SUMMARY
Vertigo in and around MRI machines has been noted for years [1, 2]. Several mechanisms have been suggested to explain these sensations [3, 4], yet without direct, objective measures, the cause is unknown. We found that all healthy human subjects lying in the static magnetic field of an MRI machine develop a robust nystagmus. Patients lacking labyrinthine function do not. Here we use the pattern of eye movements as a measure of vestibular stimulation to show that the stimulation is static (continuous, proportional to static magnetic field strength, requiring neither head movement nor dynamic change in magnetic field strength) and directional (sensitive to magnetic field polarity and head orientation). Our calculations and geometric model suggest that magnetic vestibular stimulation derives from a Lorentz force due to interaction between the magnetic field and naturally-occurring ionic currents in the labyrinthine endolymph fluid. This force pushes on the semicircular canal cupula, leading to nystagmus. We emphasize that the unique, dual role of endolymph in the delivery of both ionic current and fluid pressure, coupled with the cupula’s function as a pressure sensor, makes magnetic field induced nystagmus and vertigo possible. Such effects could confound fMRI studies of brain behavior, including resting-state brain activity.
RESULTS AND DISCUSSION
Recently, in a study of functional MRI imaging of caloric-induced vestibular responses, the eyes of some subjects were noted to drift while simply lying in the MRI magnet bore [5]. Animals show behavioral and postural changes when exposed to strong magnetic fields [6, 7]. The vestibular labyrinth is the likely target since after labyrinthectomy the animals no longer show abnormal postural responses due to the fields [8]. The vestibuloocular reflex (VOR) links labyrinthine stimulation to eye movements. Head rotation in one direction leads to eye rotation in the other, ensuring stable vision during head motion. Here, we use the link between vestibular stimulation and eye movements to investigate magnetic vestibular stimulation (MVS). Normally, labyrinthine stimulation produces nystagmus, an alternating slow drift (slow phase) and fast resetting movement (quick phase) of the eyes. We used the direction and velocity of the slow phases of nystagmus as a measure of the pattern of labyrinthine stimulation.
Previously Proposed Mechanisms and a Rationale for Investigating Their Role
Glover gives an overview and mathematical analysis of three candidate mechanisms for MVS: electromagnetic induction (EMI), magnetic susceptibility (MS) and magneto-hydrodynamics during head movements [4]. EMI (due to Faraday’s law of induction) is a voltage induced by a changing magnetic field. Although the MRI magnetic field is static, our subjects moved through the magnetic field gradient into the MRI bore, producing a change in field strength, and hence an EMI voltage, within the subject. Faster movement produces larger EMI. When there is no movement, there is no EMI.
To investigate EMI’s possible role in MVS, we placed a small coil of wire (“search coil”) near the ear to record EMI voltage. EMI was only present during subject table motion into or out of the MRI bore. When the subject lies still outside or inside the MRI bore, the EMI voltage is zero. We plotted both the EMI voltage and eye movements to compare the amplitude, direction, and timing of the eye movements relative to the EMI voltage induced by movement through the magnetic field. If EMI is the mechanism of MVS, we would expect the EMI voltage on the search coil and the slow-phase eye velocity (SPV) to be correlated. Similarly, the previously proposed magneto-hydrodynamic mechanism requires movement through the field so its effect should also be correlated with the EMI voltage. Magnetic susceptibility effects are static (require no movement), but they are not sensitive to magnetic field polarity, so reversing the field polarity relative to the subject’s head and observing whether the slow-phases change direction should reveal if MS is the underlying mechanism.
Subject Data
We placed ten healthy human subjects and two patients with no labyrinthine function in MRI machines with magnetic field strengths of three and seven tesla (T) and measured eye movements with an infrared video camera while the subjects lay still in darkness. No MRI scans were performed – only the static magnetic field was present. Darkness is essential to an uncontaminated VOR measurement, as visual cues are used by the brain to suppress the unwanted nystagmus [9]. We chose our test conditions to address the physical properties of the proposed mechanisms. We varied the speed and direction of subject movement into the bore, the duration in the bore, and the static field strength.
