Abstract
Background
In human breast cancer, a growth status switched from estrogen-dependent to growth factor-dependent is a critical step during development of acquired tamoxifen resistance. However, the molecular mechanisms underlying this switch remain poorly understood. The Wilms' tumor suppressor gene, WT1, encodes a zinc-finger protein WT1 that functions as a transcription regulator. High levels of the WT1 expression have been associated with de novo tamoxifen resistance. The goal of this study was to investigate the function of WT1 in acquired tamoxifen resistance.
Materials and Methods
A stable tamoxifen-resistance cell line MCF7TAM was established by selecting ER-positive breast cancer MCF7 cells in a medium containing tamoxifen. Western blot, cell growth assay and shRNA method were used to examine the role of WT1 in acquired tamoxifen resistance.
Results
MCF7TAM cells expressed EGFR, HER2 and WT1 at higher levels compared to tamoxifen-sensitive parental MCF7 cells. MCF7TAM cells responded weakly to estrogen stimulation, grew rapidly in the absence of estrogen and were insensitive to tamoxifen. We also established stable cell lines from MCF7TAM cells to express shRNA specific for WT1, and found expression levels of the epidermal growth factor receptor (EGFR), HER2 and estrogen receptor (ER)-α to be down-regulated in MCF7TAM cells with knocked-down levels of WT1 expression. MCF7TAM cells with WT1 expression knocked-down by shRNA still retained tamoxifen insensitivity.
Conclusion
Our results indicated that WT1 is involved in expressional regulation of the EGFR family members and ER-α during development of acquired tamoxifen resistance.
Keywords: WT1, HER2, EGFR, estrogen and antiestrogen, breast cancer
Tamoxifen (TAM) is widely used for adjuvant therapy of breast cancer and is a key drug for breast cancer chemoprevention in high-risk women. Despite the significant antineoplastic and chemopreventive activities of TAM, most breast tumors are eventually resistant to TAM therapy. Essentially, two forms of TAM resistance occur: de novo resistance and acquired (reviewed in 1, 2). Although absence of estrogen receptor-alpha (ER-α) expression is the most common de novo resistance mechanism, subsets of ER-positive tumors are already resistant to TAM by the time of diagnosis, and the de novo resistance mechanism in these ER-positive tumors is largely unknown (reviewed in 1, 2). Furthermore, most initially responsive breast tumors gradually acquire TAM resistance by loss of TAM responsiveness. However, the underlying mechanisms of breast tumors loss of TAM responsiveness are not well established. Breast tumors with acquired TAM resistance frequently but not always retain levels of ER-α expression that would still define them as ER-positive tumors (reviewed in 1, 2). Therefore, a loss of ER-α expression is not the major mechanism driving acquired TAM resistance. Up-regulation of the HER2/Neu and increased autocrine and papracrine growth factor signaling pathways are often associated with development of acquired TAM resistance (3, 4).
The Wilms' tumor susceptibility gene, WT1, at chromosome locus 11p13 (5-7) encodes a C2-H2-type zinc-finger protein, WT1. Through alternative splicing, there are four protein isoforms of WT1 that differ by the presence of one 17 amino acid insert between its transcription regulatory domain and DNA binding domain and one 3 amino acid (KTS) insert between the third and fourth zinc fingers (reviewed in 8, 9). The different isoforms are referred to as A, B, C and D; the A isoform lacks both 17 amino acid and KTS inserts; B isoform contains the 17-amino-acid insert but lacks KTS insert; C isoform lacks the 17-amino-acid insert but contains KTS insert; and D isoform contains both inserts. Mutations of WT1 were found to be associated with subsets of Wilms' tumor (reviewed in 8, 9), mesothelioma and ovarian tumor (reviewed in 10), consistent with the role of WT1 as a tumor suppressor. However, high levels of the wild-type WT1 mRNA and protein have been found in leukemia (11), lung cancer (12) and breast cancer (13-15). Breast cancer patients with tumors that highly express WT1 usually have a lower 5-year disease-free survival rate than patients with tumors of low WT1 expression (15), suggesting WT1 expression is associated with an aggressive phenotype of breast cancer. Recently, we found that WT1 positively regulated expression levels of EGFR and HER2/Neu, which contributed to TAM resistance (16). However, the biological function of WT1 in development of acquired TAM resistance is currently unknown.
