Abstract
Endothelium-dependent vasodilator function may be regarded as an index of inflammation. Endothelial dysfunction has been observed in stroke patients and has been related to stroke physiopathology, stroke subtypes, clinical severity, and outcome. Our aim was to measure systemic vascular function directly (using forearm flow mediated dilatation) in patients with acute ischemic stroke and to clarify whether recent acute ischemic stroke is associated with impaired vascular function. Patients who were not eligible for thrombolytic therapy because of delayed arrival were randomly recruited to the study after signing a consent form. All 43 patients were conscious and had an acute ischemic stroke. Brain CT was performed on admission, and clinical evaluation was carried out by a neurologist on admission and four days later. Vascular responsiveness was evaluated by ABI and by endothelial function measurements on admission. Levels of P-selectin were measured during the first 24 hrs and on day 4. Forty-three patients (28 men and 15 women) and 23 healthy men (control) were enrolled in the study. Patients were older (62.4±12.5 y vs 44.2±11.6 y, p=0.001), had worse endothelial dysfunction (–4.4±7.4% vs 16.6±7.6%, p=0.001), and had a higher BMI (28±6 vs 24±5, p=0.001). No gender effect was found in endothelial function (–5.1±7.8% vs –2.5±6.6%, p=0.25) and ABI (1.0±0.26 vs 1.0±0.5, p=0.29). However, men had lower BMIs compared to women (26.8±5.8 vs 31.4±5.5, p=0.01). The neurological scale decreased from 4.9±3.4 to 3.2±3.0 on day 4 (p=0.001). In men, it was 4.8±3.8 on admission, and decreased to 3.2±3.4 on day 4 (p=0.001). In women, it was 5.0±2.7, and decreased to 3.3±2.3 on day 4 (p=0.001). P-selectin levels were high on admission (68.0±55.5 pg/ml) and increased 4 days later (102.3±72.0 pg/ml) (p=0.01). Men had higher levels on admission (79.1± 66.7 pg/ml vs 48.9± 15.4 pg/ml, p=0.02) and rose on day 4 to 113.6±82.6 pg/ml (p=0.05); in women P-selectin increased from 48.9± 15.4 pg/ml to 83.5±46.4 pg/ml (p=0.01), without gender effect on day 4 (113.6±82.6 pg/ml [men] vs 83.5±46.4 pg/ml [women] (p=0.08)). None of the univariate models seemed statistically significant---gender (p=0.448), age (p=0.100), BMI (p=0.607), ABI (p=0.103), FMD% (p=0.456), and P-selectin (p=0.195). Patients with acute stroke had severe endothelial dysfunction during the first 24 hrs with high P-selectin levels that further increased over the first week. Vascular instability and procoagulant activity are still in progress in the first days following acute stroke and patients are at risk to develop more vascular events at that time.
Introduction
The vascular endothelium is a primary target for inflammatory mediators. Functionally, endothelial inflammatory activation is associated with a profound impairment in endothelium-dependent vasodilator function.1–3 Endothelium-dependent vasodilatation may be regarded as an integral index of all inflammatory mediators present in an individual and thus may represent the vascular phenotype of an inflamed patient. Resting ankle-brachial index (ABI) is also considered an indicator of generalized atherosclerosis, and a low ABI is associated with an increase in stroke incidence in the elderly.4 The importance of endothelial dysfunction in the pathogenesis of acute ischemic stroke remains uncertain. Acute ischemic stroke is associated with a rise in markers of endothelial activation in the systemic circulation5, 6 and with fewer circulating endothelial progenitor cells.7 Whether these results reflect an underlying primary disturbance of endothelial function in the pathogenesis of acute ischemic stroke or an acute phase response to the cerebral injury is unknown. We hypothesized that acute ischemic stroke would be associated with impairment of systemic endothelial dysfunction.
Our aim was to measure systemic vascular function directly (using forearm flow mediated dilatation) in patients with acute ischemic stroke and to clarify whether recent acute ischemic stroke is associated with impaired vascular function. Furthermore, we wanted to find out if there is systemic platelet activation in the acute phase and whether this activation is exacerbated during the first week of the acute vascular event, creating, together with endothelial activation, a prothrombotic “vulnerable vascular environment” in the frail brain.