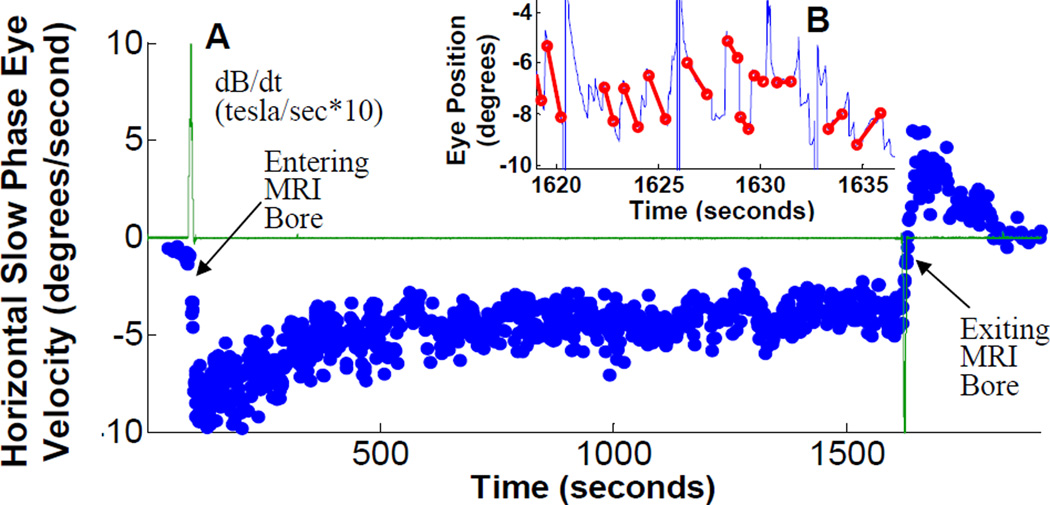
First, we confirmed that the labyrinth was necessary to elicit the nystagmus by examining two patients who had bilateral acquired loss of labyrinthine function (verified with clinical assessment and rotational and caloric laboratory testing). They were placed into the magnet in two static pitch head orientations and developed no nystagmus in either position (see Supplemental Data Figure S1). In contrast, all ten healthy subjects studied developed a robust, predominantly horizontal nystagmus in the magnet (see Supplemental Data Movie S1). While lying just outside the bore of either magnet (field strength ~0.7T), subjects had little or no nystagmus. Figure 1A shows horizontal SPV during a 25 minute trial and demonstrates the basic response in all subjects (although most trials were shorter). Most subjects reported a sense of rotation, usually after they were completely in the bore and the table stopped moving. This sensation often died away after a minute or so. Nystagmus, however, persisted, with the SPV slowly decreasing over minutes to a sustained level that remained until removal from the bore (in shorter trials, this plateau was not reached). On leaving the bore, the nystagmus direction reversed and then gradually decayed over a few minutes. The reversal, however, was not due to the reversed movement out of the bore through the magnetic field, because for short durations in the bore (e.g., Figure 3D), there was no reversal; the nystagmus just stopped. Rather, the reversal likely derives from adaptation to the persistent vestibular stimulation, similar to the reversal seen with other types of sustained vestibular stimulation [10–12].
Figure 1.
(A) Slow-phase velocity (SPV) during 25 minute trial. Data from a representative subject showing typical response. Subject is initially outside MRI bore at left of figure. EMI voltage (green line) peaks positive during subject movement into bore, and negative near end of trace as subject moves out of bore. SPV (+right, −left) peaks after in bore, settles to steady state after about 10 minutes, and reverses upon removal from bore. Inset (B) shows several seconds of the original eye position data during bore exit from which the eye velocity is derived. Slow phases are marked with a red line, and the slope of each line becomes a single blue dot on the velocity trace. The change in direction of slopes corresponds to the change in sign of SPV during bore exit. (Supplemental Figure S1 shows head position inside and outside the MRI bore, and data from patients with bilateral vestibular loss; Movie S1 shows typical eye movements during bore entry)
Figure 3.
