hippocampal-dependent spatial processing may precede the impaired parietal cortex function associated with FXTAS. In addition, parallel studies in human premutation carriers (females aged 21 – 42 years), Goodrich-Hunsaker et al.43 observed modulated cognitive impairments using a quantitative magnitude comparison task—consistent with the results of the mouse studies—suggesting a neurocognitive phenotype associated with some premutation carriers that appear much earlier than the motor/cognitive impairments associated with FXTAS.
Similar conclusions were obtained from the study of a second KI mouse model44,45 that extended the studies of dendritic morphology in cultured hippocampal neurons.41 Although both the in vitro and in vivo studies revealed consistent decreases in complexity of dendritic branching, Qin et al.44 observed increased spine density, in apparent contrast to the absence of any difference in density between WT and KI cultured neurons.41 This distinction is important; since Qin et al.44 posited that the spines in their KI mice were immature, perhaps signaling a defect in synaptic plasticity. These authors proposed further that their observations could be explained by dramatically decreased FMRP levels in their mice. However, the mouse model used by Chen et al.41 shows only a ∼40% decrease in FMRP level for the same CGG-repeat range, ∼100–150 CGG repeats,46 which is comparable to the relative FMRP levels observed in human blood lymphocytes.47–49 This decrease is nowhere near the dramatic reductions observed by Qin et al.44 Therefore, whereas reduction in FMRP may play a significant role in the Qin et al. mouse model, it is likely to play a lesser role in the Chen et al. study. The question remains as to how two very similar mouse models of the same background strain and nearly identical KI sequences can have such differences in FMRP expression. Resolution of this issue has clinical implications, since for many of the children with premutation alleles who have neurodevelopmental problems (e.g., autism, ASD, ID and ADHD), mild reductions in FMRP levels may yet be playing a role. Clearly, the involvement of FMRP in premutation-associated disorders needs further investigation.
Collectively, the mouse and human studies imply that there is a broad spectrum of clinical and cellular developmental involvement among carriers of premutation alleles, which is evident both at an early age and, most likely, in a broader cross-section of carriers than those who will eventually develop FXTAS. On the one hand, these observations provide an important foundation for understanding the known neurodevelopmental/behavioral problems suffered by some children who are carriers of a premutation allele.47,48,50–52 On the other hand, such observations also present a challenge for interpreting mouse and human neurodevelopmental studies—namely, to what extent any of the cellular and clinical findings are predictive of late-onset neurodegeneration. Thus, premutation allele status does not imply that one will necessarily develop FXTAS or associated neurocognitive decline, yet in some manner that we do not fully understand, a premutation allele increases the risk of the late-onset disorder. Indeed, the whole issue of incomplete penetrance is a major challenge to our understanding of FXTAS. In this regard, there is a need for informative biomarkers that will help predict who will develop FXTAS and how such individuals will respond to therapeutic intervention.
FXTAS versus premutation-associated disease
The uncertain relationship between FXTAS and premutation status reprises the whole issue of how one assigns a particular form of clinical involvement among premutation carriers to the canonical disorder of FXTAS, a forme fruste of the disorder, or a mechanistically distinct feature of the premutation that is not prodromal to, or co-morbid with, the disorder. Whereas it is evident that the list of co-morbid conditions with FXTAS is growing (e.g., fibromyalgia;8,9,53 autoimmune thyroid disease;8 psychopathology, including depression and anxiety disorders;54 and, sleep apnea55), the relationship(s) between the manifestation of cognitive and behavioral impairment in childhood, FXPOI in young adults,56,57 mood-instability and psychiatric conditions, and late-onset neurodegeneration remain obscure except that they are probably all related to RNA toxicity. However, the recent finding that both lowered FMRP and elevated mRNA are correlated with amygdala dysfunction in young adult males with the premutation49 suggests that additional gene dysfunction at the level of lowered FMRP, particularly for large CGG-repeat alleles, may be additive to the effect of the RNA toxicity. Thus, additional studies of the role(s) played by both RNA and protein may help to differentiate the various forms of premutation involvement.