Methods
The Institutional Review Board of the Baruch Padeh Poriya Hospital approved this study, and each study participant provided informed consent. Clinical evaluation was done by an experienced vascular neurologist, who evaluated the neurological status of the patients on admission and on day 4. Imaging studies included brain CT scanning that was performed on admission and as necessary later on. Clinical parameters comprised age, height, and weight. Vascular non-invasive methods that were used to evaluate vascular responsiveness included ABI and endothelial function, estimated by the brachial artery plethysmography method. Markers of platelet activation (levels of P-selectin) were measured twice (in the first 24 hrs and after 4 days) by enzyme-linked immunosorbent assay (ELISA).
Patients
Forty-three consecutive patients with first-ever acute ischemic stroke were enrolled in the study after signing a consent form. Participants were not eligible for thrombolytic therapy because of late arrival and were monitored for one week in the hospital. All 43 patients were conscious. Ischemic stroke was defined as a clinical stroke syndrome with either a normal CT brain scan or a recent infarct in the clinically relevant area of the brain, according to a CT brain scan performed on admission to the hospital. On admission, patients were examined by a vascular neurologist, who estimated the clinical severity according to the NIH neurological scale (NIHSS). Another clinical evaluation was carried out four days later. The neurologist was blind to the results of the vascular studies, as well as to the biochemical data (levels of soluble P-selectin that were determined on day 1 and 4).
Clinical evaluations and vascular studies were performed in the first 24 hrs of admission. Vascular studies included endothelial function measurement (flow mediated dilatation percent change [FMD%]), ABI, and body mass index (BMI).
Healthy subjects
Twenty three hospital employees served as the control group. They included physicians and nurses from the hospital. All were healthy non-smoking adults without cardiovascular risk factors and without any medical family history of cardiovascular events in the past.
Laboratory analysis
ABI: An ABI was performed while the patient lied supine and the systolic blood pressure at all four extremities were determined. To measure an ankle systolic pressure, a standard adult blood pressure cuff was placed around the ankle just above the malleoli. While using the Doppler flow-meter to monitor the signal from the posterior of the anterior tibial artery, distal to the cuff, the cuff was inflated to a pressure of approximately 30 mm Hg above the systolic pressure to occlude flow temporarily. As the cuff was slowly deflated (2 to 5 mm Hg/s), the pressure at which the Doppler flow signal was first heard and recorded was the ankle systolic pressure. An ABI was calculated by dividing the ankle systolic blood pressure by the greater of the two systolic upper extremity systolic blood pressures. An ABI of >1.0 was normal. An ABI of 0.5 to 0.9 was indicative of injury to a single arterial segment. An ABI of <0.5 was indicative of severe arterial injury or injury to multiple arterial segments.
BMI: The body mass index (BMI) is a standardized measure of the relationship of body mass to height. It has allowed for calculation of risk for adverse events in populations with different BMIs. The BMI is calculated by dividing the weight in kilograms by the (height in meters)2; the units are thus kg/m2. The upper limit of normal was identified as the point at which the risk for adverse health outcomes began to rise; the lower limit was similarly determined.
Flow Mediated Diameter percent change (FMD%): All measurements of brachial artery diameter and FMD% were performed in the morning, in a quiet and dark room and at controlled ambient temperatures between 20°C and 26°C. Studies were conducted after an overnight fast of at least 10 hrs (water was permitted), with the subject supine, and after 10 minutes of rest. The subject's right arm was comfortably immobilized in the extending position, allowing for ultrasound scanning of the brachial artery 5–10 cm above the antecubital fossa. In each examination, recording of vessel images were followed by inflation of a cuff to a supra-systolic pressure (40 to 50 mmHg above systolic pressure) for 5 min. Subsequently, the cuff was deflated and the brachial artery diameter was imaged and recorded for 3 min. An FMD% of more than 10% is considered a normal response. Lower than 10% FMD% reflect endothelial dysfunction, which means a high likelihood of developing a cardiovascular event in the future. Subjects with negative FMD% results (the artery is constricted after stress and not dilated as was expected) have the worst prognosis.
Biochemical analysis
P-selectin levels were measured by ELISA, according to the manufacturer’s instructions, on day 1 and day 4 of admission (R&D Systems Inc., 614 McKinley Place, Minneapolis, MN 55413 USA).