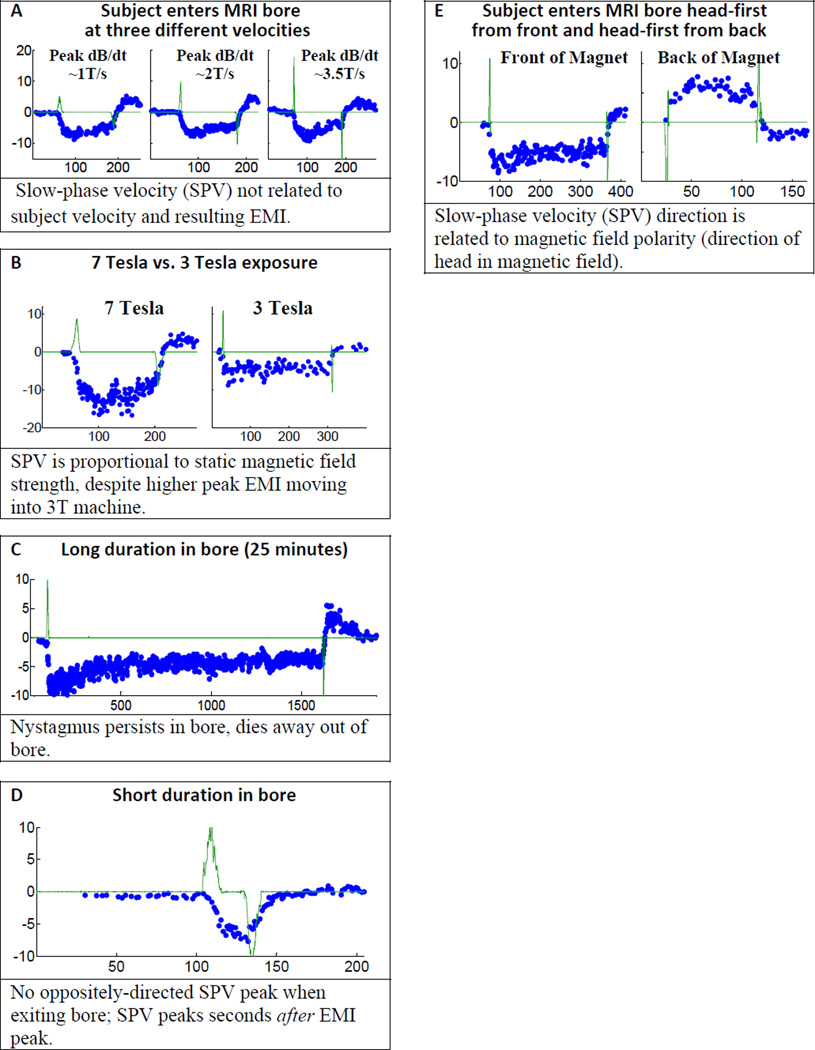
(A–D) Stimulation is due to a static mechanism. All data plots show SPV (blue dots, deg/sec) and EMI search coil voltage (green trace, tesla/sec*10, except panel A, tesla/sec*5) over time in seconds. Data shows that eye movements are not related to transient, dynamically induced EMI voltage. (E) Stimulation is due to a polarity sensitive mechanism. The SPV direction reverses when the magnetic field vector is reversed relative to the head. (see also Supplemental Data Figure S2)
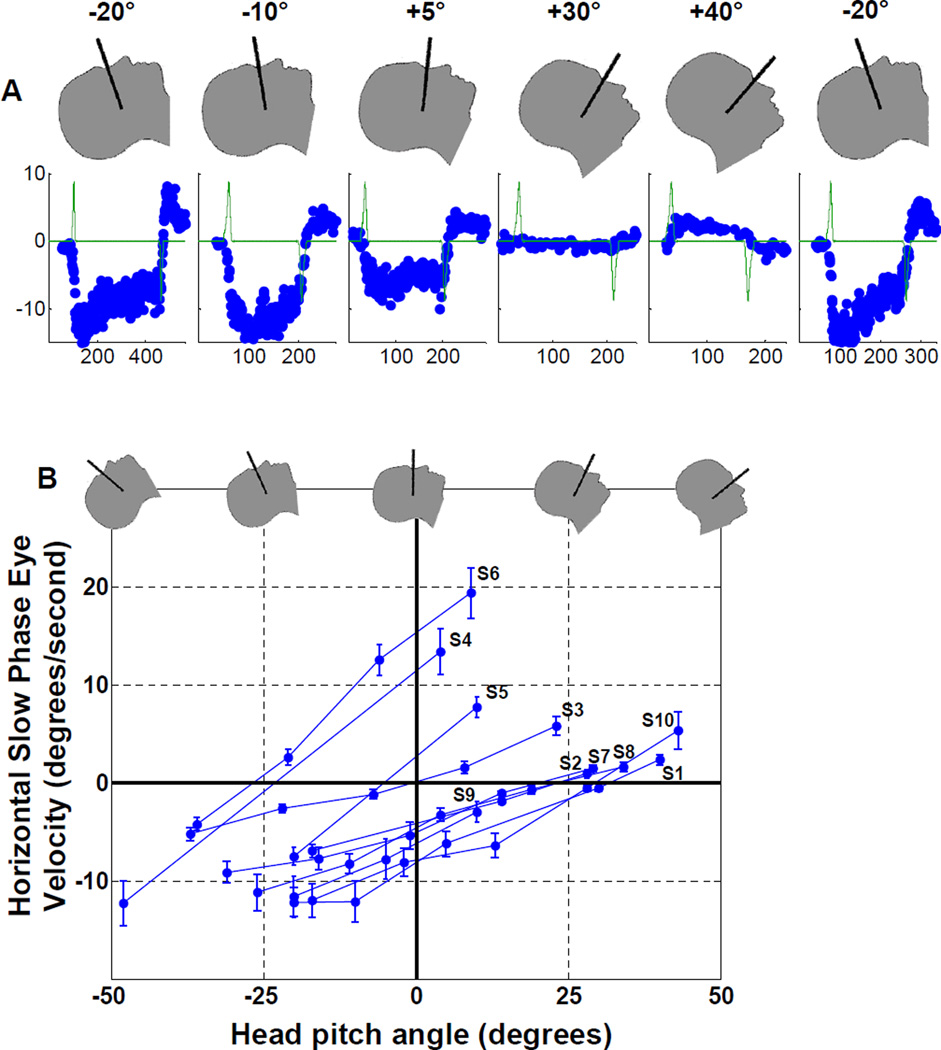
We found that the magnitude and direction of the horizontal SPV were related to static head pitch position (chin up or down). Figure 2A shows data from one subject in five different pitch positions, and Figure 2B summarizes head pitch data from all ten subjects. With the chin up, the SPV direction was leftward (negative values in the figure) in all subjects. With increasingly downward pitch positions, the SPV magnitude decreased, reached a null (no horizontal nystagmus), and eventually reversed and became rightward (positive values). While this pattern was the same for all subjects, we found null positions (where the SPV line crosses the horizontal zero SPV axis in Figure 2B) differed considerably among subjects, ranging from approximately −27 to +32 degrees.
Figure 2.
Slow-phase velocity (SPV) is related to static head pitch position (+pitch, chin towards chest). (A) Data from subject S1 in separate trials during a single session, obtained in the order shown from left to right. The first position was repeated at the end of the session to demonstrate the robust repeatability of the phenomenon. (B) SPV data for all