The MCF7 cell line, originally established from an ER-positive breast adenocarcinoma, is an estrogen-dependent breast cancer cell line that responds well to TAM. In this study, we established a subline of MCF7 cells that can grow in the presence of TAM even though they retain ER-α expression and are estrogen responsive. We also found these cells highly expressed EGFR, HER2 and WT1. The biological function of WT1 in acquired TAM resistance was studied in these cells with knocked-down levels of WT1 expression.
Materials and Methods
Cell culture and establishment of stable cell lines
Relatively low-passage MCF7 cells (<35 passages) were recently obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained at 37°C in a 5% CO2 atmosphere in Improved Modified Eagle's Medium (IMEM) supplemented with 5% fetal calf serum. The subline of MCF7 cells used in this study, MCF7TAM had been established by culturing MCF7 cells in the presence of 1 μM TAM for six months and had obtained TAM-insensitive growth.
To establish cells expressing shRNA for WT1, MCF7TAM cells were plated at a density of 1×105 cells per 60-mm dish and transfected 24 hours later with a mixture of four WT1 shRNA expression vectors purchased from the OriGene (Rockville, MD, USA), using the FuGene 6 transfection reagent (Roche Applied Sciences, Indianapolis, IN, USA). The empty expression vector was also transfected into MCF7TAM cells to serve as a control. Forty-eight hours after transfection, the cells were re-plated and selected with 500 μg/ml of G418 (Invitrogen Corporation, Carlsbad, CA, USA) for two weeks. The medium was changed every three days until colonies appeared. We established a number of clonal cell lines that highly expressed WT1, three of which are described in detail in this study (MCF7/WTSh-1, -5 and -6). More than 20 individual clones from MCF7TAM cells transfected with the empty expression vector were pooled (MCF7TAM/V) and used as a control.
To examine cell growth in the presence or absence of 17β-estradiol (E2) or TAM, cells maintained for three days in phenol red-free IMEM plus 5% dextran-charcoal-stripped fetal calf serum (HyClone, Logan, UT, USA) were treated with 1 nM of E2, or ethanol vehicle as a control. TAM (Sigma, St. Louis, MO, USA) was used in the experiments. The cells were seeded at 1×104 cells per dish in 60 mm dishes and counted for 8 days using the ADAM automatic cell counter (Digital Bio., Korea). Three dishes were used for each experiment and experiments were repeated three times.
Western blot analysis
Cells were washed with phosphate-buffered saline (PBS) and lysed with lysis buffer [50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.25 mM EDTA pH 8.0, 0.1% sodium dodecyl sulfate (SDS), 1% Triton® X-100, 50 mM NaF and the protease inhibitor cocktail from Sigma]. After adjustment to the same total protein content, cell lysates were analyzed by Western blot analysis. Forty micrograms of cell lysates were boiled for 5 minutes in SDS gel loading buffer and separated on a 12% or 10% SDS-PAGE gel. After electrophoresis, the proteins were transferred to a PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were probed with different primary antibodies, incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies and visualized with enhanced chemiluminescence (ECL) detection reagents (Amersham Pharmacia Biotech. Piscataway, NJ, USA). The same membranes were stripped and reprobed with an antibody against β-actin (I-19) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to ensure equal loading.
Anti-EGF receptor (15F8) rabbit mAb was purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-HER2/Neu antibody was purchased from Santa Cruz Biotechnology. Anti-ER-α antibody (Ab-15) was obtained from Lab Vision Products (Fremont, CA, USA). Polyclonal anti-WT1 antibody was from Invitrogen Corporation.