Statistical analysis
The chi square test and student’s t-test were used to analyze categorical variables, and a linear regression model was built to determine the independent effect of stroke, gender, and age on FMD%. Pearson correlation was used to find correlations between clinical neurological states and markers of vascular inflammation.
Results
Patients admitted with an acute ischemic stroke had severe endothelial dysfunction with increased levels of a proinflammatory/procoagulant soluble marker (P-selectin) that increased even further within four days. Interestingly, men had significantly higher levels of P-selectin than women on admission.
Forty-three patients (28 men, 15 women) were enrolled in the study after signing a consent form. Twenty-three healthy subjects (all men) served as the control group. There were significant differences between patients and subjects of the control group with respect to age (patients were older than volunteers –62.4±12.5 y vs 44.2±11.6 y, p=0.001), endothelial dysfunction (in patients it was worse —4.4±7.4% vs 16.6±7.6%, p=0.001) (Figure 1), and BMI (in patients it was higher –28±6 vs 24±5, p=0.001). No difference was noted in ABI (1.0± 0.2 vs 1.0±0.1, p=0.45) (Table 1), and no gender effect was observed with respect to age (60.7±12.8 y vs 65.7±11.4 y, p=0.19), FMD% (—5.1±7.8% vs —2.5±6.6%, p=0.25), and ABI (1.0±0.26 vs 1.0±0.5, p=0.29); however, men had a lower BMI than women (26.8±5.8 vs 31.4±5.5, p=0.01) (Table 2).
Figure 1:
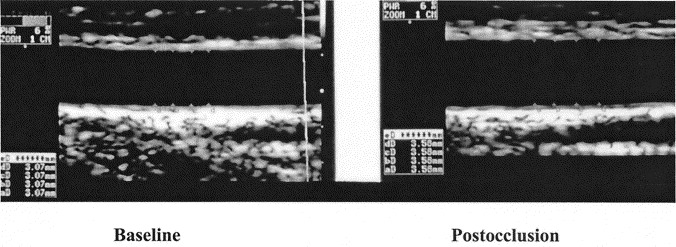
Flow Mediated Diameter Change Following Reperfusion of the Brachial Artery
A normal response in a healthy volunteer; the brachial artery was dilated in more than 10%
Table 1:
Vascular Characteristics
| Stroke patients | Control subjects | P-value | |
|---|---|---|---|
| No. | 43 | 23 | |
| Age (y) | 62.4±12.5 | 44.2±11.6 | 0.001 |
| FMD% | —4.4±7.4% | 16.6±7.6% | 0.001 |
| ABI | 1.0±0.2 | 1.0±0.1 | 0.45 |
| BMI | 28±6 | 24±5 | 0.001 |
Table 2:
Gender Effect on Vascular Characteristics
| Stroke patients | Control subjects | P-value | |
|---|---|---|---|
| No. | 28 | 15 | |
| Age | 60.7±12.8 | 65.7±11.4 | 0.19 |
| FMD% | —5.1±7.8% | —2.5±6.6% | 0.25 |
| ABI | 1.0±0.26 | 1.0±0.5 | 0.29 |
| BMI | 26.8±5.8 | 31.4±5.5 | 0.01 |
FMD% = percent change of flow mediated dilatation performed by the brachial artery method
ABI = ankle brachial index (0.9–1.4 considered normal)
BMI = body mass index
All patients in the study arrived too late for thrombolytic treatment. They were conscious and without any cognitive impairment and improved clinically within a few days of hospitalization. Generally, the neurological scale (NIHSS) on admission was 4.9±3.4, decreasing to 3.2±3.0 after four days of follow-up (p=0.001). In men, NIHSS was 4.8±3.8 on admission, declining to 3.2±3.4 on day 4 (p=0.001). In women, NIHSS on admission was 5.0±2.7, which decreased to 3.3±2.3 on day 4 (p=0.001). No gender difference was observed between NIHSS on day 1 (4.8±3.8 [men] vs 5.0±2.7 [women], p=0.8) and day 4 (3.2±3.4 [men] vs 3.3±2.3 [women], p=0.9).