ten subjects. Each data point is the average SPV over 45 seconds after the subject is completely in the bore (with standard deviation error bars). Shows consistent relationship between SPV and head pitch angle for all ten subjects (traces labeled for each subject, S1 through S10), yet reveals considerable variation in head pitch angle where SPV null occurs (where each subject line crosses the horizontal zero SPV axis). Range is from −27° for subject S6, to +32° for subject S1.
Figure 3A–D summarizes the evidence for a static mechanism underlying MVS by comparing eye movements to the dynamically induced EMI voltage (green trace on all plots) during movement into and out of the bore. We varied the speed of the subject table, the strength of the static field, and the duration in the bore. In each case, the eye movement data does not correlate with the EMI voltage, and thus favors a static, non-EMI effect. In panel A, the peak EMI voltage increased with table velocity, but SPV remained nearly the same. When exposed to 7T and 3T fields (panel B), SPV scaled with static field strength, not EMI voltage (which was actually slightly higher in the 3T magnet). When in the bore for 25 minutes (panel C), the nystagmus persisted, arguing against a transient EMI effect during entry into the bore. When quickly moved into and out of the bore (panel D), SPV did not show a strong reverse peak on exiting the bore as would be expected if the vestibular system experienced first a positive, then a negative EMI stimulus. We also note that for all subjects and all conditions, peak SPV occurred well after peak EMI (e.g., panel D), when the subject was completely in the bore and stationary.