Results
TAM-resistant MCF7 cells (MCF7TAM) exhibited estrogen-independent growth
To investigate the molecular mechanisms underlying acquired TAM resistance, we established a cell subline (MCF7TAM) from ER-positive breast cancer MCF7 cells by selecting them in the presence of 1 μM TAM for six months. Compared to the parental MCF7, MCF7TAM exhibited insensitivity to TAM (Figure 1A). In the absence of estrogen, MCF7 cells proliferated at a very slow rate while were stimulated to grow rapidly by 1 nM 17β-estradiol (E2) (Figure 1B), consistent with the fact that ER-positive MCF7 cells are estrogen-dependent that require the presence of estrogen for their optimum proliferation. MCF7TAM cells proliferated rapidly in the absence of estrogen compared to the parental MCF cells. Further growth of MCF7TAM cells was weakly stimulated by E2 (Figure 1B) compared to the parental MCF7 cells, suggesting that MCF7TAM cells exhibits estrogen-independent growth but are still estrogen responsive (Figure 1B).
Figure 1.
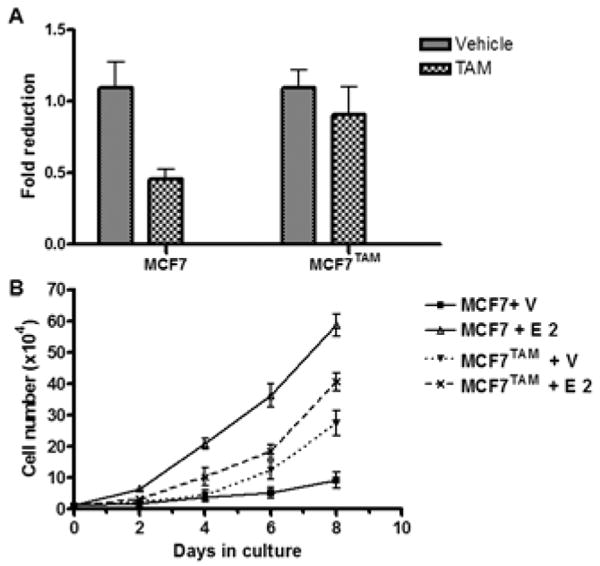
Tamoxifen resistant MCF7 cells (MCF7TAM) exhibit estrogen-independent and tamoxifen insensitive growth. (A). Cells were seeded at 1×104 cells per well in 60 mm dishes, incubated in medium containing ethanol (Vehicle) or 1 μM Tamoxifen (TAM) and counted in 12 days. Column: means of three independent experiments; bars, SE. (B). Growth rate of the parental MCF7 and MCF7TAM cells. Cells maintained in steroid-free medium were seeded at 1×104 cells per well in 6 well plates, incubated in medium containing ethanol (Vehicle) or 1 nM of 17β-estradiol (E2) and counted every other day. Data presented are means of three independent experiments; bars: SE.
MCF7TAM cells expressed high levels of EGFR, HER2 and WT1
It was well known that constitutive or enhanced expression of the EGFR family members such as EGFR and HER2 render antiestrogen resistance (17-19). Western blot analysis revealed that expression levels of HER2 and EGFR were increased in MCF7TAM cells compared to the parental MCF7 cells (Figure 2), indicating TAM resistant MCF7TAM cells gained expression of two members of the EGFR family. Accordingly, the basal levels of ERK phosphorylation were increased in MCF7TAM cells. However, the expression levels of ER-α in MCF7TAM cells were not significantly changed compared to the parental MCF7 cells (Figure 2). We also observed that the expression levels of the Wilms' tumor suppressor WT1 were greatly increased in MCF7TAM cells compared to the parental MCF7 cells (Figure 2).
Figure 2.

Tamoxifen resistant MCF7TAM cells express increased levels of EGFR, HER2 and WT1. Western blot analysis of the cell lysates from the parental MCF7 cells and MCF7TAM cells.