The broad challenge of defining the relationship between premutation status and disease formation will only be met through the identification of the common and divergent mechanisms that underlie the family of premutation disorders. Indeed, although FXTAS is principally a premutation disorder, the RNA-toxicity mechanism predicts that occasional individuals with alleles producing excess FMR1 mRNA that are outside of the premutation range may, nevertheless, manifest some characteristics of FXTAS. Consistent with this prediction, Hall et al.58 reported a case of an older adult male with fragile X syndrome who developed a progressive neurodegenerative syndrome. This individual was mosaic for allele size, with approximately 80% full mutation alleles and the remaining 20% consisting of a small premutation allele (∼70 CGG repeats). This case is interesting in its ambiguity; a preponderance of full mutation alleles giving rise to fragile X syndrome, but a minority of premutation alleles that could be responsible for his late neurodegenerative course. Less ambiguous is the report of a male with clinical and radiological features (middle cerebellar peduncle sign) of FXTAS, but who was a carrier of an unmethylated, full mutation allele.31 The recent observation of (rare) intranuclear inclusions in three older adult males with methylated, or methylation-mosaic full mutation alleles, and fragile X syndrome,59 suggests that residual RNA expression from full mutation alleles can lead to neuropathologic features normally associated with FXTAS; but, in these three cases, typical clinical features of FXTAS were not evident. However, parkinsonism is common in aging patients with fragile X syndrome60 with no difference between those with a full mutation and those with (pre/full mutation) mosaicism. Therefore the aging problems in fragile X syndrome are unlikely to be related to RNA toxicity; diminished adult neurogenesis is one possibility.61
FMR1 alleles with gray-zone expansions (i.e., between the normal and premutation ranges) may also lead to features of FXTAS in some cases,31,32,62 particularly if the FMR1 mRNA levels are elevated within that range.63 Interestingly, the rate of FXPOI is also elevated in the gray zone,56,57 again pointing to a shared mechanism. These cases, lying outside of the premutation range, underscore the possibility of additional genetic and/or environmental modifiers, perhaps the same factors that determine penetrance within the premutation range. However, it should be noted that the majority of studies have not found increased premutation or gray zone alleles in patients with parkinsonism.64–70 Thus, there remains a fundamental uncertainty with respect to the involvement of gray-zone alleles in the neurodegenerative phenotypes (e.g., FXTAS, PD) that will require either much larger studies or a clear mechanistic understanding of how such alleles might contribute to disease pathogenesis.
Concluding remarks
FXTAS is an important neurodegenerative disorder, not only from the standpoint of its clinical severity, but also because of what it can tell us about shared mechanisms across the spectrum of late-onset identification of mechanistic associations with various domains of clinical involvement (e.g., reproductive failure, cognitive impairment) that are far removed from its core neurologic features of tremor and ataxia. The elucidation of these shared mechanisms (e.g., mitochondrial dysfunction39) should lead to improved disease biomarkers and novel therapeutic approaches, not only for the more common neurodegenerative disorders (PD, AD), but also for mechanistically linked neurodevelopmental involvement.
Acknowledgments
I wish to thank the families and patients who have participated in and supported our research and Dr. Randi Hagerman for penetrating discussions. In addition, I would like to acknowledge general/infrastructural support from the University of California, Davis M.I.N.D. Institute.
Footnotes
Funding: IRC grant [UL1 DE019583; RL1 AG032119; RL1 AG032115] from the National Institutes of Health (NIH Roadmap Interdisciplinary Research Consortium) and an individual NICHD grant to P.J.H. [R01 HD040661].
Competing Interests: Paul Hagerman is a non-compensated collaborator with Asuragen, Inc. and with Pacific Biosciences. With Dr. Flora Tassone, he holds a US patent for expanded-CGG screening. There are no other conflicts of interest to report.