Figure 3E shows that MVS is polarity-sensitive, arguing against the MS mechanism. When the head was exposed to a magnetic field of opposite polarity by having the subject enter the back of the MRI bore, the nystagmus direction reversed. While MS forces can be significant in strong magnetic fields, even for diamagnetic substances that make up most biological tissue [13], the direction of MS force does not reverse when magnetic field polarity is reversed. Also, MS translational forces are negligible in the nearly homogeneous field at the center of the magnet. If translational MS forces were the cause of magnetic vestibular stimulation, we would expect to see strong nystagmus outside the magnet, and little or no nystagmus once the head reaches the center of the magnet. Instead, we see the opposite. Supplemental Figure S2 panels F and G show that the horizontal SPV direction is robust, so that MS torque forces, even with unintentional head tilts or mispositioning in the bore, cannot explain the reversal seen in 3E. These observations exclude magnetic susceptibility as the underlying mechanism.
Finally, we found that vertical nystagmus is produced when the head is tilted in the magnetic field, right ear to shoulder (SPV downward) or left ear to shoulder (SPV upward): this was the case in all subjects tested (Supplemental Figure S2).
In our analysis, differentiating among the underlying mechanisms of MVS only required observing whether nystagmus is present, its direction, and its relationship to EMI voltage. Since our signal of interest (SPV) is robust (all slow-phase velocity plots are taken from single trials; we did not average or combine trial or subject data), we could make these determinations easily, without data pooling or statistical analysis. We found that the pattern of eye movements under different conditions argues against previously proposed mechanisms that depend upon movement in the field or on magnetic susceptibility. Rather, our data imply a static, polarity sensitive mechanism. We propose that Lorentz forces (which are polarity sensitive and do not require subject movement or a changing magnetic field) are the best explanation.
The Lorentz Force
A continuous Lorentz force in the labyrinthine semicircular canals presupposes a continuous, baseline ionic current flowing through the endolymph fluid into the hair cells, and requires no head movement or changing magnetic fields. Previous investigations have mentioned magneto-hydrodynamics (MHD) as a possible cause for MRI induced vertigo. The Lorentz force is a component in the MHD equations, but the MHD conditions previously considered required active head movement to induce current within the endolymph; static Lorentz forces due to natural, continuous ionic hair cell currents were not considered [3, 4].
The Lorentz equation F = LJ×B relates the current (J, amperes) and magnetic field (B, tesla) vectors to the imparted fluid force vector (F, newtons). L (meters) is the scalar distance across which the current travels. The vector cross product (×) means that the force is at right angles to both the current and magnetic field vectors. In order for the Lorentz hypothesis to be viable, there must be a source of continuous current within the inner ear. When exposed to the MRI magnetic field, this current must produce a Lorentz force sufficient to cause nystagmus. Indeed, the labyrinth provides a unique physiological environment and anatomical arrangement in which Lorentz forces can arise and produce neural stimulation [14]. Endolymph is an unusual extracellular fluid, having a high concentration of potassium ions, which fills the internal chamber of the labyrinth and bathes the apical surface of the vestibular hair cells [15]. Endolymph serves a dual purpose. It carries ionic current for the mechanoelectrical transduction function of the vestibular hair cells [16–19]. It also conveys rotational force through each semicircular canal to its cupula, the differential pressure sensor in the ampulla. Given the numbers of hair cells in the utricle and each ampulla [20–23], the resting current of each hair cell, and the availability of roughly 1mm of travel distance for the current above the hair cell sensory epithelium, we compute pressure on the cupula due to the Lorentz force in the 7T magnet to be as high as 0.002Pa to 0.02Pa (pascals), which is well above the nystagmus threshold of 0.0001Pa [24] (seeSupplemental Experimental Procedures for complete calculation). We emphasize that the Lorentz force is within the volume of endolymph fluid, not within the hair cells themselves. This fluid force pushes against the cupula to stimulate its attached hair cells, but does not significantly stimulate hair cells directly.