Knockdown of WT1 expression down-regulated expression of EGFR, HER2 and ER-a
Recently, we reported that forced expression of recombinant WT1 in MCF7 cells induced expression of HER2 and EGFR (16). To determine if WT1 is also the cause of the elevated expression of HER2 and EGFR in MCF7TAM cells, we transfected MCF7TAM cells that express high levels of endogenous WT1 with a mixture of four WT1 shRNA expression vectors. We established three clonal cell lines that expressed WT1 shRNA (MCF7TAM/WTSh-1, -5 and -6). We also generated a cell line from a mixture of more than twenty clones transfected with the empty expression expression vector (MCF7TAM/V). Western blot analysis using the antibody against WT1 confirmed that the WT1 protein (∼52 kDa) was dramatically down-regulated in three stable cell lines but not in the parental MCF7TAM cells and control MCF7TAM cells transfected with the empty vector (MCF7TAM/V) (Figure 3A). The expression levels of EGFR and HER2 were also dramatically down-regulated in WT1 knocked-down MCF7TAM cells compared to the parental MCF7TAM and MCF7TAM/V cells (Figure 3A). Intriguingly, ER-α expression was also significantly down-regulated in WT1 knocked-down MCF7TAM cells (Figure 3A). These results strongly suggested that WT1 is involved in the enhanced expression of EGFR and HER2 in acquired TAM-resistant MCF7TAM cells and WT1 is also involved in regulation of ER-α expression during development of acquired TAM resistance.
Figure 3.
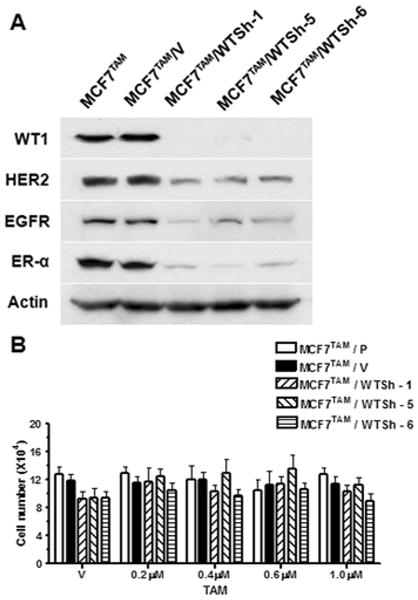
Knockdown of WT1 expression in MCF7TAM cells using the shRNA method down-regulates expression levels of EGFR, HER2 and ER-α, and has no effect on TAM resistance. (A). Western blot analysis of the lysates from the parental MCF7 cells (MCF7), MCF7TAM cells transfected with the empty expression vector (MCF7TAM/V) and clonal cell lines from MCF7TAM cells transfected with WT1 shRNA expression vectors (MCF7TAM/WTSh-1, -5 and -6). (B). Cells were seeded at 1×104 cells per 60mm dishes, incubated in medium containing ethanol (Vehicle), or indicated concentrations of TAM and counted in 12 days. Column: means of three independent experiments; bars, SE.
MCF7TAM cells with knocked-down levels of WT1 expression retain TAM resistance
To examine whether down-regulation of WT1 sensitizes MCF7TAM to TAM treatment, the parental MCF7TAM, MCF7TAM/V and MCF7TAM cells with WT1 expression knocked-down by the shRNA method were maintained in the absence of estrogen and treated with TAM. The growth rate of each cell line was determined by counting the number of cells after 8 days. We found that the WT1 ‘null’ MCF7TAM cells remained resistant to TAM (Figure 3B). These results suggested that down-regulation of ER-α expression in the MCF7TAM cells with WT1 expression knocked-down turned these cells into ER-negative breast cancer cells that are resistant to TAM.
Discussion
The switch of breast tumor growth from an estrogen-dependent to a growth factor-dependent phenotype is associated with a progression from a benign form to a more aggressive form of the disease. Endocrine therapy such as antiestrogen TAM that binds and antagonizes ER-α has been used to treat both early and advanced ER-positive breast cancer patients for almost three decades. Unfortunately, however, about 50% of patients with advanced primary breast tumors that are ER positive do not respond to first-line treatment with TAM and thus have de novo resistance to TAM therapy (17). Of those that initially respond to TAM, most of them eventually will develop estrogen independence and acquire resistance to TAM. Further understanding of the molecular mechanisms underlying the development of acquired TAM resistance will allow us to overcome these obstacles.