P-selectin levels were high on admission and increased further on day 4 (from 68.0±55.5 pg/ml to 102.3±72.0 pg/ml, p=0.01) (Table 4). Interestingly, men had higher P-selectin levels on admission compared to women (79.1± 66.7 pg/ml vs 48.9± 15.4 pg/ml, p=0.02), even though their NIHSS scores were similar. In male patients, P-selectin increased further on day 4---from 79.1±66.7 pg/ml to 113.6±82.6 pg/ml (p=0.05), and in female patients, P-selectin increased from 48.9±15.4 pg/ml to 83.5±46.4 pg/ml, (p=0.01) (Table 4). Although there was a gender difference in the P-selectin level on day 1, no such difference was observed on day 4---the average P-selectin level in men was 113.6±82.6 pg/ml, while that of women was 83.5±46.4 pg/ml (p=0.08) (Table 3). P-selectin level in healthy humans is between 3.12 and 12.5 pg/ml; thus, all patients with an acute stroke had higher than normal levels of P-selectin on admission, which further increased during their first week of hospitalization.
Table 3:
P-selectin Levels (pg/ml)
| Day 1 | Day 4 | P-value | |
|---|---|---|---|
| Overall | 68.0±55.5 | 102.3±72.0 | 0.01 |
| Men | 79.1±66.7 | 113.6±82.6 | 0.05 |
| Women | 48.9±15.4 | 83.5±46.4 | 0.01 |
| P-value | *0.02 | **0.08 | 0.45 |
P-value = the difference in P-selectin level on day 1 between men and women
P-value = the difference in P-selectin level on day 4 between men and women
A linear regression model was built to determine the independent effect of CVA, gender and age on FMD%. CVA remained an independent correlate even after controlling for age and gender. None of the univariate models seemed statistically significant---gender (p=0.448), age (p=0.100), BMI (p=0.607), ABI (p=0.103), FMD% (p=0.456), and P-selectin (p=0.195). Since no univariate model seemed statistically significant, no multivariate model was built.
Discussion
The main findings of this study are that patients with an acute ischemic stroke have severe endothelial dysfunction within the first 24 hrs of admission and very high P-selectin levels on admission (mainly men) that increase further during the first week of hospitalization, despite the general trend of clinical neurological improvement.
These data support the hypothesis that acute stroke is associated with a worsening in systemic vascular function. Our data, which demonstrated severe endothelial dysfunction and platelet activation during the first week of ischemic stroke, are consistent with studies that have examined E-selectin8–10and P-selectin8 in acute stroke. Possible reasons for acute activation of endothelial cells and platelets following an ischemic stroke are previous recent infections, inflammation and activation of a symptomatic atherosclerotic plaque, atherothrombosis after plaque eruption, and ischemic neuronal damage causing expression of factors that promote a cascade of events involving these markers. Our results demonstrate a strong independent association between endothelial activation and an increased concentration of platelet activation marker in ischemic acute stroke, suggesting that vascular inflammation and activation of a symptomatic atherosclerotic plaque and/or atherothrombosis after plaque erosion are the explanation for the acute vascular event.
There is a growing body of evidence suggesting that endothelial dysfunction is associated with cardiovascular morbidity and mortality.11, 12 The concept of endothelial dysfunction should be extended beyond blood vessels into the vascular wall and even into the level of endothelial stem cells. A meta-analysis found that coronary and peripheral endothelial dysfunction can predict cardiovascular events similarly, 13 and that the observation that cardiovascular events may occur remotely from the site in which the endothelial dysfunction was detected14 demonstrates the systemic nature of endothelial dysfunction and its pivotal role in prediction of cardiovascular events. Moreover, the lack of direct correlation between endothelial dysfunction and traditional risk factors may support the concept that endothelial dysfunction may be regarded as an integrated risk of all risk factors (traditional and non-traditional) and may serve as a sensitive bio-assay.15
In acute stroke, the increase in P-selectin level altogether with severe endothelial dysfunction, despite the significant clinical improvement, may suggest that subclinical vascular instability is in progress, and that the patient is vulnerable and at risk of developing future vascular events. The clinical improvement that was observed and defined by the NIHSS during the first few days of hospitalization does not mean that the vascular “story” is over, and precautions should be taken in order to prevent future deterioration and recurrent vascular events (including death).