Geometric Model and Head Pitch Simulation
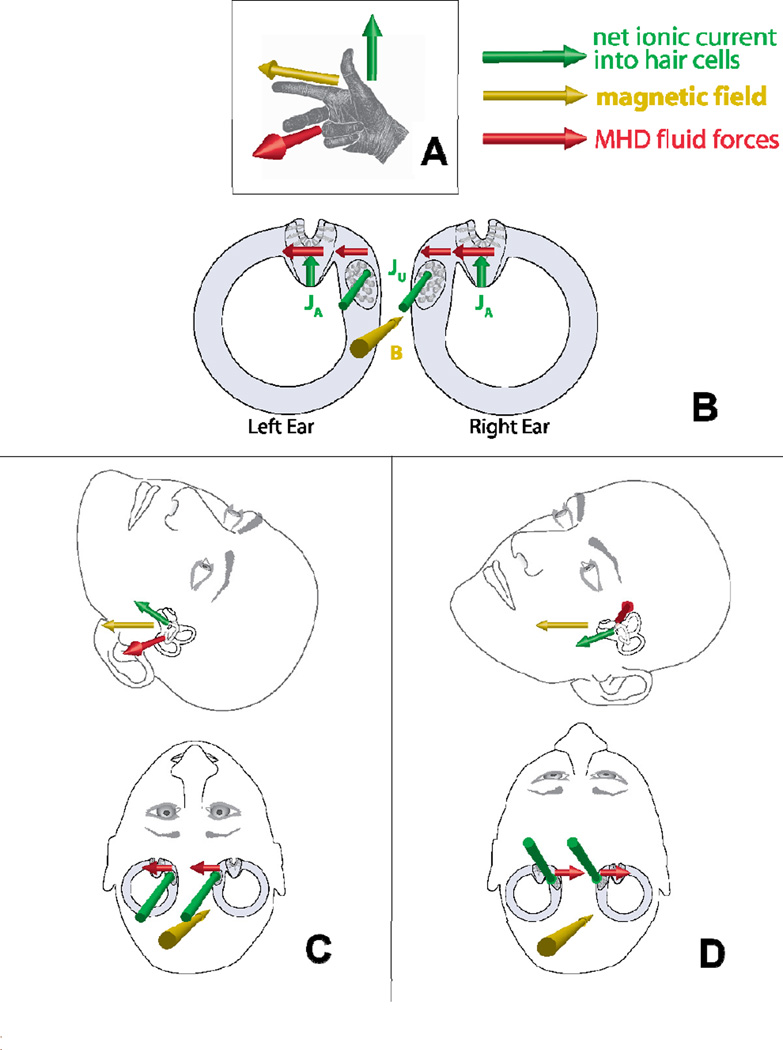
We have established that the amount of Lorentz force is sufficient, but still must show that the direction of force correctly accounts for the observed nystagmus. There is a perpendicular relationship between the direction of net ion current flow into the hair cells (which are, on average, oriented perpendicular to the walls of the canal) and the direction of pressure that stimulates the cupulae (around the torus of the canals). This matches the orthogonal relationship between current and force vectors in the Lorentz equation, making the canals geometrically conducive to these forces. While ion current flowing into the utricle contributes to the Lorentz fluid forces that deflect the cupula, the utricle is not coupled to fluid pressure by a closing membrane like the cupula. Therefore, Lorentz forces acting on the utricle itself would contribute negligibly to the nystagmus. Since our subjects exhibited a predominantly horizontal nystagmus in the bore, it likely arises from excitation of the lateral semicircular canals. Our simplified anatomical model (Figure 4) shows the geometric relationship among the lateral canal and utricle, the magnetic field, the ion current vectors (which point toward the hair cells in the utricle and the lateral canal ampulla), and the resulting Lorentz forces that are transmitted by the endolymph to the cupula that acts as a pressure transducer. Note that the force in both ears is always in the same direction, just as during actual head rotation. In other words, induction of nystagmus in the magnetic field does not require an inherent imbalance between the left and right labyrinths. We conclude that model simulation of head pitch is in good agreement with data (seeSupplemental Experimental Procedures for details of mathematical model computations), and correctly predicts that a) horizontal SPV varies with head pitch, b) direction of SPV changes with head pitch, c) the “null” (zero SPV) position can vary due to anatomical variation of the utricle and ampulla, d) SPV directions are correct around the null (head pitched up produces leftward SPV, down produces rightward), and e) SPV varies smoothly with head pitch.