In the present study, we found that TAM-resistant MCF7 cells (MCF7TAM) exhibit estrogen- independent growth and are less sensitive to estrogen stimulation compared to the parental MCF7 cells. The MCF7TAM cells also express increased levels of both EGFR and HER2, members of the ErbB family. It is well established that in ER-positive breast tumors, overexpression of EGFR and/or HER2 is associated with resistance to endocrine therapy (18-20). However, the mechanisms whereby ER-positive breast cancer cells gain EGFR and HER2 expression during development of acquired TAM resistance are largely unknown. Here, our data suggested that the Wilms' tumor gene product WT1 may play an important role in the upregulation of EGFR and HER2 expression in ER-positive breast cancer cells. WT1 was initially discovered as a tumor suppressor gene of the genitourinary system. The biological function of WT1 outside of the genitourinary system remains controversy. Mutations of the WT1 gene have been reported in different types of cancer, such as mesothelioma, ovarian and prostate cancer and acute myeloid leukemia (reviewed in 9, 10). In recent years, however, accumulating evidence has indicated a potentially oncogenic role of WT1 in leukemia and breast cancer (reviewed in 21). WT1 expression was found in primary breast tumors (13-15) and high levels of WT1 expression were shown to predict a poor prognosis in breast cancer patients (15), consistent with a putative oncogenic role of WT1. WT1 is a dual transcription regulator and functions to activate or suppress gene transcription depending on cell and gene context (22-26), which may provide an explanation of WT1 function as both a tumor suppressor and oncogene. Previously, Zapata-Benavides et al (27) found that expression levels of WT1 correlated with the proliferation of several lines of breast cancer cells and down-regulation of WT1 expression using WT1 antisense oligonuleotides led to growth inhibition of breast cancer cells, suggesting a growth- promoting role of WT in breast cancer. In this study, we found knocked-down levels of WT1 expression in MCF7TAM cells resulted in a decreased expression of EGFR and HER2, suggesting that WT1 positively regulates their expression. This result is in a good agreement with our previous report that forced expression of recombinant WT1 in MCF7 cells led to an increased expression of EGFR and HER2 (16). Taken together, our results demonstrated that WT1 functions as a switch from TAM responsiveness to TAM resistance.
The molecular mechanisms by which WT1 activates EGFR and HER2 expression are not clear. It has been reported WT1 activates the EGFR promoter activity in neuron cells (23). It is thus possible that WT1 may activate EGFR expression directly in breast cancer cells. The HER2 promoter sequence is G+C rich. The 300 bp upstream of the mRNA start site has G+C content of 59% (28). It is also worth noting that a pentanucleotide sequence GGAGG appears eight times in the region upstream of the mRNA start site in the HER2 promoter region (28). In the EGFR promoter, the complement of this sequence, CCTCCTCC (the ‘TCC’ motif), appears four times upstream to the first start codon, which was required for WT1-mediated transcription activation of the EGFR gene (23). It is reasonable to speculate that the same ‘TCC’ motif in the HER2 promoter may be used for WT1 binding and activation of HER2 expression.
Recently, Han et al. reported that WT1 down-regulated ER-α expression and mediated antiestrogen resistance (29). On the contrary, here we observed significant down-regulation of ER-α expression in the WT1 ‘null’ MCF7TAM cells, suggesting that WT1 expression is required for optimum ER-α expression and knock-down of WT1 expression turns ER-positive breast cancer MCF7 cells into ER-negative cells. This may provide a molecular explanation for the observation that these WT ‘null’ cells were still resistant to TAM. The mechanisms underlying these contradictory results are not clear. In the previous report (29), recombinant expression of WT1 isoforms B and D were used for experiments. In this report, we used WT1 shRNA for all four isoforms of WT1, which may provide an explanation for the distinct effects of WT1 on ER-α expression observed. Previously, it was reported different WT1 isoforms have distinct effects on mammary epithelial cells (30). Thus, it is possible that different WT1 isoforms are involved in development of acquired TAM resistance observed in our experiments.
In summary, our results demonstrated that the Wilms' tumor suppressor WT1 induces EGFR and HER2 expression in breast cancer cells that acquired TAM resistance. These findings are consistent with the oncogenic role of WT1 in breast cancer progression and suggest that acquired WT1 expression may act as a switch to activate EGFR and HER2 expression. Future examination of WT1 expression together with EGFR and HER2 assessment might be useful as a new diagnostic marker for choosing the most appropriate treatment and as a prognosis factor in breast cancer patients under endocrine therapy.