Levels of soluble P-selectin can be measured in plasma and are derived from platelets and from endothelial cells. P-selectin is an adhesion molecule stored within the α-granules of platelets and the Weibel-Palade bodies of endothelial cells.16 After stimulation, P-selectin quickly redistributes to the surface of endothelial cells, mediating early inflammatory cell adhesion.17–20
In a prospective epidemiological trial of apparently healthy women, elevated baseline levels of soluble P-selectin were associated with risk of future myocardial infarction, stroke, coronary revascularization, and cardiovascular death. This association was independent of age and smoking status, and persisted after control for lipid and non-lipid cardiovascular risk factors. These data provide a strong clinical confirmation of the importance of P-selectin in the processes of atherogenesis and future vascular occlusion.21
Platelet activation and platelet-leukocyte interaction have been shown to be increased in stroke patients.22, 23 Intercellular communication is established between surface glycoproteins on the leukocyte (the P-selectin glycoprotein ligand 1 [PSGL-1]).24–27 This glycoprotein is mainly expressed on polymorphonuclear leukocytes and monocytes. By interacting with platelets, leukocytes can enhance platelet aggregation and thromboxane release,25 as well as promote recruitment of activated platelets.28 Local inflammation in the stroke site contributes to the migration of leukocytes into damaged cells in the brain through up-regulation of adhesion molecules like P-selectin. The intense local inflammation enhances apoptosis and facilitates death of neurons, which could have been saved if timely interventions were done.29
On the basis of our findings, we propose that endothelial cell/platelet/leukocyte interactions represent important mechanism in the development of increased thrombogenesis. This increased thrombogenesis leads to vascular occlusion and stroke evolution, and to a potentially cytotoxic inflammatory reaction in the injured brain tissue, eventually expanding the ischemic/damaged brain tissue. This cascade of events is likely to continue and enhance during the first few days following an acute vascular event and is apt to negatively affect the clinical outcome. Interestingly, the fact that men with acute stroke had higher P-selectin levels during the first 24 hrs of admission may suggest that there is a gender effect in ischemic stroke. Men may have higher systemic markers of inflammation, making them more vulnerable to develop vascular events. Another possible mechanism could be that men respond more vigorously to an acute vascular event with an intense inflammatory response. One way or another, the intense inflammatory response may lead to a greater neurological insult, and men seem to be in a higher risk to develop more vascular events in the first week following an acute vascular event.
Summary
Severe endothelial dysfunction was detected both in men and in women, with an intense vascular response following an acute stroke. High P-selectin level within the first 24 hrs that further increase during the first week after stroke suggests that vascular instability still exists and that patients with acute stroke are vulnerable and at higher risk during this period.
Study limitations
The small population studied and the fact that only men (younger than the patients) served as the control group are the main limitations of this study. Larger studies with more variable populations are needed to validate our findings, and these studies should be carried out for longer periods of time, in order to estimate more clearly the duration of patient vulnerability. Such investigations will improve prevention and risk reduction strategies following an acute stroke.
Disclosure
The authors have nothing to declare.