Figure 4.
Geometric model using Lorentz forces. (A) Right-hand rule relationship among current (green), magnetic field (yellow), and resulting Lorentz force (red). (B) Two-dimensional view of lateral canals, ampulla, and utricle, looking through top of head (vertical canals not shown), in head pitch-up position, with resulting Lorentz forces to the left (same orientation as panel C). The sign of the utricular force contribution depends on head pitch in the magnetic field as shown in C and D. (C) Two three-dimensional views of the same head pitch up position (utricle current vector pointing slightly upward), with resulting utricular Lorentz force to subject left. (D) Head pitch down (utricle current vector pointing slightly down), and utricular Lorentz force to subject right. (see also Supplemental Figure S3)
Implications of MVS induced nystagmus
Our findings and analysis have important implications for understanding the effects of magnetic fields on the vestibular system as well as on the interpretation of functional imaging studies in general. We emphasize that the dual role of endolymph in the delivery of both ionic current and fluid pressure, coupled with the function of the cupula as a pressure sensor, makes MVS-induced nystagmus possible. MVS-induced nystagmus and vertigo should be considered as imaging techniques use progressively stronger magnetic fields, which leads to stronger Lorentz forces. MVS-induced nystagmus carries important ramifications and caveats for functional MRI studies, not only of the vestibular system[25], but of cognition, motor control, and perception in general. Indeed, vestibular stimulation induced by the magnetic field in healthy subjects simply lying in the bore could activate many brain areas related to vision, eye movements, and the perception of the position and motion of the body. Whether the eyes are open or closed, the vestibular system is stimulated and engaged in the MRI bore. If the eyes are open, there is also a cascade of activity first in the visual system, which detects motion of images on the retina, and in turn engages the networks, both immediate and long-term (adaptive), that suppress unwanted nystagmus. Furthermore, the level and areas of activation of the brain by MVS could depend upon the magnitude and direction of nystagmus produced by each subject, which in turn would depend on the anatomical features of an individual’s labyrinth as well as static head orientation in the magnetic field. Since the magnitude of the induced nystagmus depends upon magnet field strength, any effects of MVS on functional imaging could differ among subjects in 7T, 3T and 1.5T magnets. MVS is polarity sensitive, so the magnetic field polarity relative to the head (which is not standardized among MRI manufacturers) determines the nystagmus direction. These potential confounds emphasize the importance of considering MVS-induced nystagmus in studies of ‘baseline’ resting-state brain activity and other behavioral paradigms exploring vision, control of eye movements and adaptation to unwanted motor behavior. Finally, MVS is a potential noninvasive and comfortable way to stimulate the labyrinth for vestibular diagnosis, and possibly as an aid to vestibular rehabilitation.
HIGHLIGHTS.
All subjects in MRI machine develop a robust nystagmus and often a sense of motion
Nystagmus strength depends on static field strength, not motion through the field
Horizontal nystagmus direction depends on head pitch angle and MRI field polarity
Modeling suggests MRI nystagmus is due to Lorentz forces pushing on the cupula
Supplementary Material
Acknowledgements
We thank Ruth Anne Eatock for her advice regarding hair cell ionic currents. This work was supported by the Johns Hopkins Brain Science Institute, the Leon Levy Foundation, the Schwerin family foundation, and the Charles Lott family. MRI experiments were performed at the F. M. Kirby Research Center at the Kennedy Krieger Institute. Joe Gillen was supported in part by NIH/NCRR grant P41 RR015241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schenck JF. Human exposure to 4.0-Tesla magnetic fields in a whole-body scanner. Med. Phys. 1992;19:1089. doi: 10.1118/1.596827. [DOI] [PubMed] [Google Scholar]