Acknowledgments
This work was funded by the Nebraska Tobacco Settlement Biomedical Research Program Award (LB-595) to Z.Y. Wang and NIH grant DK070016 (Z.Y.Wang).
References
- 1.Clark RB, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2005;53:25–71. [PubMed] [Google Scholar]
- 2.Clarke RB, Liu MC, Bouker KB, Gu ZP, Lee RY, Zhu YL, Shaar TC, Gomez B, O'Brien K, Wang Y, Hilakivi-Clarke LA. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 3.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowske MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (ERB-B2) and mitogen-activated protein kinases enhances tamoxifen action against HER-2 overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 4.Carlomagno C, Perrone F, Gallo C, De Laurentitis M, Lauria R, Morabito A, Pettinato G, Panico L, D'Antonio A, Bianco AR, De Placido S. c-ERB-B2 overexpression decreases the benefit of adjuvant tamoxifen. J Clin Oncol. 1996;14:2702–2708. doi: 10.1200/JCO.1996.14.10.2702. [DOI] [PubMed] [Google Scholar]
- 5.Bonetta L, Kuehn SE, Huang A, Law DJ, Kalikin LM, Koi M, Reeve AE, Brownstein BH, Yeger H, Williams BR. Wilms' tumor locus on 11p13 defined by multiple CpG island-associated transcripts. Science. 1990;250:994–997. doi: 10.1126/science.2173146. [DOI] [PubMed] [Google Scholar]
- 6.Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990;60:509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- 7.Gessler M, Poustka A, Cavenee W, Neve RL, Orkin SH, Bruns GA. Homozygous deletion in Wilms' tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990;343:774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- 8.Coppes MJ, Campbell CE, Williams BR. The role of WT1 in Wilms' tumorigenesis. FASEB J. 1993;7:886–895. doi: 10.1096/fasebj.7.10.8393819. [DOI] [PubMed] [Google Scholar]
- 9.Lee SB, Haber DA. Wilms' tumor and the WT1 gene. Exp Cell Res. 2001;264:74–99. doi: 10.1006/excr.2000.5131. [DOI] [PubMed] [Google Scholar]
- 10.Little M, Wells C. A clinical overview of WT1 gene mutations. Hum Mutat. 1997;9:209–225. doi: 10.1002/(SICI)1098-1004(1997)9:3<209::AID-HUMU2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Ogawa H, Sonoda Y, Kimura T, Sakabe H, Oka Y, Miyake S, Tamaki H, Oji Y, Yamagami T, Tatekawa T, Soma T, Kishimoto T, Sugiyama H. Aberrant overexpression of the Wilms' tumor gene (WT1) in human leukemia. Blood. 1997;89:1405–1412. [PubMed] [Google Scholar]
- 12.Oji Y, Miyoshi S, Maeda H, Hayashi S, Tamaki H, Nakatsuka S, Yao M, Takahashi E, Nakano Y, Hirabayashi H, Shintani Y, Oka Y, Tsuboi A, Hosen N, Asada M, Fujioka T, Murakami M, Kanato K, Motomura M, Kim EH, Kawakami M, Ikegame K, Ogawa H, Aozasa K, Kawase I, Sugiyama H. Overexpression of the Wilms' tumor gene WT1 in de novo lung cancers. Int J Cancer. 2002;100:297–303. doi: 10.1002/ijc.10476. [DOI] [PubMed] [Google Scholar]
- 13.Silberstein GB, Horn KV, Strickland P, Roberts CT, Jr, Daniel CW. Altered expression of the WT1 Wilms' tumor suppressor gene in human breast cancer. Proc Natl Acad Sci USA. 1997;94:8132–8137. doi: 10.1073/pnas.94.15.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb DM, Evron E, Patel CB, Sharma PM, Niranjan B, Buluwela L, Weitzman SA, Korz D, Sukumar S. Wilms' tumor suppressor gene (WT1) is expressed in primary breast tumors despite tumor-specific promoter methylation. Cancer Res. 2001;61:921–925. [PubMed] [Google Scholar]
- 15.Miyoshi Y, Ando A, Egawa C, Taguchi T, Tamaki Y, Tamaki H, Sugiyama H, Noguchi S. High expression of Wilms' tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin Cancer Res. 2002;8:1167–1171. [PubMed] [Google Scholar]