References
- 1.Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald AE, Palacios M, Griffin GE, Deanfield JE, MacAllister RJ, Vallance P. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 2.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 3.Fitchtlscherer S, Rossig L, Breuer S, Vasa M, Dimmeler S, Zeiher AM. Tumor necrosis factor antagonism with etanercept improves systemic endothelial vasoreactivity in patients with advanced heart failure. Circulation. 2001;104:3023–3025. doi: 10.1161/hc5001.101749. [DOI] [PubMed] [Google Scholar]
- 4.Roquer J, Segura T, Serena J, Castillo J. Endothelial dysfunction vascular disease and stroke: the ARTICO study. Cerebrovascular Disease. 2009;1(27 suppl):25–37. doi: 10.1159/000200439. [DOI] [PubMed] [Google Scholar]
- 5.Cherian P, Hankey GJ, Eikelboom JW, Thom J, Baker RI, McQuillan A, Staton J, Yi Q. Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke. 2003;34(9):2132–2137. doi: 10.1161/01.STR.0000086466.32421.F4. [DOI] [PubMed] [Google Scholar]
- 6.Lip GYH, Blann AD, Farooqi IS, Zarifis J, Sagar G, Beevers DG. Sequential alterations in haemorheology, endothelial dysfunction, platelet activation and thrombogenesis in relation to prognosis following acute stroke: the West Birmingham Stroke Project. Blood Coagulation & Fibrinolysis. 2002;13(4):339–347. doi: 10.1097/00001721-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Shef F, O’Rourke F, Nasser AM, Schwindt B, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36(1):151–153. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 8.Frijns CJM, Kappelle LJ, van Gijn J, Niewenhius HK, Sixma JJ, Fijnheer R. Soluble adhesion molecules in ischemic stroke and in carotid atherosclerosis. Stroke. 1997;28:2214–2218. doi: 10.1161/01.str.28.11.2214. [DOI] [PubMed] [Google Scholar]
- 9.Fassbender K, Bertsch T, Mielke O, Muhlhauser F, Hennerici M. Adhesion molecules in cerebrovascular disease: evidence for an inflammatory endothelial activation and large and small vessel disease. Stroke. 1999;30:1647–1650. doi: 10.1161/01.str.30.8.1647. [DOI] [PubMed] [Google Scholar]
- 10.Blann A, Kumar P, Krupinski J, McCollom C, Beevers DG, Lip GY. Soluble intercellular adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 and von Willebrand factor in stroke. Blood Coag Fibrinol. 1999;10:277–284. doi: 10.1097/00001721-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Widlansky ME, Gokce N, Keaney JF, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 12.Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 13.Lerman A, Zeiher AM. Endothelial Function : Cardiac Events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 14.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial function is associated an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 15.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 16.Wagner DD. The Webel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993;70:105–110. [PubMed] [Google Scholar]
- 17.Frenette PS, Wagner DD. Molecular medicine: adhesion molecules. N Engl J Med. 1996;334:1526–1529. doi: 10.1056/NEJM199606063342308. [DOI] [PubMed] [Google Scholar]
- 18.Bevilacqua MP, Neson RM, Mannori G, et al. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–378. doi: 10.1146/annurev.med.45.1.361. [DOI] [PubMed] [Google Scholar]
- 19.Frenette PS, Johnson RC, Hynes RO, et al. Platelets roll in stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci USA. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson-Tidey RR, McGregor JL, Taylor PR, et al. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques: co-expression with intercellular adhesion molecule-1. Am J Pathol. 1994;144:952–961. [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–495. doi: 10.1161/01.cir.103.4.491. [DOI] [PubMed] [Google Scholar]
- 22.Zeller JA, Tschoepe D, Kessler C. Circulating platelets show increased activation in patients with acute cerebral ischemia. Thromb Haemost. 1999;81:373–377. [PubMed] [Google Scholar]
- 23.Marquardt L, Ruf A, Mansmann U, Winter R, Schuler M, Buggle F, Mayer H, Grau AJ. Course of platelet activation markers after ischemic stroke. Stroke. 2002;33:2570–2574. doi: 10.1161/01.str.0000034398.34938.20. [DOI] [PubMed] [Google Scholar]
- 24.Sarma J, Laan CA, Alam S, Jha A, Fox KA, Dransfield I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;105:2166–2171. doi: 10.1161/01.cir.0000015700.27754.6f. [DOI] [PubMed] [Google Scholar]
- 25.Faraday N, Scharpf RB, Dodd-o JM, Martinez EA, Rosenfeld BA, Dorman T. Leukocytes can enhance platelet-mediated aggregation and thromboxane release via interaction of P-selectin glycoprotein ligand 1 with P-selectin. Anesthesiology. 2001;94:145–151. doi: 10.1097/00000542-200101000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Evangelista V, Manarini S, Rotondo S, Martelli N, Polischuk R, McGregor JL, de Gaetano G, Cerletti C. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and beta 2 integrin CD11b/CD18. Blood. 1996:4183–4194. [PubMed] [Google Scholar]
- 27.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:97–103. [PubMed] [Google Scholar]
- 28.Maugeri N, Evangelista V, Celardo A, Dell’Elba G, Martelli N, Piccardoni P, de Gaetano G, Cerletti C. Polymorphonuclear leukocyte-platelet interaction: role of P-selectin in thromboxane B2 and leukotriene C4 cooperative synthesis. Thromb Haemost. 1994;72:450–456. [PubMed] [Google Scholar]
- 29.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]