- 2.Heilmaier C, Theysohn JM, Maderwald S, Kraff O, Ladd ME, Ladd SC. A large-scale study on subjective perception of discomfort during 7 and 1.5T MRI examinations. Bioelectromagnetics. 2011 doi: 10.1002/bem.20680. [DOI] [PubMed] [Google Scholar]
- 3.Schenck JF. Health and Physiological Effects of Human Exposure to Whole-Body Four-Tesla Magnetic Fields during MRI. AnnNYAcad. Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- 4.Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: A theoretical and experimental investigation. Bioelectromagnetics. 2007;28:349–361. doi: 10.1002/bem.20316. [DOI] [PubMed] [Google Scholar]
- 5.Marcelli V, Esposito F, Aragri A, Furia T, Riccardi P, Tosetti M, Biagi L, Marciano E, Di Salle F. Spatio-temporal pattern of vestibular information processing after brief caloric stimulation. Eur. J. Radiol. 2009;70:312–316. doi: 10.1016/j.ejrad.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Houpt TA, Carella L, Gonzalez D, Janowitz I, Mueller A, Mueller K, Neth B, Smith JC. Behavioral effects on rats of motion within a high static magnetic field. Physiol. Behav. 2011;102:338–346. doi: 10.1016/j.physbeh.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houpt TA, Houpt CE. Circular swimming in mice after exposure to a high magnetic field. Physiology & Behavior. 2010;100:284–290. doi: 10.1016/j.physbeh.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cason AM, Kwon B, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol. Behav. 2009;97:36–43. doi: 10.1016/j.physbeh.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Leigh RJ, Zee DS. The Neurology of Eye Movements. 4th ed. USA: Oxford University Press; 2006. [Google Scholar]
- 10.Young LR, Oman CM. Model for vestibular adaptation to horizontal rotation. Aerospace Med. 1969;40:1076–1080. [PubMed] [Google Scholar]
- 11.Malcolm R, Jones GM. A quantitative study of vestibular adaptation in humans. Acta Oto-Laryngol. 1970;70:126–135. doi: 10.3109/00016487009181867. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J. Neurophys. 1971;34:635. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- 13.Geim A. Everyone’s magnetism. Physics Today. 1998;51:36–39. [Google Scholar]
- 14.Rabbitt RD, Damiano ER. A Hydroelastic Model of Macromechanics in the Endolymphatic Vestibular Canal. J. Fluid Mech. 1992;238:337–369. [Google Scholar]
- 15.Ghanem TA, Breneman KD, Rabbitt RD, Brown HM. Ionic composition of endolymph and perilymph in the inner ear of the oyster toadfish, Opsanus tau. Biol. Bull. 2008;214:83–90. doi: 10.2307/25066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- 17.Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. 1983;3:962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J. Physiol. 1998;506:159–173. doi: 10.1111/j.1469-7793.1998.159bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vollrath MA, Eatock RA. Time Course and Extent of Mechanotransducer Adaptation in Mouse Utricular Hair Cells: Comparison With Frog Saccular Hair Cells. J. Neurophys. 2003;90:2676–2689. doi: 10.1152/jn.00893.2002. [DOI] [PubMed] [Google Scholar]
- 20.Rosenhall U. Vestibular macular mapping in man. Ann. Otol. Rhinol. Laryngol. 1972;81:339. doi: 10.1177/000348947208100305. [DOI] [PubMed] [Google Scholar]
- 21.Rosenhall U. Mapping of the cristae ampullares in man. Ann. Otol. Rhinol. Laryngol. 1972;81:882–889. doi: 10.1177/000348947208100622. [DOI] [PubMed] [Google Scholar]
- 22.Merchant SN, Velázquez-Villaseñor L, Tsuji K, Glynn RJ, Wall C, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative vestibular hair cell data. Ann. Otol. Rhinol. Laryngol. Suppl. 2000;181:3–13. doi: 10.1177/00034894001090s502. [DOI] [PubMed] [Google Scholar]
- 23.Watanuki K, Schuknecht HF. A Morphological Study of Human Vestibular Sensory Epithelia. Arch. Otolaryngol. 1976;102:583–588. [PubMed] [Google Scholar]
- 24.Oman CM, Young LR. The physiological range of pressure difference and cupula deflections in the human semicircular canal. Theoretical considerations. Acta. Otolaryngol. 1972;74:324–331. doi: 10.3109/00016487209128458. [DOI] [PubMed] [Google Scholar]
- 25.Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008;131:2538–2552. doi: 10.1093/brain/awn042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.