- 16.Wang L, Wang ZY. The Wilms' tumor suppressor WT1 A-isoform induces estrogen independent growth and antiestrogen resistance in ER-positive breast cancer MCF7 cells. Oncology Reports. 2010;23:1109–1117. doi: 10.3892/or_00000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JMW. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer. 2004;11:623–641. doi: 10.1677/erc.1.00778. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, Barrow D, McClelland RA, Jones HE, Wakeling AE, Gee JMW. Modulation of epidermal growth factor receptor In endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr Relat Cancer. 2001;8:175–182. doi: 10.1677/erc.0.0080175. [DOI] [PubMed] [Google Scholar]
- 19.Grazia A, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocrine Reviews. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor R, Powles T, Humphreys J, Bettelheim R, Dowsett M, Casey A, Neville A, Coombes R. Effects of endocrine therapy on steroid-receptor content of breast cancer. Br J Cancer. 1982;45:80–84. doi: 10.1038/bjc.1982.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Han Y, Saiz FS, Minden MD. A tumor suppressor and oncogene the WT1 story. Leukemia. 2007;21:868–876. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZY, Qiu QQ, Deuel TF. The Wilms' tumor gene product WT1 activates or suppresses transcription through separate functional domains. J Bio Chem. 1993;268:9172–9175. [PubMed] [Google Scholar]
- 23.Liu XW, Gong LJ, Guo LY, Katagiri Y, Jiang H, Wang ZY, Johnson AC, Guroff G. The Wilms' tumor gene product WT1 mediates the down-regulation of the rat epidermal growth factor receptor by nerve growth factor in PC12 cells. J Bio Chem. 1998;273:27047–27050. doi: 10.1074/jbc.M008776200. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, San-Marina S, Liu J, Minden MD. Transcriptional activation of c-myc proto-oncogene by WT1 protein. Oncogene. 2004;23:6933–6941. doi: 10.1038/sj.onc.1207609. [DOI] [PubMed] [Google Scholar]
- 25.Hewitt SM, Hamada S, McDonnell TJ, Rauscher FJ, III, Saunders GF. Regulation of the proto-oncogenes bcl-2 and c-myc by the Wilms' tumor suppressor gene WT1. Cancer Res. 1995;55:5386–5389. [PubMed] [Google Scholar]
- 26.Englert C, Hou X, Maheswaran S, Bennett P, Ngwu C, Re GG, Garvin AJ, Rosner MR, Haber DA. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 1995;14:4662–4675. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zapata-Benavides P, Tuna M, Lopez-Berestein G, Tari AM. Down-regulation of Wilms'tumor 1 protein inhibits breast cancer proliferation. Biochem Biophys Res Commun. 2002;295:784–790. doi: 10.1016/s0006-291x(02)00751-9. [DOI] [PubMed] [Google Scholar]
- 28.Tal M, King CR, Kraus MH, Ullrich A, Schlessinger J, Givollt D. Human HER2 (neu) promoter: evidence for multiple mechanisms for transcriptional initiation. Mol Cell Biol. 1987;7:2597–2601. doi: 10.1128/mcb.7.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han YQ, Lin Y, Suarez-Saiz F, San-Matina S, Cui J, Minden MD. Wilms' tumor 1 suppressor gene mediates antiestrogen resistance via down-regulation of estrogen receptor-α expression in breast cancer cells. Mol Cancer Res. 2008;6:1347–1355. doi: 10.1158/1541-7786.MCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 30.Simpson LA, Thompson KA, Loeb DM. Isoforms of Wilms' tumor suppressor (WT1) have distinct effects on mammary epithelial cells. Oncogene. 2007;26:3423–3430. doi: 10.1038/sj.onc.1210127. [DOI] [PubMed] [Google Scholar]