References
- 1.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 2.Hall DA, Berry-Kravis E, Jacquemont S, et al. Initial diagnoses given to persons with the fragile X associated tremor/ataxia syndrome (FXTAS) Neurology. 2005;65:299–301. doi: 10.1212/01.wnl.0000168900.86323.9c. [DOI] [PubMed] [Google Scholar]
- 3.Kenneson A, Zhang F, Hagedorn CH, et al. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 4.Tassone F, Hagerman RJ, Taylor AK, et al. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagerman PJ, Hagerman RJ. The fragile-X premutation: A maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willemsen R, Levenga J, Oostra B. CGG repeat in the FMR1 gene: size matters. Clin Genet. 2011;80:214–225. doi: 10.1111/j.1399-0004.2011.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagerman R, Au J, Hagerman P. FMR1 premutation and full mutation molecular mechanisms related to autism. J Neurodev Disord. 2011;3:211–224. doi: 10.1007/s11689-011-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17:1359–1362. doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chonchaiya W, Au J, Schneider A, et al. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012;131(4):581–589. doi: 10.1007/s00439-011-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JE, Cooper TA. Pathogenic mechanisms of myotonic dystrophy. Biochem Soc Trans. 2009;37:1281–1286. doi: 10.1042/BST0371281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler TM, Thornton CA. Myotonic dystrophy: RNA-mediated muscle disease. Curr Opin Neurol. 2007;20:572–576. doi: 10.1097/WCO.0b013e3282ef6064. [DOI] [PubMed] [Google Scholar]
- 13.Sellier C, Rau F, Liu Y, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29:1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla G, Lin CH, Han A, et al. Sam68 regulates a set of alternatively spliced exons during neurogenesis. Mol Cell Biol. 2009;29:201–213. doi: 10.1128/MCB.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paronetto MP, Achsel T, Massiello A, et al. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176:929–939. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin P, Duan R, Qurashi A, et al. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qurashi A, Li W, Zhou JY, et al. Nuclear accumulation of stress response mRNAs contributes to the neurodegeneration caused by fragile X premutation rCGG repeats. PLoS Genet. 2011;7:e1002102. doi: 10.1371/journal.pgen.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalili K, Del Valle L, Muralidharan V, et al. Pur alpha is essential for postnatal brain development and developmentally coupled cellular proliferation as revealed by genetic inactivation in the mouse. Mol Cell Biol. 2003;23:6857–6875. doi: 10.1128/MCB.23.19.6857-6875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sofola OA, Jin P, Qin Y, et al. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muslimov IA, Patel MV, Rose A, et al. Spatial code recognition in neuronal RNA targeting: Role of RNA-hnRNP A2 interactions. J Cell Biol. 2011;194:441–457. doi: 10.1083/jcb.201010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian M, Rage F, Tabet R, et al. G-quadruplex RNA structure as a signal for neurite mRNA targeting. EMBO Rep. 2011;12:697–704. doi: 10.1038/embor.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellier C, Hagerman P, Willemsen R, et al. 12th International Fragile X Conference; July 21–25; Detroit, MI. 2010. DROSHA/DGCR8 sequestration by expanded CGG repeats leads to global micro-RNA processing alteration in FXTAS patients [abstract] [Google Scholar]
- 23.Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14:2162–2167. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 24.McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Medicine (Baltimore) 2011;90:1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- 25.Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331:550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napoli E, Ross-Inta C, Wong S, et al. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011;20:3079–3092. doi: 10.1093/hmg/ddr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross-Inta C, Omanska-Klusek A, Wong S, et al. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429:545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Arocena D, Yang JE, Brouwer JR, et al. Fibroblast phenotype in male carriers of FMR1 premutation alleles. Hum Mol Genet. 2010;19:299–312. doi: 10.1093/hmg/ddp497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins EC, Tassone F, Ye L, et al. Reduced telomere length in older men with premutation alleles of the fragile X mental retardation 1 gene. Am J Med Genet A. 2008;146A:1543–1546. doi: 10.1002/ajmg.a.32342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall DA, Howard K, Hagerman R, et al. Parkinsonism in FMR1 premutation carriers may be indistinguishable from Parkinson disease. Parkinsonism Relat Disord. 2009;15:156–159. doi: 10.1016/j.parkreldis.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loesch DZ, Godler DE, Evans A, et al. Evidence for the toxicity of bidirectional transcripts and mitochondrial dysfunction in blood associated with small CGG expansions in the FMR1 gene in patients with parkinsonism. Genet Med. 2011;13:392–399. doi: 10.1097/GIM.0b013e3182064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loesch DZ, Khaniani MS, Slater HR, et al. Small CGG repeat expansion alleles of FMR1 gene are associated with parkinsonism. Clin Genet. 2009;76:471–476. doi: 10.1111/j.1399-0004.2009.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourgeois JA, Coffey SM, Rivera SM, et al. A review of fragile X premutation disorders: Expanding the psychiatric perspective. J Clin Psychiatry. 2009;70:852–862. doi: 10.4088/JCP.08r04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seritan AL, Nguyen DV, Farias ST, et al. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): comparison with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1138–1144. doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinta SJ, Andersen JK. Nitrosylation and nitration of mitochondrial complex I in Parkinson's disease. Free Radic Res. 2011;45:53–58. doi: 10.3109/10715762.2010.509398. [DOI] [PubMed] [Google Scholar]
- 36.Lin TK, Liou CW, Chen SD, et al. Mitochondrial dysfunction and biogenesis in the pathogenesis of Parkinson's disease. Chang Gung Med J. 2009;32:589–599. [PubMed] [Google Scholar]
- 37.Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson's disease. Exp Neurol. 2009;218:247–256. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Z, Wood NW. Cell death pathways in Parkinson's disease: Role of mitochondria. Antioxid Redox Signal. 2009;11:2135–2149. doi: 10.1089/ars.2009.2624. [DOI] [PubMed] [Google Scholar]
- 39.Schon EA, Przedborski S. Mitochondria: The next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham CL, Martínez Cerdeño V, Navarro Porras E, et al. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum Molec Genet. 2011;20:64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19:196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunsaker MR, Wenzel HJ, Willemsen R, et al. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009;123:1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodrich-Hunsaker N, Wong L, McLennan Y, et al. Young adult female fragile X premutation carriers show age-and genetically-modulated cognitive impairments. Brain Cogn. 2011;75:255–260. doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin M, Entezam A, Usdin K, et al. A mouse model of the fragile X premutation: Effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011;42:85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunsaker MR, Arque G, Berman RF, et al. Mouse models of the fragile X premutation and the fragile X associated tremor/ataxia syndrome. Results Probl Cell Differ. 2012;54:255–269. doi: 10.1007/978-3-642-21649-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brouwer JR, Huizer K, Severijnen LA, et al. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008;107:1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tassone F, Hagerman RJ, Taylor AK, et al. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000;91:144–152. doi: 10.1002/(SICI)1096-8628(20000313)91:2<144::AID-AJMG14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 48.Goodlin-Jones BL, Tassone F, Gane LW, et al. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25:392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Hessl D, Wang JM, Schneider A, et al. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry. 2011;70:859–865. doi: 10.1016/j.biopsych.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aziz M, Stathopulu E, Callias M, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. 2003;121:119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- 51.Farzin F, Perry H, Hessl D, et al. Autism Spectrum Disorders and Attention-Deficit/Hyperactivity Disorder in Boys with the Fragile X Premutation. J Dev Behav Pediatr. 2006;27:S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 52.Hagerman R, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Molec Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leehey M, Hagerman P. Fragile X-associated tremor/ataxia syndrome. In: Vinken P, Bruyn G, editors. Handbook of Clinical Neurology. New York: Wiley Interscience Division; 2012. pp. 373–386. [Google Scholar]
- 54.Roberts J, Mazzocco MM, Murphy MM, et al. Arousal modulation in females with fragile X or Turner syndrome. J Autism Dev Disord. 2008;38:20–27. doi: 10.1007/s10803-007-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamlin A, Liu Y, Nguyen D, et al. Sleep apnea in fragile X premutation carriers with and without FXTAS. Am J Med Genet Part B: Neuropsychiatric Genetics. 2011;156:23–928. doi: 10.1002/ajmg.b.31237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodega B, Bione S, Dalpra L, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21:952–957. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- 57.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117:376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 58.Hall D, Pickler L, Riley K, et al. Parkinsonism and cognitive decline in a fragile X mosaic male. Mov Disord. 2010;25:1523–1524. doi: 10.1002/mds.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunsaker MR, Greco CM, Tassone F, et al. Rare intranuclear inclusions in the brains of 3 older adult males with fragile X syndrome: Implications for the spectrum of fragile X-associated disorders. J Neuropathol Exp Neurol. 2011;70:462–469. doi: 10.1097/NEN.0b013e31821d3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Utari A, Adams E, Berry-Kravis E, et al. Aging in fragile X syndrome. J Neurodev Disord. 2010;2:70–76. doi: 10.1007/s11689-010-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Y, Shan G, Guo W, et al. Fragile X mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall DA, Berry-Kravis E, Zhang W, et al. FMR1 gray-zone alleles: Association with Parkinson's disease in women. Mov Disord. 2011;26:1900–1906. doi: 10.1002/mds.23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loesch DZ, Bui QM, Huggins RM, et al. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet. 2007;44:200–204. doi: 10.1136/jmg.2006.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cilia R, Kraff J, Canesi M, et al. Screening for the presence of FMR1 premutation alleles in women with parkinsonism. Arch Neurol. 2009;66:244–249. doi: 10.1001/archneurol.2008.548. [DOI] [PubMed] [Google Scholar]
- 65.Deng H, Le W, Jankovic J. Premutation alleles associated with Parkinson disease and essential tremor. JAMA. 2004;292:1685–1686. doi: 10.1001/jama.292.14.1685-b. [DOI] [PubMed] [Google Scholar]
- 66.Kraff J, Tang HT, Cilia R, et al. Screen for excess FMR1 premutation alleles among males with parkinsonism. Arch Neurol. 2007;64:1002–1006. doi: 10.1001/archneur.64.7.1002. [DOI] [PubMed] [Google Scholar]
- 67.Kurz MW, Schlitter AM, Klenk Y, et al. FMR1 alleles in Parkinson's disease: Relation to cognitive decline and hallucinations, a longitudinal study. J Geriatr Psychiatry Neurol. 2007;20:89–92. doi: 10.1177/0891988706297737. [DOI] [PubMed] [Google Scholar]
- 68.Reis AH, Ferreira AC, Gomes KB, et al. Frequency of FMR1 premutation in individuals with ataxia and/or tremor and/or parkinsonism. Genet Mol Res. 2008;7:74–84. doi: 10.4238/vol7-1gmr357. [DOI] [PubMed] [Google Scholar]
- 69.Tan EK, Zhao Y, Puong KY, et al. Fragile X premutation alleles in SCA, ET, and parkinsonism in an Asian cohort. Neurology. 2004;63:362–363. doi: 10.1212/01.wnl.0000130199.57181.7b. [DOI] [PubMed] [Google Scholar]
- 70.Toft M, Aasly J, Bisceglio G, et al. Parkinsonism, FXTAS, and FMR1 premutations. Mov Disord. 2005;20:230–233. doi: 10.1002/mds.20297. [DOI] [PubMed] [Google Scholar]